Photoperiod Conditions Modulate Serum Oxylipins Levels in Healthy and Obese Rats: Impact of Proanthocyanidins and Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
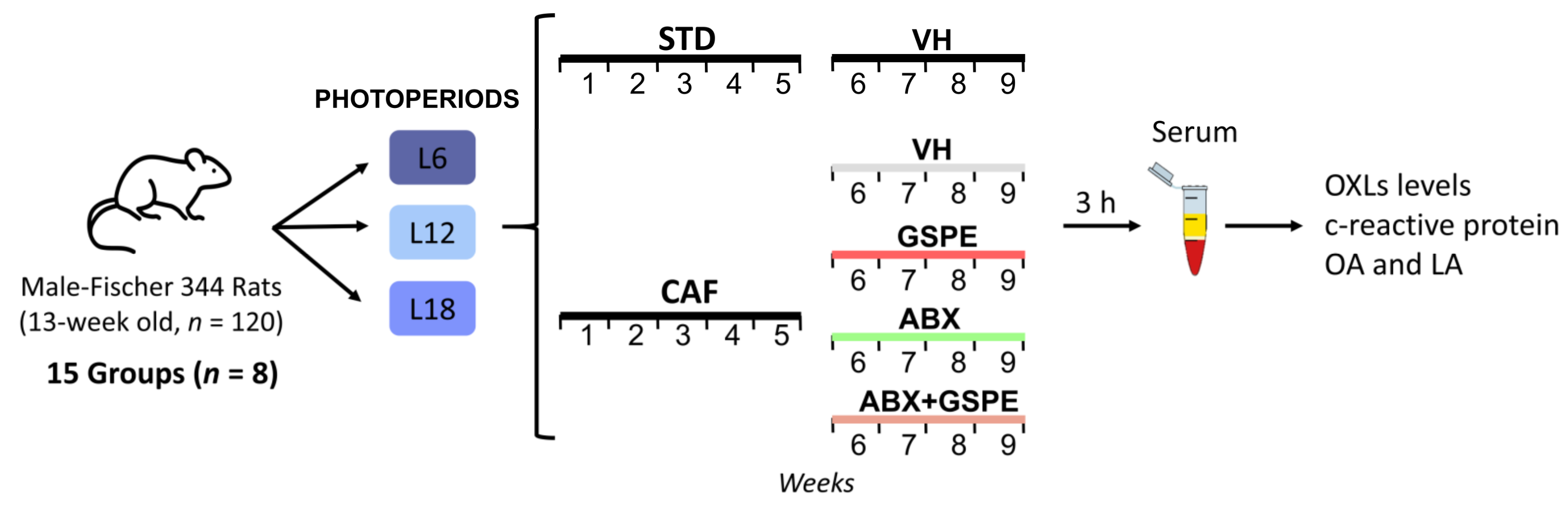
2.1. Experimental Design
- STD-fed rats (72% carbohydrate, 8% lipid, and 19% protein; Safe-A04c, Rosenberg, Germany) receiving a daily oral dose of vehicle (VH, condensed milk diluted with water in 1:5 proportion).
- Cafeteria diet (CAF)-fed rats receiving a daily oral dose of VH.
- CAF-fed rats administered a daily oral dose of GSPE (25 mg/kg BW dissolved in VH).
- CAF-fed rats receiving an antibiotic cocktail (ABX) in drinking water.
- CAF-fed rats receiving both a daily oral dose of GSPE and ABX in drinking water.
