Abstract
Sechium edule (Cucurbitaceae) is a commercial species of chayote and is just one of several species in the genus Sechium, whose extracts inhibit proliferation in tumor cell lines. The capacity of the wild species Sechium chinantlense (SCH) as an antitumor agent is unknown, as is the mechanism of action. In the present study, HeLa cervical cancer and HaCaT normal cell lines were treated with SCH and cell proliferation was inhibited in both cell lines in a dose-dependent manner similar to the effect of the antineoplastic agent cisplatin (Cis). Additionally, SCH arrested cell cycle progression but only in HeLa cells and induced apoptosis, as shown by phosphatidylserine translocation and caspase-3 activation, while Cis did so in both cell lines. Exploration of the mechanism of action of SCH in HeLa cells suggests that apoptosis was mediated by the intrinsic signaling pathway since there was no activation of caspase-8, but there was a release of cytochrome-c. These findings suggest that the SCH extract has the potential to selectively kill tumor cells by promoting apoptosis, without harming nontumor cells.
1. Introduction
Cancer is a growing health problem worldwide; according to the statistics of the International Agency for Research on Cancer [AIIC] of 2020, it is estimated that there were more than 19 million cases worldwide and 9.9 million cancer deaths. The recurrence rate of cervical cancer is still high in developing countries, and this disease is the seventh most common cancer that affects the total population and the fourth most common in women worldwide. In 2020, there were 604,127 new cases and 341,831 cases of deaths [1]. Of the global cervical cancer deaths each year, 90% occur in low- and middle-income countries [2].
Some hallmarks of cancer are uncontrolled growth and apoptosis evasion [3], which are the major hurdles in defeating cancer. Apoptosis is the cell’s natural mechanism of programmed cell death and is mainly regulated by intrinsic or extrinsic pathways. In both pathways, apoptosis is carried out by caspases (cysteine aspartyl-specific proteases), which can function as initiators or executors that cleave target proteins, eventually leading to the death of the cell [4]. The pathways of apoptosis are highly regulated; the extrinsic pathway is death receptor (DR)-mediated apoptosis with a high concentration of caspase-6, 7, and 8 but the intrinsic pathway is mediated by cytochrome-c release from mitochondria and activation of caspase-9, which stimulates the effector caspase-3 [5].
Chemotherapy is used in patients with cervical cancer to suppress DNA duplication or damage DNA in order to kill cancer cells that divide rapidly, but it is also used as an addition to definitive locoregional treatments (surgery or radiotherapy) to improve patient outcome and as a palliative therapy for patients with recurrent or de novo metastatic disease. These treatment modalities are applied independent of the histology of the disease, which may impact prognosis as well as response to treatment [6]. The optimal approach to the treatment of locally advanced cervical cancer is concurrent chemotherapy with radiotherapy (CCRT). The benefit of adding concurrent chemotherapy to radiation therapy (RT) is greater in earlier stages of the disease. Cisplatin is the most preferred agent for concurrent chemoradiotherapy, other agents have been tried for CCRT, but none have been found to be as effective or superior to cisplatin. In patients who cannot tolerate cisplatin, 5-FU (fluorouracil) is an alternative. The therapy for cervical cancer is changing with increasing knowledge regarding disease biology, particularly genomics and immunology. In locally advanced cervical cancers, CCRT with cisplatin remains the standard of care, but other drugs, such as bevacizumab, have been shown to improve survival. Immunotherapy agents such as pembrolizumab show promise in the treatment of advanced disease [7]. While concurrent chemoradiation has improved prognoses for women with locally advanced cervical cancer, outcomes remain poor for women with node-positive, recurrent, and metastatic disease [8]. Additionally, adverse side effects, such as bone marrow dysfunction inhibition, neurological, cardiac, pulmonary, and renal toxicity; and nausea, vomiting, and alopecia, reduce quality of life [9]. Efforts have always been focused on discovering new anticancer agents from medicinal plants and have successfully resulted in several experimental models using natural products for the treatment [10]. The fruit of Sechium edule (Jacq.) Sw, also known as “chayote”, is part of the human diet, but in ethnomedicine, it is also recommended for the treatment of cancer [9]. Several edible varietal groups have been described with different antiproliferative effects on different tumor cell lines [11], but the potential of the wild species Sechium chinantlense (Lira & F. Chiang) is unknown. Therefore, in this work, the antiproliferative and apoptosis-inducing potential of the S. chinantlense extract was compared in the human cervical HeLa cancer cell line and the nontumorigenic HaCaT epithelial cell line.
2. Materials and Methods
2.1. Processing of Fruits and Preparation of Extract
The acquired S. chinantlense fruits were collected at horticultural maturity, 18 ± 2 days after the anthesis stage, by the Interdisciplinary Research Group on Sechium edule in Mexico, A. C. (GISeM), at the Germplasm Bank, located in Veracruz, Mexico (19°49′ N; 97°21′ W), in 2013, and was authenticated by Cadena-Iñiguez J. at the National Seed Inspection and Certification Service (SNICS; Secretariat of Agriculture, Livestock, Rural Development, Fisheries and Food, Mexico).
The fruits were cut into flakes. In this procedure, the whole fruit was used, which also included parts of the exocarp and the seed. Later, the flakes were dried at 40 °C in an air circulation oven (BLUE-M, Electronic Company, Blue Island, IL, USA) until completely dehydrated. They were finally ground (in a common mill), to a particle size of 2 mm. Subsequently, a discontinuous extraction was carried out using 1.5 kg of the ground material, which was macerated in methanol at room temperature (20 ± 2 °C), and every 48 h, the solvent was changed. Finally the extract was concentrated in a rotary evaporator (IKA® RV10, BUCHI R-114, Flawil, Switzerland) at 50 °C and the extract was recovered. The previous step was repeated 25 times until the solvent showed no color, and the extract was stored in an amber bottle, which was kept at room temperature. Of this extract, 71.2 mg was weighed in a tube and solubilized with 1 mL of PBS (phosphate buffer solution), and centrifuged at 2000 rpm for 5 min, and the supernatant was passed through a sterile filter and finally diluted in more PBS to obtain the following concentrations of the extract 0.0, 0.15, 0.3, 0.6, 1.2, 2.5, and 5 μg/mL.
2.2. Cell Culture and Culture Conditions
In this study, 2 types of cells were used, HeLa, which is a cell line from an adenocarcinoma of human cervical epithelial tissue, and HaCaT, a keratinocyte cell line from nontumorigenic human skin epithelial tissue; both were obtained from the American Type Culture Collection (ATCC) (The Global Bioresource Center, Manassas, VA, USA). The HeLa and HaCaT cell lines were cultured at densities of 1 × 105 and 7 × 104 cells/mL, respectively, with reseeding every 48 h. They were maintained in glass Petri dishes (Pyrex, Corning, NY, USA) with Iscove’s Modified Dulbecco’s Medium (IMDM) culture medium (GIBCO-BRL Invitrogen, Grand Island, NY, USA) and supplemented with 10% fetal bovine serum (FBS) (Invitrogen GIBCO-BRL HyClone, Carlsbad, CA, USA) in an incubator (Thermo Forma, Marietta, OH, USA) at 37 °C with an atmosphere of 5% CO2 and 95% humidity [12]. All the experiments were carried out under these same culture conditions. Depending on the experiment, the appropriate concentrations of the S. chinantlense extracts or the control antineoplastic cisplatin (Cis) diluted in PBS (Blastolem RU® 10 mg/10 mL, Teva Pharma AG, Basel, Switzerland) were used. For all experiments, 3 independent tests were performed with 3 repetitions per condition; in the case of histograms, a representative test is shown.
2.3. Antiproliferative Activity Assay
HeLa and HaCaT cells (2 × 104 cells/well) were seeded onto 96-well plates (Corning Costar, St. Louis, MO, USA) and exposed for 72 h to 0.0, 0.15, 0.30, 0.6, 1.2, 2.5 or 5 μg/mL of extracts prepared from S. chinantlense or cisplatin as control antineoplastic agent. Next, they were fixed with 1.1% glutaraldehyde, stained with 0.1% crystal violet solution (Sigma-Aldrich, St. Louis, MO, USA), washed with distilled water, and solubilized with 10% acetic acid [13], after which the absorbance was measured at 570 nm using a plate reader (Tecan Spectra, Grödig, Austria).
The results were plotted as percent proliferation relative to 0.0 μg/mL of extract. From these assays, the average inhibition concentration of cell proliferation by 50% (IC50) was determined, which was calculated using the linear regression equation and used in subsequent assays.