2.2. GSPE
2.3. C-Reactive Protein Analysis
2.4. OXLs Serum Analysis
2.5. Fatty Acids Serum Analysis
2.6. Statistical Analysis
3. Results
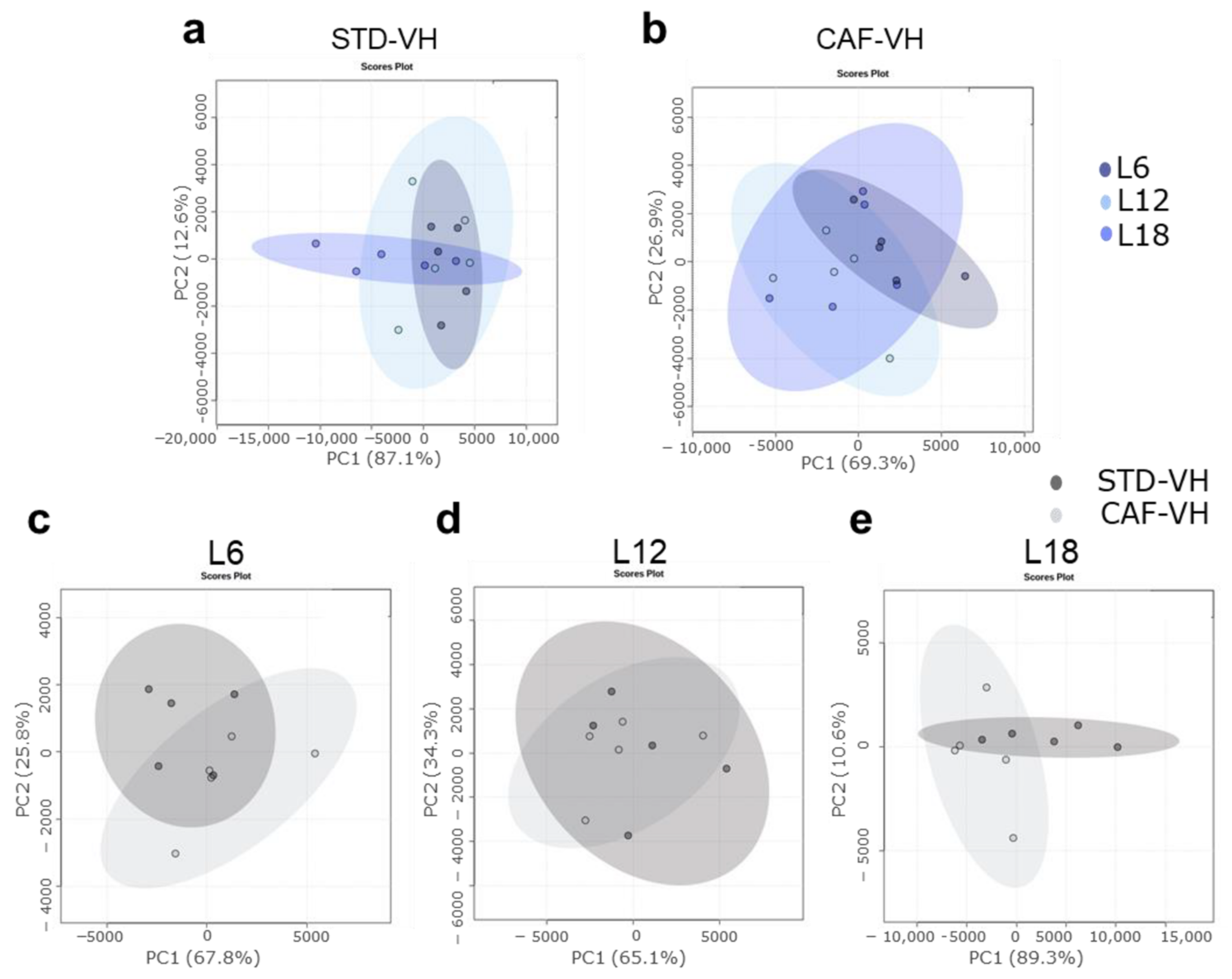
3.1. OXLs Levels Are Affected by Photoperiods Conditions Depending on the Healthy or Obese Condition. CAF Effects Are Different Depending on the Photopeiod Condition
3.2. Photoperiod Effects on OXLs Serum Levels in Obese Rats Is Influenced by Gut Microbiota
3.3. GSPE Significantly Altered PGE2 Serum Levels in Obese Rats in a Photoperiod Dependent Manner
3.4. GSPE Mitigated ABX-Mediated Changes in Serum OXLs Levels in CAF-Fed Rats in a Photoperiod Dependent Manner
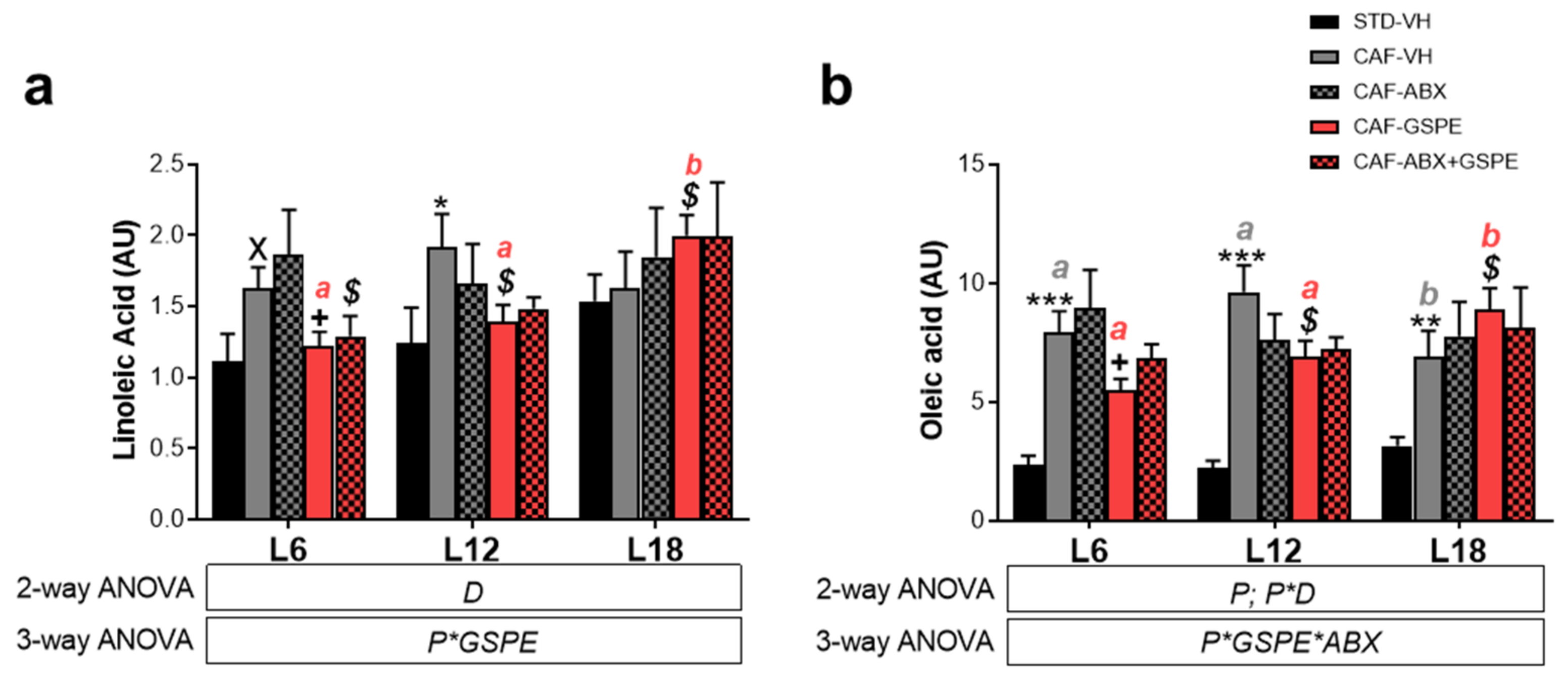
3.5. LA and OA Were Affected by Diet and GSPE Differently Depending on Photoperiod Conditions
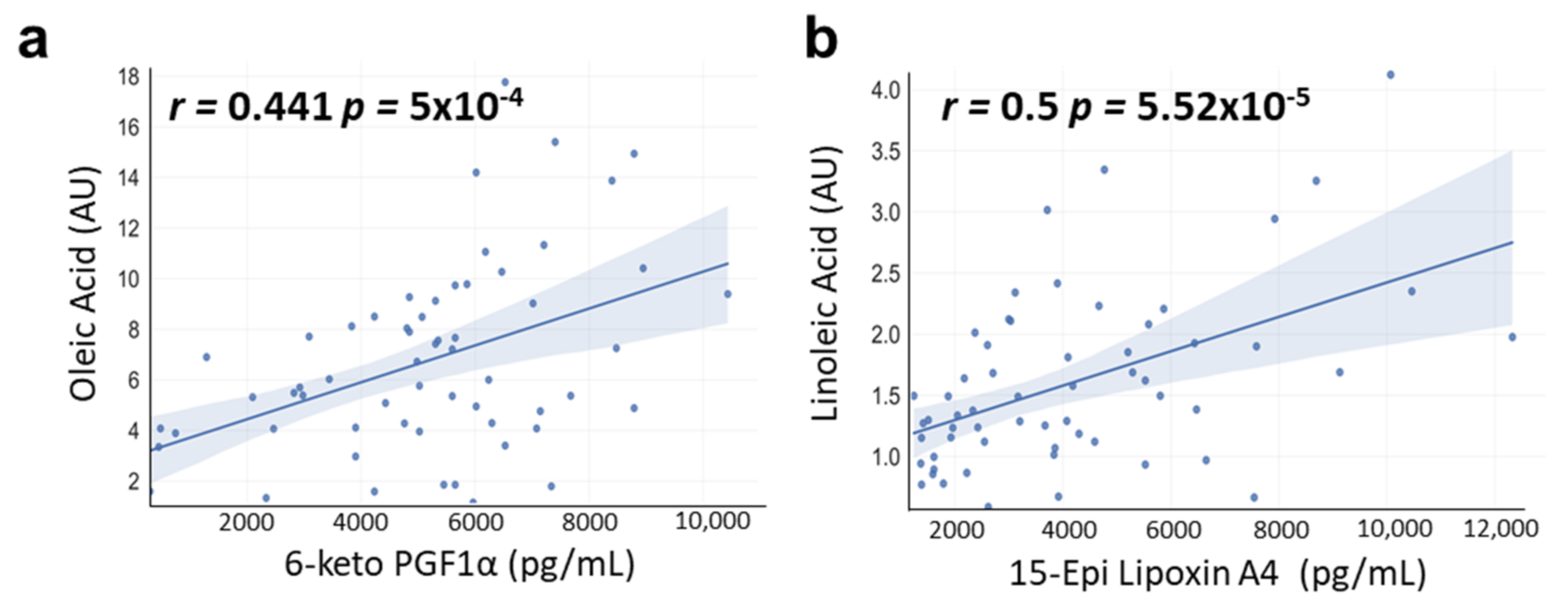
3.6. Correlations between OA and LA with OXLs Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rácz, B.; Dušková, M.; Stárka, L.; Hainer, V.; Kunešová, M. Links between the Circadian Rhythm, Obesity and the Microbiome. Physiol. Res. 2018, 67, S409–S420. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K.G.; Maneval, A.C.; Cable, E.C.; Tuohy, M.C.; Scasny, A.J.; Sterina, E.; Love, J.A.; Riggle, J.P.; Malamut, L.K.; Mukerji, A.; et al. Circadian and Circannual Timescales Interact to Generate Seasonal Changes in Immune Function. Brain. Behav. Immun. 2020, 83, 33–43. [Google Scholar] [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between Light at Night, Melatonin Secretion, Sleep Deprivation, and the Internal Clock: Health Impacts and Mechanisms of Circadian Disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. The Exposure to Different Photoperiods Strongly Modulates the Glucose and Lipid Metabolisms of Normoweight Fischer 344 Rats. Front. Physiol. 2018, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Y.; Yang, D.B.; Xu, Y.C.; Gronning, M.O.L.; Zhang, F.; Wang, D.H.; Speakman, J.R. Photoperiod Induced Obesity in the Brandt’s Vole (Lasiopodomys Brandtii): A Model of “Healthy Obesity”? Dis. Model. Mech. 2016, 9, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Kobayashi, M. The Relationship between Obesity and Seasonal Variation in Body Weight among Elementary School Children in Tokyo. Econ. Hum. Biol. 2006, 4, 253–261. [Google Scholar] [CrossRef]
- Moreno, J.P.; Johnston, C.A.; Chen, T.A.; O’Connor, T.A.; Hughes, S.O.; Baranowski, J.; Woehler, D.; Baranowski, T. Seasonal Variability in Weight Change during Elementary School. Obesity 2015, 23, 422–428. [Google Scholar] [CrossRef]