2.4. Discrimination of Apoptosis
The procedure was conducted according to the methodology provided in PE Annexin V Apoptosis Detection Kit (BD PharmingenTM, Franklin Lakes, NJ, USA). Cells were incubated with the S. chinantlense extract at the IC50 value or with cisplatin for 72 h. All adhering and floating cells were harvested and then suspended in 1X binding buffer at a concentration of 1 × 105 cells/mL. This cell suspension was transferred to an Annexin V-7AAD conjugate solution, and propidium iodide (PE) was added. The cells were incubated in the dark for 15 min at room temperature. The fluorescence of Annexin V-7AAD and PE (532 and 488 nm, respectively) was analyzed using a BD FACSAria II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
2.5. Cell Cycle Analysis
Cells were incubated with the S. chinantlense extract at the IC50 value or cisplatin for 72 h. All adhering and floating cells were harvested and washed twice with phosphate-buffered saline (PBS), then, 5 × 105 cells were resuspended in ice-cold 70% ethanol and incubated at 4 °C for 12 h. Then, the cells were washed twice with PBS, and incubated with propidium iodide/RNAse solution was added to the cells and incubated in the dark for 1.0 h at room temperature before being analyzed by flow cytometry (BD FACSAria II Biosciences, NJ, USA). The analysis, histograms, and percentages of cell phases were obtained using Flowing Software version 2.5.1 (Perttu Terho, Turku Centre for Biotechnology University of Turku, Finland).
2.6. Caspase-8 Activity Assay
The procedure was conducted according to the methodology provided in the Caspase-8 Colorimetric Assay Kit (R&D Systems®, Minneapolis, MN, USA). Cells were incubated with the S. chinantlense extract at the IC50 value or cisplatin for 24 h. All adhering and floating cells were harvested, and 2 × 106 cells were resuspended in an ice-cold lysis buffer for 10 min. Then, the cells were centrifuged, and the supernatant was transferred onto 96-well plates (Corning Costar, St. Louis, MO, USA) with 2X Reaction Buffer 8 and a colorimetric substrate of caspase-8. After 2 h at 37 °C, the absorbance was read at 490 nm using a microplate spectrophotometer (Thermo ScientificTM, MultiskanTM GO, Madrid, Spain).
2.7. p53 and Caspase-3 Activity Assays
HeLa and HaCaT cells were incubated with the S. chinantlense extract at IC50 value or cisplatin for 48 h. Then, all cells were harvested and washed with PBS. A total of 5 × 105 cells were incubated with ice-cold BD Cytofix/CytopermTM for 20 min and then washed twice with 1X BD Perm/WashTM. The subsequent steps were performed according to the manufacturer’s instructions provided in the PE Active Caspase-3 Apoptosis Kit (BD PharmingenTM NJ, USA). Additionally, in a set-aside sample of cells, an Alexa Fluor®647 Mouse anti-p53 (ack382) antibody (BD PhosflowTM, Piscataway, NJ, USA) was used, which recognizes the acetylated lysine, residue 382, in the C-terminal region of p53. After 30 min in the dark, we washed both samples (the samples stained with caspase-3 PE and p53 Alexa Fluor®647) before analyzing them using a BD FACSAria II flow cytometer (Biosciences, NJ, USA).
2.8. Cytochrome-c Release Assay
The concentration of cytochrome-c was determined by using the Human Cytochrome-c Immunoassay Quantikine® ELISA (R&D Systems, Inc, Minneapolis, MN, USA). Cytochrome-c measurement was performed according to the manufacturer’s instructions. HeLa and HaCaT cells were incubated with the S. chinantlense extract at the IC50 value or cisplatin for 24 h. Then, 1 × 106 cells were washed twice with PBS and incubated in lysis buffer for 1 h at room temperature with gentle shaking. Subsequently, the cells were centrifuged, and the supernatant was transferred onto 96-well plates (Corning Costar, St. Louis, MO, USA) with RD5P (1:10) diluent calibrator and after 2 h at room temperature, all samples were washed four times, and human cytochrome-c conjugate was added. After 2 h at room temperature and another four rounds of washing, we added substrate solution and incubated the mixture for 30 min in the dark at room temperature. The absorbance was read at 490 nm using a microplate spectrophotometer (Thermo ScientificTM, MultiskanTM GO, Madrid, Spain).
2.9. Statistical Analysis
Values are expressed as the mean ± S.D. for control and experimental samples and statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s test. For this analysis, IBM SPSS Statistics Software Package version 18.0 (Armonk, NY, USA) was used. The values were considered as statistically significant if the p value was equal to or less than 0.05.
3. Results
3.1. Sechium chinantlense Extract Causes Dose-Dependent Inhibition of the Proliferation of HeLa and HaCaT Cell Lines
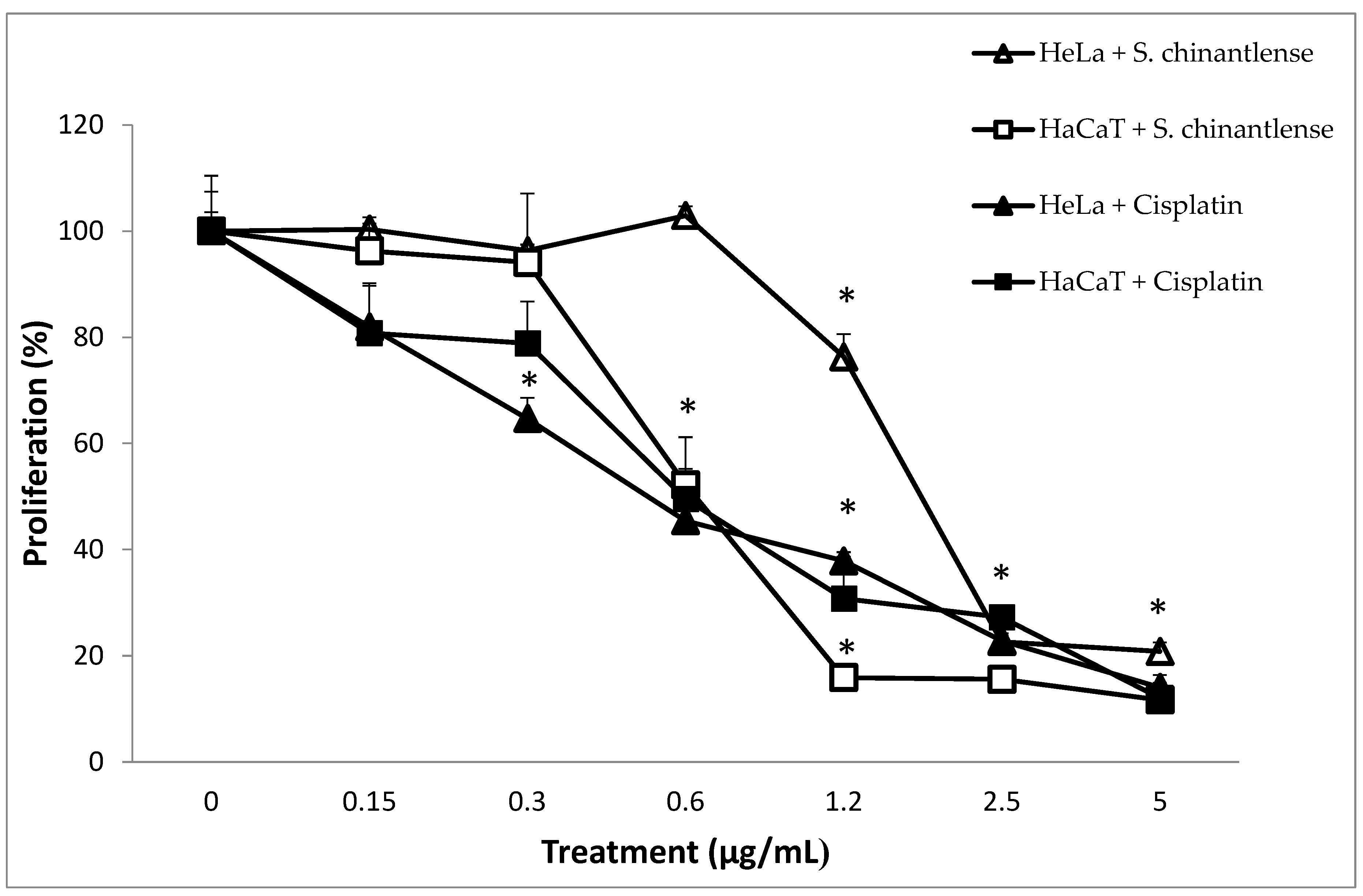
We evaluated the effect of S. chinantlense extract on a human cervical cancer cell line, HeLa, and a nontumorigenic epithelial cell line, HaCaT, using a crystal violet assay. The results showed a dose-dependent effect on cell proliferation of both cell lines upon treatment with increasing concentrations of S. chinantlense, and from 2.5 µg/mL of S. chinantlense extract, proliferation was inhibited by approximately 75% in both cell lines (Figure 1). Additionally, similar results were obtained when 0.3 µg/mL and 0.6 µg/mL of the antineoplastic agent cisplatin were used in HeLa and HaCaT cells, respectively. Using these dose-response results, the mean inhibition concentration (IC50) of S. chinantlense extract and cisplatin was calculated (Table 1), which was used in subsequent assays.