- Moreno, J.P.; Crowley, S.J.; Alfano, C.A.; Hannay, K.M.; Thompson, D.; Baranowski, T. Potential Circadian and Circannual Rhythm Contributions to the Obesity Epidemic in Elementary School Age Children. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, T.J.; Prendergast, B.J. Photoperiodic time measurement and seasonal immunological plasticity. Front. Neuroendocrinol. 2016, 37, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Weil, Z.M.; Borniger, J.C.; Cisse, Y.M.; Abi Salloum, B.A.; Nelson, R.J. Neuroendocrine Control of Photoperiodic Changes in Immune Function. Front. Neuroendocrinol. 2015, 37, 108–118. [Google Scholar] [CrossRef]
- Mao, C.; Xu, Y.; Shi, L.; Guo, S.; Jin, X.; Yan, S.; Shi, B. Effects of Photoperiod Change on Melatonin Secretion, Immune Function and Antioxidant Status of Cashmere Goats. Animals 2019, 9, 766. [Google Scholar] [CrossRef] [Green Version]
- Bilbo, S.D.; Dhabhar, F.S.; Viswanathan, K.; Saul, A.; Yellon, S.M.; Nelson, R.J. Short Day Lengths Augment Stress-Induced Leukocyte Trafficking and Stress-Induced Enhancement of Skin Immune Function. Proc. Natl. Acad. Sci. USA 2002, 99, 4067–4072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prendergast, B.J.; Kampf-Lassin, A.; Yee, J.R.; Galang, J.; McMaster, N.; Kay, L.M. Winter Day Lengths Enhance T Lymphocyte Phenotypes, Inhibit Cytokine Responses, and Attenuate Behavioral Symptoms of Infection in Laboratory Rats. Brain. Behav. Immun. 2007, 21, 1096–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myrianthefs, P.; Karatzas, S.; Venetsanou, K.; Grouzi, E.; Evagelopoulou, P.; Boutzouka, E.; Fildissis, G.; Spiliotopoulou, I.; Baltopoulos, G. Seasonal Variation in Whole Blood Cytokine Production after LPS Stimulation in Normal Individuals. Cytokine 2003, 24, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Gassen, J.; Proffitt Leyva, R.P.; Mengelkoch, S.; White, J.D.; Peterman, J.L.; Prokosch, M.L.; Bradshaw, H.K.; Eimerbrink, M.J.; Corrigan, E.K.; Cheek, D.J.; et al. Day Length Predicts Investment in Human Immune Function: Shorter Days Yield Greater Investment. Psychoneuroendocrinology 2019, 107, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Colas, R.A.; Shinohara, M.; Dalli, J.; Chiang, N.; Serhan, C.N. Identification and Signature Profiles for Pro-Resolving and Inflammatory Lipid Mediators in Human Tissue. Am. J. Physiol.—Cell Physiol. 2014, 307, C39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajeyah, A.A.; Griffiths, W.J.; Wang, Y.; Finch, A.J.; O’Donnell, V.B. The Biosynthesis of Enzymatically Oxidized Lipids. Front. Endocrinol. 2020, 11, 591819. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [Green Version]