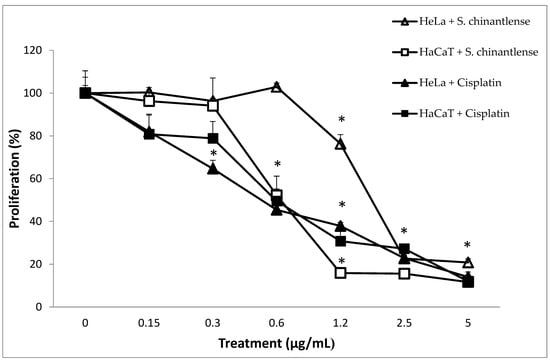
Figure 1.
Effect of different S. chinantlense extract concentrations on the proliferation of HeLa and HaCaT cell lines. The proliferation of HeLa and HaCaT cells was evaluated after 72 h in the presence of S. chinantlense extract or cisplatin. Average values of three independent experiments ± S.D. * p < 0.01 ANOVA—Tukey’s test with respect to the control of each cell line.

Table 1.
Mean concentration of inhibition of cell proliferation [IC50] (µg/mL) induced by S. chinantlense extract or cisplatin in HeLa and HaCaT cell lines.
3.2. Effects of S. chinantlense Extract on the Cell Cycle of HeLa and HaCaT Cell Lines
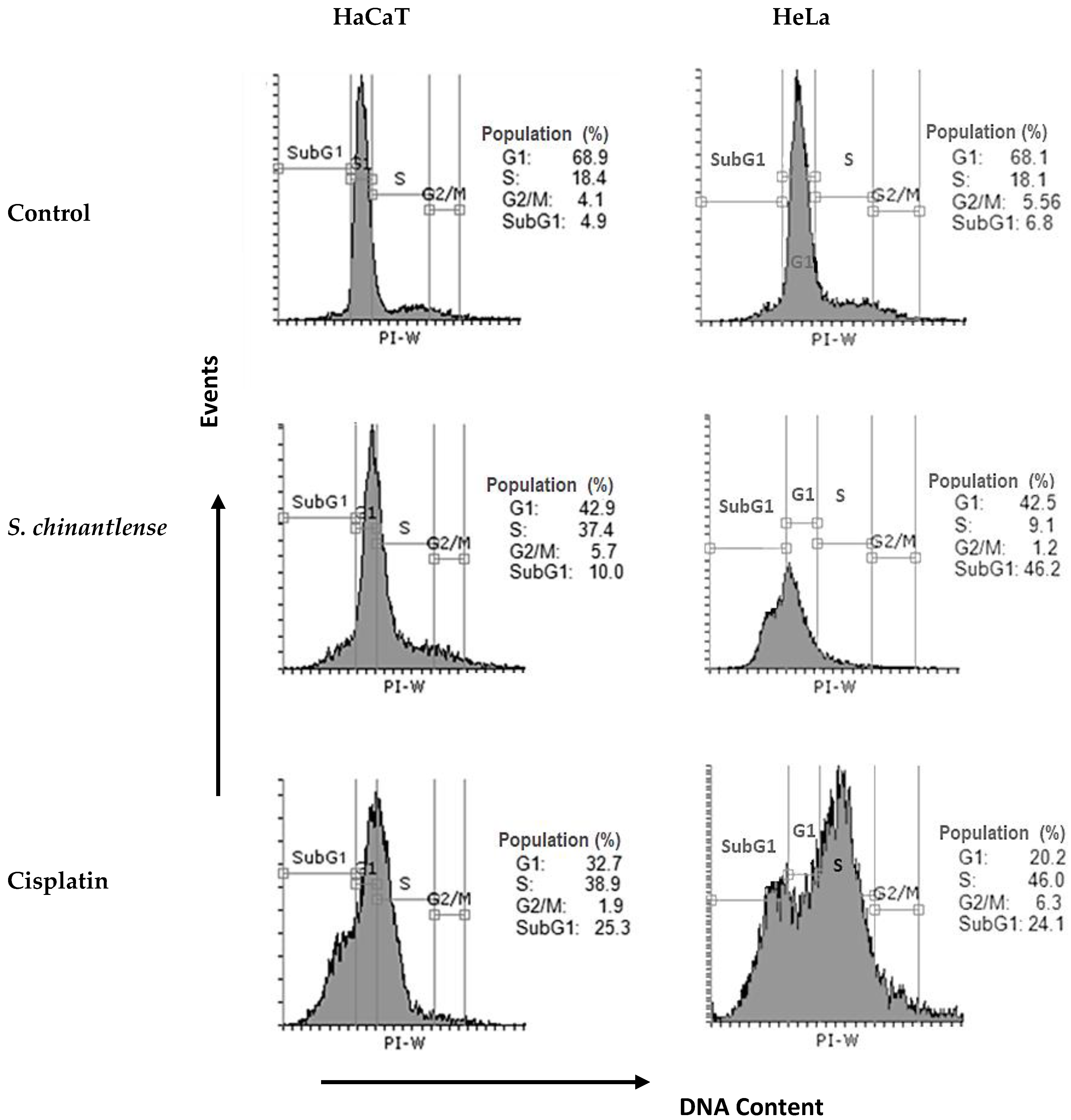
Since S. chinantlense extract and cisplatin inhibited cell proliferation, the effect of each respective IC50 on cell cycle progression in HeLa and HaCaT cell lines was evaluated. The evaluation indicated that S. chinantlense extract induced an increase in the percentage of cells in the SubG1 phase in HeLa cells and the percentage of cells in S phase in HaCaT cells, while cisplatin increased the S and SubG1 phases in both cell lines (Figure 2).
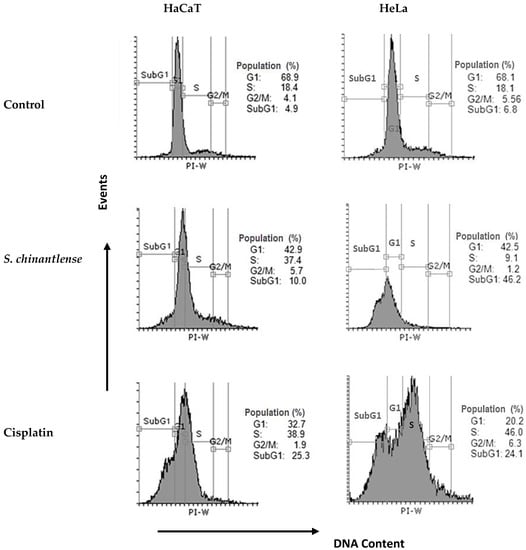
Figure 2.
Effects of S. chinantlense extract or cisplatin at the IC50 value on cell cycle distribution in HeLa and HaCaT cells. Histograms represent flow cytometric analysis of untreated control, S. chinantlense extract-treated, and cisplatin-treated HeLa and HaCaT cells. The X-axis represents the intensity of PI staining, which is directly proportional to the amount of DNA in cells, and the Y-axis represents cell number.