- Liakh, I.; Janczy, A.; Pakiet, A.; Korczynska, J.; Proczko-Stepaniak, M.; Kaska, L.; Sledzinski, T.; Mika, A. One-Anastomosis Gastric Bypass Modulates the Serum Levels of pro- and Anti-Inflammatory Oxylipins, Which May Contribute to the Resolution of Inflammation. Int. J. Obes. 2021, 46, 408–416. [Google Scholar] [CrossRef]
- Hasturk, H.; Schulte, F.; Martins, M.; Sherzai, H.; Floros, C.; Cugini, M.A.; Chiu, C.J.; Hardt, M.; Van Dyke, T. Safety and Preliminary Efficacy of a Novel Host-Modulatory Therapy for Reducing Gingival Inflammation. Front. Immunol. 2021, 12, 704163. [Google Scholar] [CrossRef]
- Fishbein, A.; Hammock, B.D.; Serhan, C.N.; Panigrahy, D. Carcinogenesis: Failure of Resolution of Inflammation? Pharmacol. Ther. 2021, 218, 107670. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Shinde, S.; Tsai, I.J.; Croft, K.D.; Beilin, L.J.; Puddey, I.B.; Mori, T.A. Effect of Weight Loss on Neutrophil Resolvins in the Metabolic Syndrome. Prostaglandins. Leukot. Essent. Fatty Acids 2019, 148, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Ostermann, A.I.; Rund, K.; Thoms, S.; Blume, C.; Stahl, F.; Hahn, A.; Schebb, N.H.; Schuchardt, J.P. Influence of Weight Reduction on Blood Levels of C-Reactive Protein, Tumor Necrosis Factor-α, Interleukin-6, and Oxylipins in Obese Subjects. Prostaglandins Leukot. Essent. Fat. Acids 2016, 106, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Tans, R.; Bande, R.; van Rooij, A.; Molloy, B.J.; Stienstra, R.; Tack, C.J.; Wevers, R.A.; Wessels, H.J.C.T.; Gloerich, J.; van Gool, A.J. Evaluation of Cyclooxygenase Oxylipins as Potential Biomarker for Obesity-Associated Adipose Tissue Inflammation and Type 2 Diabetes Using Targeted Multiple Reaction Monitoring Mass Spectrometry. Prostaglandins Leukot. Essent. Fat. Acids 2020, 160, 102157. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Arreaza-Gil, V.; Cortés-Espinar, A.J.; Soliz-Rueda, J.R.; Mulero, M.; Muguerza, B.; Arola-Arnal, A.; Arola, L.; Torres-Fuentes, C. Impact of Gut Microbiota on Plasma Oxylipins Profile under Healthy and Obesogenic Conditions. Clin. Nutr. 2021, 40, 1475–1486. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef]
- Gjermeni, E.; Kirstein, A.S.; Kolbig, F.; Kirchhof, M.; Bundalian, L.; Katzmann, J.L.; Laufs, U.; Blüher, M.; Garten, A.; Le Duc, D. Obesity-An Update on the Basic Pathophysiology and Review of Recent Therapeutic Advances. Biomolecules 2021, 11, 1462. [Google Scholar] [CrossRef]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut Microbiota Confers Host Resistance to Obesity by Metabolizing Dietary Polyunsaturated Fatty Acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef] [Green Version]
- Hartung, N.M.; Fischer, J.; Ostermann, A.I.; Willenberg, I.; Rund, K.M.; Schebb, N.H.; Garscha, U. Impact of Food Polyphenols on Oxylipin Biosynthesis in Human Neutrophils. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2019, 1864, 1536–1544. [Google Scholar] [CrossRef]
- García-Flores, L.A.; Medina, S.; Gómez, C.; Wheelock, C.E.; Cejuela, R.; Martínez-Sanz, J.M.; Oger, C.; Galano, J.M.; Durand, T.; Hernández-Sáez, Á.; et al. Aronia–Citrus Juice (Polyphenol-Rich Juice) Intake and Elite Triathlon Training: A Lipidomic Approach Using Representative Oxylipins in Urine. Food Funct. 2018, 9, 463–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Gillitt, N.D.; Chen, G.Y.; Zhang, Q.; Sha, W.; Kay, C.D.; Chandra, P.; Kay, K.L.; Lila, M.A. Blueberry and/or Banana Consumption Mitigate Arachidonic, Cytochrome P450 Oxylipin Generation During Recovery From 75-Km Cycling: A Randomized Trial. Front. Nutr. 2020, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Arreaza-Gil, V.; Escobar-Martínez, I.; Suárez, M.; Bravo, F.; Muguerza, B.; Arola-Arnal, A.; Torres-Fuentes, C. Gut Seasons: Photoperiod Effects on Fecal Microbiota in Healthy and Cafeteria-Induced Obese Fisher 344 Rats. Nutrients 2022, 14, 722. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Arola, L.; Muguerza, B.; Arola-Arnal, A. Exposure of Fischer 344 Rats to Distinct Photoperiods Influences the Bioavailability of Red Grape Polyphenols. J. Photochem. Photobiol. B Biol. 2019, 199, 111623. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and Polyphenols: Roles and Diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef] [Green Version]
- Arreaza-Gil, V.; Escobar-Martínez, I.; Muguerza, B.; Aragonès, G.; Suárez, M.; Torres-Fuentes, C.; Arola-Arnal, A. The Effects of Grape Seed Proanthocyanidins in Cafeteria Diet-Induced Obese Fischer 344 Rats Are Influenced by Faecal Microbiota in a Photoperiod Dependent Manner. Food Funct. 2022, 13, 8363–8374. [Google Scholar] [CrossRef]
- Liu, M.; Yun, P.; Hu, Y.; Yang, J.; Khadka, R.B.; Peng, X. Effects of Grape Seed Proanthocyanidin Extract on Obesity. Obes. Facts 2020, 13, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Q.; Li, T.; Ren, D.; Yang, X. Grape Seed Proanthocyanidins Reduced the Overweight of C57BL/6J Mice through Modulating Adipose Thermogenesis and Gut Microbiota. Food Funct. 2021, 12, 8467–8477. [Google Scholar] [CrossRef]
- Pascual-Serrano, A.; Arola-Arnal, A.; Suárez-García, S.; Bravo, F.I.; Suárez, M.; Arola, L.; Bladé, C. Grape Seed Proanthocyanidin Supplementation Reduces Adipocyte Size and Increases Adipocyte Number in Obese Rats. Int. J. Obes. 2017, 41, 1246–1255. [Google Scholar] [CrossRef] [Green Version]