3.3. S. chinantlense Extract Increased the Presence of Active p53 in HeLa Cells
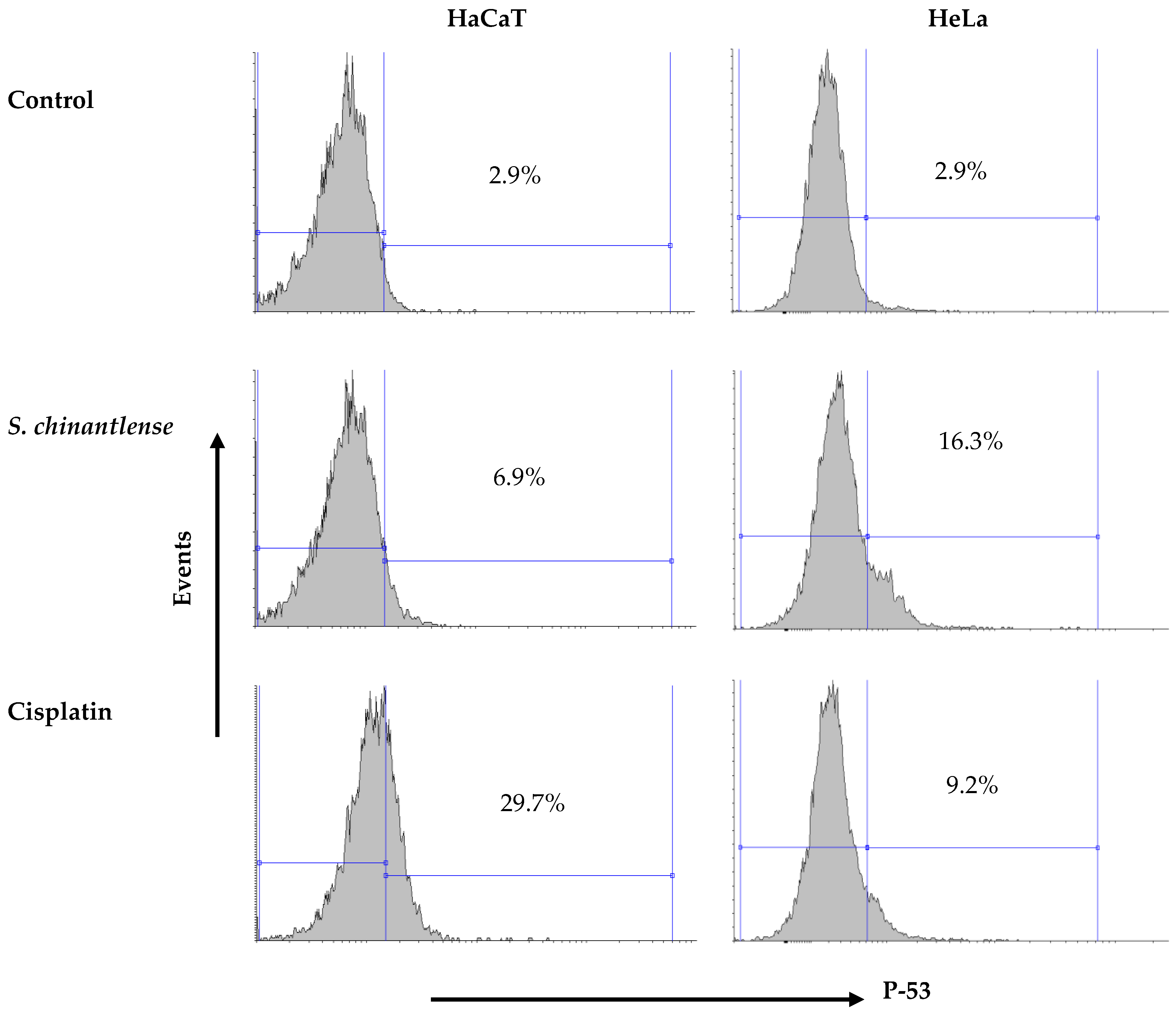
The arrest of HeLa cells in SubG1 phase in the cell cycle suggests DNA damage and this event may be related to p53 activation. The incubation of both cell lines in the presence of the S. chinantlense extract or cisplatin at IC50 showed that both treatments increased the presence of the active form of p53 in both cell lines, but while the S. chinantlense extract had a greater effect on HeLa tumor cells (16.3% vs. 6.9% in HaCaT cells), cisplatin had a greater effect on nontumorigenic HaCaT cells (29.7% vs. to 9.2% for HeLa cells) (Figure 3).
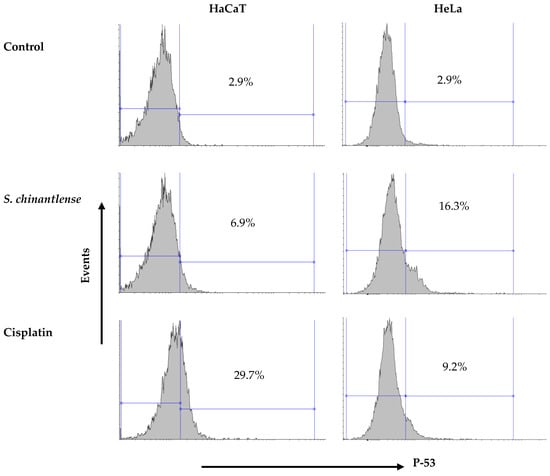
Figure 3.
Effect of incubation with S. chinantlense extract or cisplatin at the IC50 value for 48 h on the level of active p53 in HeLa and HaCaT cell lines.
3.4. S. chinantlense Extract Induces Apoptosis in HeLa Cells
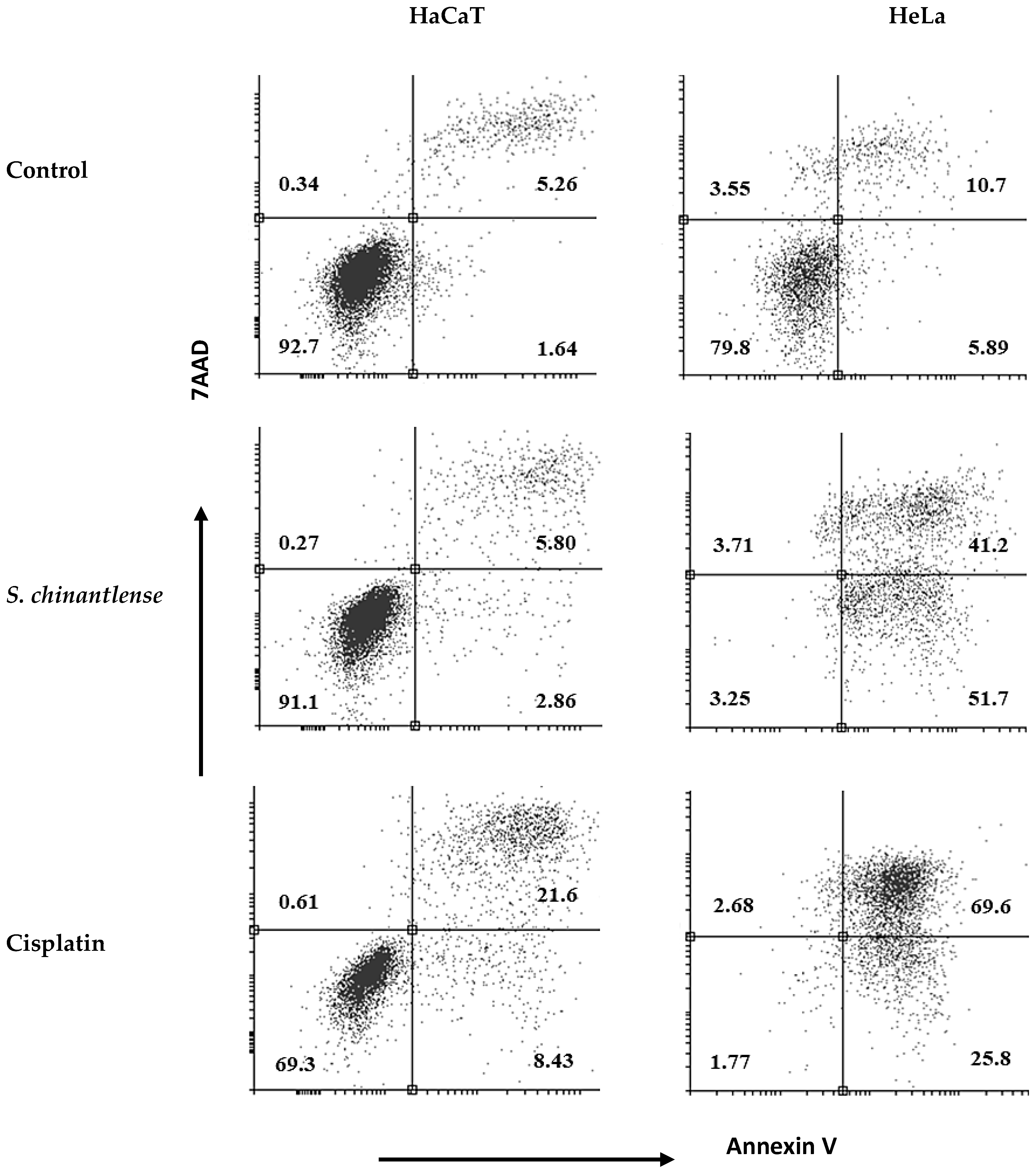
To determine if the S. chinantlense extract could induce apoptosis, we used annexin V-FITC and PI staining, and the stained cells were analyzed by flow cytometry. The translocation of phosphatidylserine, indicated by annexin V coupled to FITC, showed that exposure of HeLa cells to S. chinantlense extract increased early apoptotic cells (lower-right quadrants) to 51.7% vs. 5.8% in the control and increased late apoptotic cells (upper -right quadrants) to 41.2% vs. 10.7% in the control; in contrast, this effect was minimal in HaCaT cells, with early apoptotic cells to 2.86% vs. 1.64% in the control and late apoptotic cells to 5.80 vs. 5.26 in the control. Cisplatin induced apoptosis in both cell lines (Figure 4). These results suggested that the S. chinantlense extract could be effective in remarkably increasing the apoptosis of HeLa cancer cells at the early and late stages.