- Dorris, S.L.; Peebles, R.S. PGI 2 as a Regulator of Inflammatory Diseases. Mediators Inflamm. 2012, 2012, 926968. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular Mechanisms Underlying Prostaglandin E2-Exacerbated Inflammation and Immune Diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Pirault, J.; Bäck, M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekheri, M.; El Kebir, D.; Edner, N.; Filep, J.G. 15-Epi-LXA4 and 17-Epi-RvD1 Restore TLR9-Mediated Impaired Neutrophil Phagocytosis and Accelerate Resolution of Lung Inflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 7971–7980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieran, N.E.; Maderna, P.; Godson, C. Lipoxins: Potential Anti-Inflammatory, Proresolution, and Antifibrotic Mediators in Renal Disease. Kidney Int. 2004, 65, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Oh, D.Y.; Bandyopadhyay, G.; Lagakos, W.S.; Talukdar, S.; Osborn, O.; Johnson, A.; Chung, H.; Mayoral, R.; Maris, M.; et al. LTB4 Promotes Insulin Resistance in Obese Mice by Acting on Macrophages, Hepatocytes and Myocytes. Nat. Med. 2015, 21, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Picklo, M.J.; Idso, J.; Seeger, D.R.; Aukema, H.M.; Murphy, E.J. Comparative Effects of High Oleic Acid vs High Mixed Saturated Fatty Acid Obesogenic Diets upon PUFA Metabolism in Mice. Prostaglandins Leukot. Essent. Fat. Acids 2017, 119, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caimari, A.; Del Bas, J.M.; Crescenti, A.; Arola, L. Low Doses of Grape Seed Procyanidins Reduce Adiposity and Improve the Plasma Lipid Profile in Hamsters. Int. J. Obes. 2012, 37, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Margalef, M.; Pons, Z.; Iglesias-Carres, L.; Arola, L.; Muguerza, B.; Arola-Arnal, A. Gender-Related Similarities and Differences in the Body Distribution of Grape Seed Flavanols in Rats. Mol. Nutr. Food Res. 2016, 60, 760–772. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serha, C.N. Specialized Pro-Resolving Mediator Network: An Update on Production and Actions. Essays Biochem. 2020, 64, 443. [Google Scholar] [CrossRef] [PubMed]