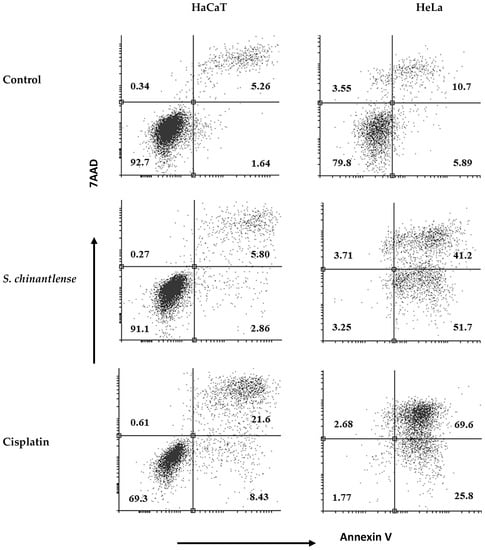
Figure 4.
Apoptosis analysis upon treatment of HeLa and HaCaT cells with S. chinantlense extract at the IC50 value. The distribution of cells undergoing early and late apoptosis together with viable cells was determined at 72 h in comparison to control or cisplatin at the IC50 value, using Annexin V-FITC and propidium iodide flow cytometric analysis.
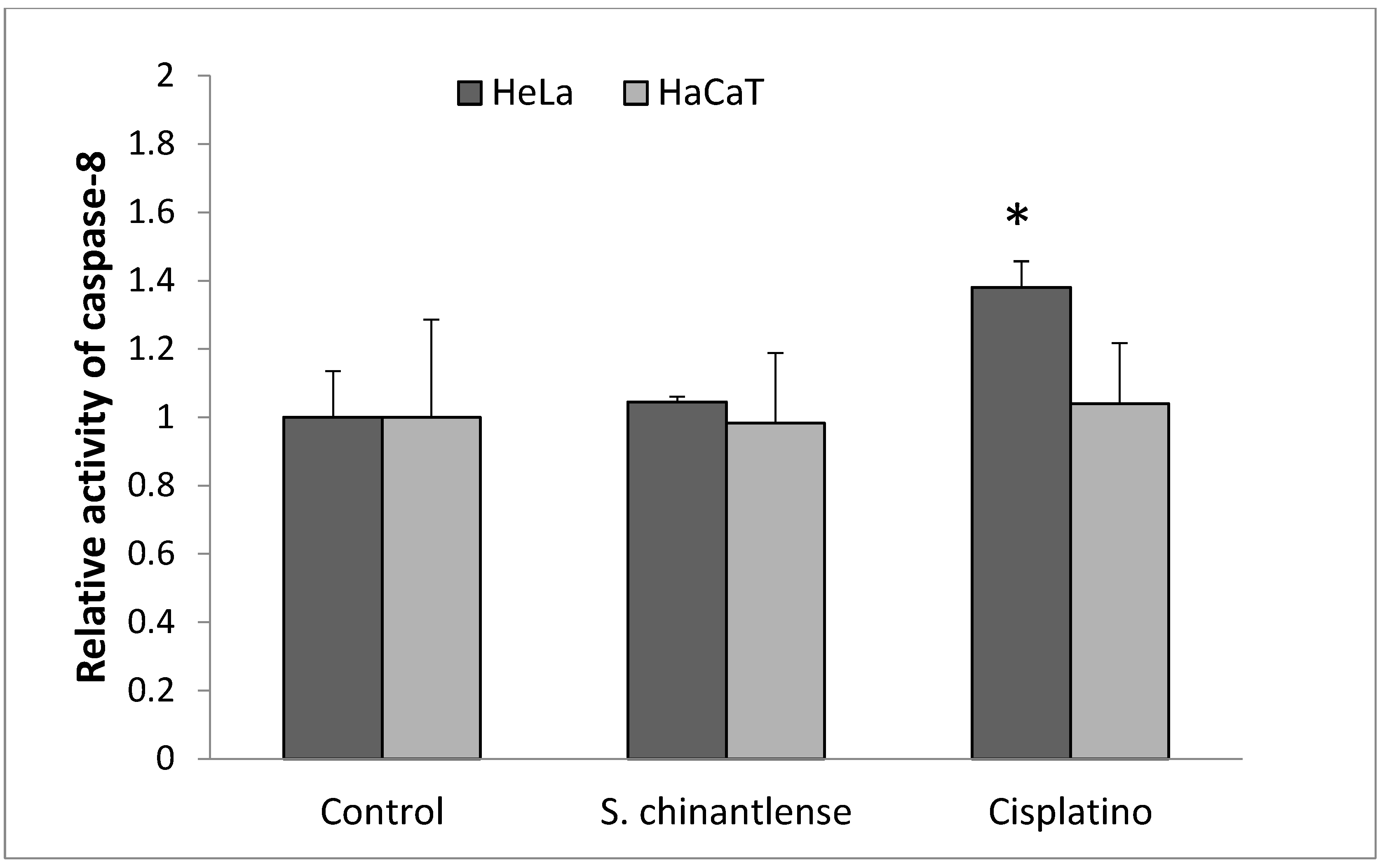
3.5. Active Caspase-8 Is Not Increased in HeLa or HaCaT Cells Treated with S. chinantlense Extract
Caspase-8 is considered a crucial modulator of the extrinsic pathway of apoptosis. As shown in Figure 5, S. chinantlense extract did not significantly increase caspase-8 activity in HeLa or HaCaT cells, after cisplatin treatment for 24 h, caspase-8 activity increased in HeLa cells but not in HaCaT cells.
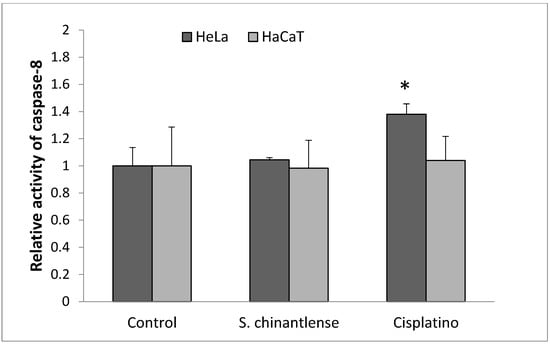
Figure 5.
Activation of caspase-8 in HeLa cancer cells after incubation with S. chinantlense extract or cisplatin at the IC50 value for 24 h. The values are presented as the mean ± S.D., where ∗ indicates a significant difference relative to the control (p < 0.05).
3.6. S. chinantlense Extract Induces the Release of Cytochrome c in HeLa and HaCaT Cells
In the intrinsic pathway, p53 is related to cytochrome-c release from mitochondria. Here, both S. chinantlense extract and cisplatin increased the release of cytochrome c in both HeLa and HaCaT cell lines (Table 2), suggesting that apoptosis activation occurred through the intrinsic pathway, even in HaCaT cells, which exhibited a small percentage of cell death when treated with S. chinantlense extract.

Table 2.
Mean concentration of cytoplasmic cytochrome c (μg/mL) in HeLa and HaCaT cell lines induced by S. chinantlense extract or cisplatin at the IC50 value for 24 h.
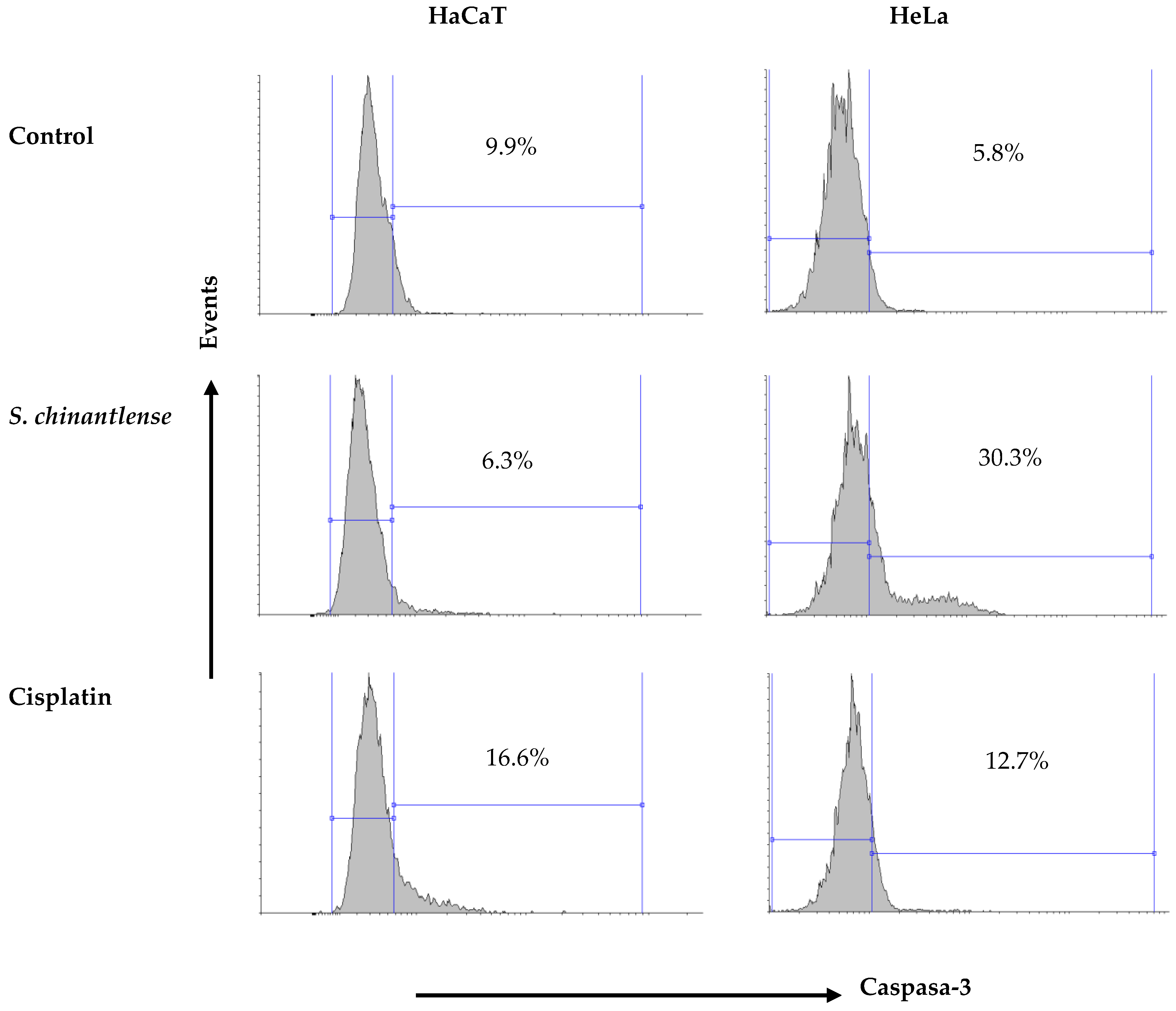
3.7. S. chinantlense Extract Induces an Increase in Active Caspase-3 in HeLa Cancer Cells but Not in Nontumorigenic HaCaT Cells
Caspase-3 is responsible for most of the apoptotic effects, and upon activation, it can induce cleavage and DNA breakage and finally lead to the death of cells. Cisplatin induced caspase-3 activation in both HeLa and HaCaT cells (12.7% and 16.6%, respectively) while exposure to the S. chinantlense extract induced the activation of caspase-3 in the HeLa cancer cell line but not in the HaCat cell line (30.3% and 6.3%, respectively) (Figure 6).
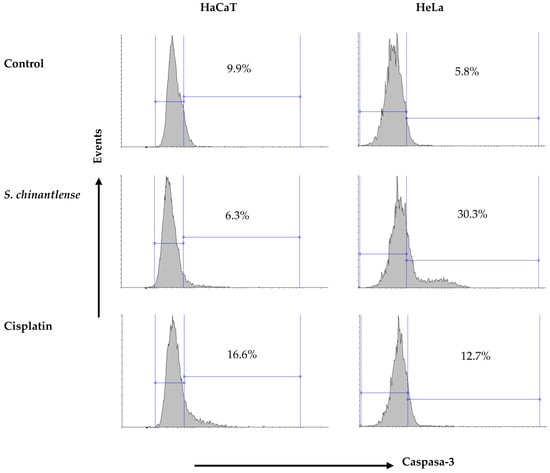
Figure 6.
Activation of caspase-3 in HeLa and HaCaT cell lines after incubation with S. chinantlense extract or cisplatin at the IC50 value for 48 h.
4. Discussion
This study shows that S. chinantlense extract inhibits proliferation in the cancer cell line HeLa and in the nontumorigenic cell line HaCaT. Additionally, the extract increased the percentage of cells in the SubG1 phase, translocation of phosphatidylserine (PS), release of cytochrome-c, and activation of p53 and caspase-3 (Figure 2, Figure 3, Figure 4 and Figure 6; Table 2. All these events are characteristic of cells entering apoptosis [14]. The data obtained in this study show that cisplatin induces the same biological response in HeLa cells, which has been widely reported [15,16]. These results allow us to suggest that the S. chinantlense extract induces death by apoptosis in HeLa tumor cells in the same manner as the antineoplastic agent cisplatin. Additionally, as has been previously reported, cisplatin is cytotoxic in HeLa cells but also in HaCaT cells [16]. In contrast, in HaCaT cells, the S. chinantlense extract induces the release of cytochrome c and arrest of the cell cycle in S phase but does not induce PS translocation or activate p53 or caspase-3, suggesting that S. chinantlense, although it has an antiproliferative effect, does not induce apoptosis in the nontumorigenic HaCaT cell line, but it does in HeLa cells. This differential effect has already been reported before. Allium atroviolaceum extract induces a cytotoxic effect on HeLa cells without affecting the normal cell line 3T3 [17], and resveratrol induces cell death in leukemic cells via caspases without affecting normal lymphocytes [18]. Most likely, what prevented HaCaT cells exposed to S. chinantlense extract from presenting the same apoptotic events, may be due to the presence of the inhibitor of apoptosis (IAP), since it has been shown that IAPs can inhibit apoptosis despite the release of cytochrome-c [19].
In some tumor cell lines, the release of cytochrome-c, a promoter of apoptosis via the intrinsic pathway, is promoted by plant phytochemicals [20]. Examples include lycopene, an abundant carotenoid phytochemical in tomatoes [21]; capsaicin, a phenolic compound from chili peppers (Capsicum sp.), that induces a rapid increase in reactive oxygen species followed by a disruption of mitochondrial membrane potential [22]; luteolin and curcumin found in celery and turmeric, respectively, which depolarize mitochondria and induce apoptosis in human tumor cells [23,24]; the crude extract of A. atroviolaceum, which induces the activation of caspase-9 and caspase-3. These examples all indicate that the mitochondrial pathway is involved in apoptosis induction in HeLa cells by plant phytochemicals [17]. A preliminary analysis of the phytochemical content in the S. chinantlense extract revealed that the main components are terpenes, flavonoids (flavones and flavonols), saponins and tannins [25] and that other phytochemicals reported in the species S. edule, such as peroxidases, sterols, phenols, polyphenols, fatty acid esters, carotenoids, vitamin c, antioxidants, and cucurbitacins, are also present [5,26,27,28,29,30]. This set of phytochemicals has been isolated individually from other plants and is reported to have anti-allergenic, anti-inflammatory, antimutagenic, antioxidant, antiviral, and antitumor and antileukemic effects [5,27,31,32,33,34,35,36,37], which allows us to assume that S. chinantlense, as it contains some of these compounds, such as cucurbitacins, is responsible for its antineoplastic activity.
It has also been reported that cucurbitacins show anticancer activity [29,38,39,40,41,42,43,44,45,46], via induction of cytochrome-c release, activation of caspase-3 and caspase-9, and apoptosis through the intrinsic pathway [47], and there are no reports that show that they activate the death receptor of the extrinsic pathway. Cucurbitacins have been present in the Cucurbitaceae family and widely in some of its genera, such as Sechium, to which S. chinantlense belongs [47]. Therefore, the apoptotic effect of the S. chinantlense extract may occur through the intrinsic pathway activated by cucurbitacins, as the main phytochemical responsible for the antineoplastic activity of the S. chinantlense extract. If so, it could serve as an adjuvant and complementary treatment, to increase the therapeutic efficacy of antineoplastic drugs and reduce the toxicity generated to the organs [48] and, therefore, reduce the side effects derived from chemotherapy treatment. Therefore, it is necessary to continue this research using in vivo models.