- Tourdot, B.E.; Ahmed, I.; Holinstat, M. The Emerging Role of Oxylipins in Thrombosis and Diabetes. Front. Pharmacol. 2014, 4, 176. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhao, B.; Huang, L.; Shen, Q.; Ma, L.; Chen, Y.; Wu, T.; Fu, Z. Effects of Altered Photoperiod on Circadian Clock and Lipid Metabolism in Rats. Chronobiol. Int. 2017, 34, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. Intake of an Obesogenic Cafeteria Diet Affects Body Weight, Feeding Behavior, and Glucose and Lipid Metabolism in a Photoperiod-Dependent Manner in F344 Rats. Front. Physiol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Ruf, T.; Arnold, W. Daily and Seasonal Rhythms in Human Mucosa Phospholipid Fatty Acid Composition. J. Biol. Rhythms 2015, 30, 331–341. [Google Scholar] [CrossRef]
- Król, K.; Tomaszewska-Zaremba, D.; Herman, A.P. Photoperiod-Dependent Effect of Inflammation on Nocturnal Gene Expression of Proinflammatory Cytokines and Their Receptors in Pars Tuberalis of Ewe. J. Anim. Feed Sci. 2016, 25, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Penner, A.L.; Waytt, V.; Winter, T.; Leng, S.; Duhamel, T.A.; Aukema, H.M. Oxylipin Profiles and Levels Vary by Skeletal Muscle Type, Dietary Fat and Sex in Young Rats. Appl. Physiol. Nutr. Metab. 2021, 46, 1378–1388. [Google Scholar] [CrossRef]
- Ferdouse, A.; Leng, S.; Winter, T.; Aukema, H.M. Dietary N-6 and n-3 PUFA Alter the Free Oxylipin Profile Differently in Male and Female Rat Hearts. Br. J. Nutr. 2019, 122, 252–261. [Google Scholar] [CrossRef]
- Aljada, A. Endothelium, Inflammation, and Diabetes. Metab. Syndr. Relat. Disord. 2004, 1, 3–21. [Google Scholar] [CrossRef]
- Rahman, M.S. Prostacyclin: A Major Prostaglandin in the Regulation of Adipose Tissue Development. J. Cell. Physiol. 2019, 234, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Kuwata, H.; Akatsu, M.; Yamakawa, Y.; Ochiai, T.; Yoda, E.; Nakatani, Y.; Yokoyama, C.; Hara, S. Involvement of Prostacyclin Synthase in High-Fat-Diet-Induced Obesity. Prostaglandins Other Lipid Mediat. 2021, 153, 106523. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.D.; Sathyanarayana, P.; Konduru, S.; Ye, Y.; Birnbaum, Y.; Bajaj, M. The Effect of Pioglitazone Treatment on 15-Epi-Lipoxin A4 Levels in Patients with Type 2 Diabetes. Atherosclerosis 2012, 223, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jin, F.; Zhang, J.; Li, Z.; Yu, D. Lipoxin A4 Promotes Adipogenic Differentiation and Browning of Mouse Embryonic Fibroblasts. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Börgeson, E.; Johnson, A.M.F.; Lee, Y.S.; Till, A.; Syed, G.H.; Ali-Shah, S.T.; Guiry, P.J.; Dalli, J.; Colas, R.A.; Serhan, C.N.; et al. Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metab. 2015, 22, 125–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Zhang, L.; Jia, S.; Tang, X.; Fu, H.; Li, W.; Liu, C.; Zhang, H.; Cheng, Q.; Zhang, Y. Seasonal Variations in the Composition and Functional Profiles of Gut Microbiota Reflect Dietary Changes in Plateau Pikas. Integr. Zool. 2022, 17, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Gao, H.; Qin, W.; Song, P.; Wang, H.; Zhang, J.; Liu, D.; Wang, D.; Zhang, T. Marked Seasonal Variation in Structure and Function of Gut Microbiota in Forest and Alpine Musk Deer. Front. Microbiol. 2021, 12, 2460. [Google Scholar] [CrossRef]