As we mentioned before, many plant species have been shown to have antiproliferative effects by using high concentrations of extracts; however, A. atroviolaceum is among some of the notable exceptions with effectiveness at low concentrations but still requires an IC50 of 75 μg/mL to induce apoptosis in HeLa cells [17]. In this sense, S. chinantlense extract has a much higher antiproliferative potential since it has an IC50 11 times lower than that required for an extract of oncological interest, according to the National Cancer Institute of the United States States [49]. It should be noted that the inhibition potential of S. chinantlense is similar to that of cisplatin, with a difference of 1.1 μg/mL between their IC50 values, and S. chinantlense has the advantage of being a natural extract that is considered less toxic to normal cells [24]. It should be noted that the S. edule wild varietal group extract has an IC50 of 1170 μg/mL [11], so the S. chinantlense extract is 600 times more effective as an antitumor agent, with similar in effectiveness in inhibiting the proliferation of the same cervical cancer line to that reported in a new cultivar of S. edule called “Perla Negra”, where the IC50 is 1.85 μg/mL [50]. Thus, the antitumor potential of the Sechium genus is evidenced by another study carried out with a hybrid of S. edule in leukemia cell lines, and the extract showed an antiproliferative and proapoptotic effect with an IC50 lower than 1.3 μg/mL [51]. Finally, in the WEHI-3 leukemic line, the S. chinantlense extract inhibited cell proliferation with an IC50 of 0.3 μg/mL, while 3.56 μg/mL was required to inhibit normal bone marrow cells [25], which correlates with our finding of the differential selective toxic effect against the HeLa tumor cell line compared to the nontumorigenic HaCaT cell line, so it would be important to analyze this differential effect between tumors cells and nontumorigenic cells in other types of tumor in the future. In the same way, in this study, the observation that S. chinantlense has a lower effect on normal cells indicates that it could also have fewer side effects, and it is necessary to perform more expansive experiments and even clinical studies to further explore the potential of S. chinantlense alone and in combination with the antineoplastic cisplatin agent.
5. Conclusions
In this study, Sechium chinantlense extract shows antitumor activity similar to that of antineoplastic agent cisplatin in eliminating neoplastic cells by inducing apoptosis, but S. chinantlense extract has certain selectivity for cervical cancer cells over nontumorigenic keratinocytes, which indicates the value of continuing the biomedical investigation of this wild species of the Sechium genus.
Author Contributions
Conceptualization, E.S.-O. and I.A.-S.; methodology, A.R.R.-M. and I.A.-S.; investigation, A.R.R.-M.; data curation, G.G.-G.; writing—original draft preparation, A.R.R.-M.; writing—review and editing, E.S.-O. and E.L.-M.; supervision, J.C.-I., I.S.-C., A.M.-G., B.W.-S. and E.S.-O.; project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was in part supported by Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México (DGAPA-UNAM, PAPIIT IA205222 and IN229820).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors sincerely thank Sandra Salazar Aguilar of the FES-Zaragoza, UNAM, Ph.D., for her assistance in writing this article. Rivera-Martínez acknowledges the scholarship granted by the National Council of Science and Technology (CONACyT). Rivera-Martínez acknowledges the Graduate Program in Biological Sciences at the National Autonomous University of Mexico (UNAM) for the training received during her studies. Finally, we especially thank GISeM, A.C., for the kind donation of the Sechium chinantlense fruit.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- The Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 6 August 2022).
- OMS (Organización Mundial de la Salud). Cáncer Cervicouterino. 2022. Available online: https://www.who.int/es/news-room/fact-sheets/detail/cervical-cancer (accessed on 6 August 2022).
- Hanahan, D.; Weinberg, A.R. The hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Cascales-Angosto, M. Bases moleculares de la apoptosis. Anal. Real. Acad. Nal. Farm. 2003, 69, 36–64. [Google Scholar]
- Liontos, M.; Kyriazoglou, A.; Dimitriadis, I.; Dimopoulos, M.A.; Bamias, A. Systemic therapy in cervical cancer: 30 years in review. Crit. Rev. Oncol. Hematol. 2019, 137, 9–17. [Google Scholar] [CrossRef]
- Gopu, P.; Antony, F.; Cyriac, S.; Karakasis, K.; Oza, A.M. Updates on systemic therapy for cervical cancer. Indian J. Med. Res. 2021, 154, 293–302. [Google Scholar] [PubMed]
- Feng, C.H.; Mell, L.K.; Sharabi, A.B.; McHale, M.; Mayadev, J.S. Immunotherapy with radiotherapy and chemoradiotherapy for cervical cancer. Semin. Radiat. Oncol. 2020, 30, 273–280. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef]
- Mann, S.; Sarkar, A.; Sharma, A.; Gupta, R.K.; Biswas, S. Antitumor Activity of Choerospondias axillaris Fruit Extract by Regulating the Expression of SNCAIP and SNCA on MDA-MB-231 Cells. Asian Pac. J. Cancer Prev. 2022, 23, 1577–1586. [Google Scholar] [CrossRef]
- Cadena Iñiguez, J.; Soto-Hernández, M.; Torres-Salas, A.; Aguiñiga-Sánchez, I.; RuizPosadas, L.; Rivera-Martínez, A.R.; Santiago-Osorio, E. The anti-proliferative effect of chayote varieties (Sechium edule (Jacq.) Sw.) on tumour cell lines. J. Med. Plant Res. 2013, 7, 455–460. [Google Scholar]
- Morgan, S.J.; Darling, D.C. Cultivo de Células Animals; Acriba: Zaragoza Spain, 1995; p. 159. [Google Scholar]
- Gillies, R.G.; Didier, N.; Dentosn, M. Determination of cell number in monolayer cultures. Anal. Biochem. 1986, 159, 109–113. [Google Scholar] [CrossRef]
- Ulukaya, E.; Acilan, C.; Yilmaz, Y. Apoptosis: Why and how does it occur in biology? Cell. Biochem. Funct. 2011, 29, 468–480. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Ortiz-Lazareno, P.C.; Lerma-Diaz, J.M.; Dominguez-Rodriguez, J.R.; Jave-Suarez, L.F.; Aguilar-Lemarroy, A.C.; Celis-Carrillo, R.; Toro-Arreola, S.; Castellanos-Esparza, Y.C.; Bravo-Cuellar, A. Pentoxifylline sensitizes human cervical tumor cells to cisplatin-induced apoptosis by suppressing NFkappaB and decreased cell senescence. BMC Cancer 2011, 11, 1–15. [Google Scholar] [CrossRef]
- Mohanty, S.; Huang, J.; Basu, A. Enhancement of cisplatin sensitivity of cisplatinresistant human cervical carcinoma cells by Bryostatin-1. Clin. Cancer Res. 2005, 11, 6730–6737. [Google Scholar]
- Khazaei, S.; Esa, N.M.; Ramachandran; Hamid, R.A.; Pandurangan, A.K.; Etemad, A.; Ismail, P. In vitro antiproliferative and apoptosis inducing effect of Allium atroviolaceum bulb extract on breast, cervical, and liver cancer cells. Front. Pharmacol. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Clément, M.V.; Hirpara, J.L.; Chawdhury, S.H.; Pervaiz, S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998, 92, 996–1002. [Google Scholar] [CrossRef]
- Deveraux, Q.L.; Roy, N.; Stennicke, H.R.; Van Arsdale, T.; Zhou, Q.; Srinivasula, S.M.; Alnemri, E.S.; Salvesen, G.S.; Reed, J.C. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998, 17, 2215–2223. [Google Scholar] [CrossRef]
- Flores-Balcázar, C.; Rosales-Pérez, S.; Sánchez, C.; Gallardo-Alvarado, L.; Gordillo-Bastidas, D. Nutrientes de la Dieta y Apoptosis como Mecanismos Reguladores del Cáncer. Arch. Med. 2015, 11, 1698–9465. [Google Scholar]
- Hantz, H.L.; Young, L.F.; Martin, K.R. Physiologically attainable concentrations of lycopene induce mitocondrial apoptosis in LNCaP human prostate cancer cells. Exp. Biol. Med. 2005, 230, 171–179. [Google Scholar] [CrossRef]
- Macho, A.; Calzado, M.A.; Munoz-Blanco, J.; Gomez-Diaz, C. Selective induction of apoptosis by capsaicin in transformed cells: The role of reactive oxygen species and calcium. Cell. Death. Differ. 1999, 6, 155–165. [Google Scholar] [CrossRef]
- Cheng, A.C.; Huang, T.C.; Lai, C.S.; Pan, M.H. Induction of apoptosis by luteolin through cleavage of Bcl-2 family in human leukemia HL-60 cells. Eur. J. Pharmacol. 2005, 509, 1–10. [Google Scholar] [CrossRef]
- Pan, M.H.; Ghai, G.; Ho, C.T. Food bioactives, apoptosis, and cancer. Mol. Nutr. Food. Res. 2008, 52, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Aguiñiga, S.I. Potencial Antileucémico In Vitro de Extractos de Cuatro Genotipos de Sechium spp. (Cucurbitaceae). Master’s Thesis, Colegio de Posgraduados, Texcoco, Mexico, 2013. [Google Scholar]
- Cadena-Iñiguez, J. Caracterización Morfoestructural, Fisiológica, Química y Genética de Diferentes tipos de Chayote (Sechium edule (Jacq.) Sw). Ph.D. Thesis, Colegio de Postgraduados, Texcoco, Mexico, 2005. [Google Scholar]
- Cadena-Iñiguez, J.; Arévalo, L.; Avedaño, C.; Soto, M.; Ruíz, L.; Santiago, E.; Acosta, M.; Cisneros, V.; Aguirre, J.; Ochoa, D. Production, genetics, postharvest managment and pharmacological characteristics of Sechium edule (Jacq.) Sw. Fresh. Prod. 2007, 1, 41–53. [Google Scholar]
- Monroy-Vázquez, M.E.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Santiago-Osorio, E.; Ruiz-Posadas, L.M.; Rosas-Acevedo, H. Estudio biodirigido de un extracto alcohólico de frutos de Sechium edule (Jacq.). Swartz. Agrociencia 2009, 43, 777–790. [Google Scholar]
- Aguiñiga-Sánchez, I.; Cadena-Íñiguez, J.; Santiago-Osorio, E.; Gómez-García, G.; Mendoza-Núñez, V.M.; Rosado-Pérez, J.; Ruíz-Ramos, M.; Cisneros-Solano, V.M.; Ledesma-Martínez, E.; Delgado-Bordonave, A.d.J.; et al. Chemical analyses and in vitro and in vivo toxicity of fruit methanol extract of Sechium edule var. nigrum spinosum. Pharm. Biol. 2017, 55, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Pinho, O.; Ferreira, I.M.; Delerue-Matos, C. Chayote (Sechium edule): A review of nutritional composition, bioactivities, and potential applications. Food Chem. 2019, 275, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.A.; Polo, N.A.E.; Contreras, M.C.R.; Maldonado, R.L. Análisis fitoquímico preliminar y determinación de las actividades anti-inflamatoria y cardiaca de Sechium edule. Rev. Col. Ciencias. Químico Farm. 1986, 15, 79–82. [Google Scholar]
- Yen, G.C.; Chen, H.Y.; Peng, H.H. Evaluation of the cytotoxicity, mutagenicity and antimutagenicity of emerging edible plants. Food. Chem. Toxic. 2001, 39, 1045–1053. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and anti-inflamatory activities of cucurbitacinas from Cucurbita andreana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Setzer, W.N.; Setzer, M.C. Plant-derived triterpenoids as potential antineoplastic agents. Mini Review. Med. Chem. 2003, 3, 540–556. [Google Scholar]
- Siciliano, T.; De Tomáis, N.; Morelli, I.; Braca, A. Study of flavonoids of Sechium edule (Jacq.) Swartz (cucurbitaceae) different edible organs by liquid chromatography photodiode array mass spectrometry. J. Agric. Food. Chem. 2004, 52, 6510–6515. [Google Scholar] [CrossRef]
- Ordoñez, A.A.L.; Gómez, J.D.; Cudmani, N.M.; Vattuone, M.A.; Isla, M.I. Antimicrobial activity of nine extracts of Sechium edule (Jacq.) Sw. Microb. Ecol. Health. Dis. 2003, 15, 33–39. [Google Scholar]
- Ordoñez, A.A.L.; Gómez, J.D.; Vattuone, M.A.; Isla, M.I. Antioxidant activities of Sechium edule (Jacq.) Sw. extracts. Food. Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Dantas, I.N.; Gadelha, G.C.M.; Chaves, D.C.; Monte, F.J.Q.; Pessoa, C.; De Moraes, M.O.; Costa-Cotufo, L.V. Studies on the cytotoxicity of cucurbitacins isolated from Cayaponia racemosa (Cucurbitaceae). Z. Naturforsch, C. Biosciences 2006, 61, 643–646. [Google Scholar]
- Li, D.; Ikeda, T.; Nohara, T.; Liu, J.; Wen, Y.; Sakamoto, T.; Nonaka, G. Cucurbitane glycosides from unripe fruits of Siraitia grosvenori. Chem. Pharm. Bull. 2007, 55, 1082–1086. [Google Scholar] [CrossRef]
- Chen, J.; Tian, R.; Qiu, M.; Lu, L.; Zheng, Y.; Zhang, Z. Trinorcucurbitane and cucurbitane triterpenoids from the roots of Momordica charantia. Phytochemistry 2008, 69, 1043–1048. [Google Scholar] [CrossRef]
- Wang, D.C.; Xiang, H.; Li, D.; Gao, H.; Cai, H.; Wu, L.J.; Deng, X.M. Purinecontaining cucurbitane triterpenoids from Cucurbita pepo cv. dayangua. Phytochemistry 2008, 69, 1434–1438. [Google Scholar] [CrossRef]
- Soto-Hernández, M.; Cadena-Iñiguez, J.; Arévalo-Galarza, L.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Ruíz-Posadas, L.; del Mar Ruíz-Posadas, L. Lead compounds from Cucurbitaceae for the treatment of cáncer. In Phytochemicals—Isolation, Characterization and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; IntechOpen: Rijeka, Croatia, 2015; pp. 289–303. [Google Scholar]
- Klungsaeng, S.; Kukongviriyapan, V.; Prawan, A.; Kongpetch, S.; Senggunprai, L. Cucurbitacin B induces mitochondrial-mediated apoptosis pathway in cholangiocarcinoma cells via suppressing focal adhesion kinase signaling. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 271–278. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, W.; Hao, W.; Ren, G.; Lu, J.; Chen, X. Cucurbitacin B induces DNA damage, G2/M phase arrest, and apoptosis mediated by reactive oxygen species (ROS) in leukemia K562 cells. Anti Cancer. Agents. Med. Chem. 2014, 14, 1146–1153. [Google Scholar] [CrossRef]
- Qu, Y.; Cong, P.; Lin, C.; Deng, Y.; Li-Ling, J.; Zhang, M. Inhibition of paclitaxel resistance and apoptosis induction by cucurbitacin B in ovarian carcinoma cells. Oncol. Lett. 2017, 14, 145–152. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Gao, M.X.; Yang, K. Cucurbitacin B inhibits cell proliferation and induces apoptosis in human osteosarcoma cells via modulation of the JAK2/STAT3 and MAPK pathways. Exp. Ther. Med. 2017, 14, 805–812. [Google Scholar] [CrossRef]
- Delgado-Tiburcio, E.E.; Cadena-Iñiguez, J.; Santiago-Osorio, E.; Ruiz-Posadas, L.d.M.; Castillo-Juárez, I.; Aguiñiga-Sánchez, I.; Soto-Hernández, M. Pharmacokinetics and Biological Activity of Cucurbitacins. Pharmaceuticals 2022, 15, 1325. [Google Scholar] [CrossRef] [PubMed]
- Arafa, H.M.M. Possible contribution of β-glucosidase and caspases in the cytotoxicity of glufosfamide in colon cancer cells. Eur. J. Pharmacol. 2009, 616, 58–63. [Google Scholar] [CrossRef]
- Abu-Dahab, R.; Afifi, F. Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7). Sci. Pharm. 2007, 75, 121–136. [Google Scholar] [CrossRef]
- Salazar-Aguilar, S.; Ruiz-Posadas, L.D.M.; Cadena-Iñiguez, J.; Soto-Hernández, M.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Rivera-Martínez, A.R.; Aguirre-Medina, J.F. Sechium edule (Jacq.) Swartz, a New Cultivar with Antiproliferative Potential in a Human Cervical Cancer HeLa Cell Line. Nutrients 2017, 9, 798. [Google Scholar] [CrossRef] [PubMed]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Ruíz-Posadas, L.M.; Cadena-Zamudio, J.D.; González-Ugarte, A.K.; Steider, B.W.; Santiago-Osorio, E. Fruit extract from a Sechium edule hybrid induce apoptosis in leukaemic cell lines but not in normal cells. Nutr. Cancer 2015, 67, 250–257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).