- Maurice, C.F.; Cl Knowles, S.; Ladau, J.; Pollard, K.S.; Fenton, A.; Pedersen, A.B.; Turnbaugh, P.J. Marked Seasonal Variation in the Wild Mouse Gut Microbiota. ISME J. 2015, 9, 2423–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Chlorogenic Acid Protects Against Indomethacin-Induced Inflammation and Mucosa Damage by Decreasing Bacteroides-Derived LPS. Front. Immunol. 2020, 11, 1125. [Google Scholar] [CrossRef]
- Néia, V.B.M.J.C.; Ambrosio-Albuquerque, E.P.; Figueiredo, I.L.; Boeing, J.S.; Da Silva, T.C.; Lewandowski, V.; Ribeiro, R.P.; Visentainer, J.E.L.; Visentainer, J. V Impact of Cafeteria Diet on the Composition of Fatty Acids in Zebrafish (Danio Rerio) Fillets. Artic. J. Braz. Chem. Soc 2018, 29, 1183–1188. [Google Scholar] [CrossRef]
- Haastrup, A.; Gadegbeku, C.A.; Zhang, D.; Mukhin, Y.V.; Greene, E.L.; Jaffa, A.A.; Egan, B.M. Lipids Stimulate the Production of 6-Keto-Prostaglandin f(1alpha) in Human Dorsal Hand Veins. Hypertension 2001, 38, 858–863. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arreaza-Gil, V.; Ávila-Román, J.; Escobar-Martínez, I.; Muguerza, B.; Suárez, M.; Arola-Arnal, A.; Torres-Fuentes, C. Photoperiod Conditions Modulate Serum Oxylipins Levels in Healthy and Obese Rats: Impact of Proanthocyanidins and Gut Microbiota. Nutrients 2023, 15, 707. https://doi.org/10.3390/nu15030707
Arreaza-Gil V, Ávila-Román J, Escobar-Martínez I, Muguerza B, Suárez M, Arola-Arnal A, Torres-Fuentes C. Photoperiod Conditions Modulate Serum Oxylipins Levels in Healthy and Obese Rats: Impact of Proanthocyanidins and Gut Microbiota. Nutrients. 2023; 15(3):707. https://doi.org/10.3390/nu15030707
Chicago/Turabian StyleArreaza-Gil, Verónica, Javier Ávila-Román, Iván Escobar-Martínez, Begoña Muguerza, Manuel Suárez, Anna Arola-Arnal, and Cristina Torres-Fuentes. 2023. "Photoperiod Conditions Modulate Serum Oxylipins Levels in Healthy and Obese Rats: Impact of Proanthocyanidins and Gut Microbiota" Nutrients 15, no. 3: 707. https://doi.org/10.3390/nu15030707
APA StyleArreaza-Gil, V., Ávila-Román, J., Escobar-Martínez, I., Muguerza, B., Suárez, M., Arola-Arnal, A., & Torres-Fuentes, C. (2023). Photoperiod Conditions Modulate Serum Oxylipins Levels in Healthy and Obese Rats: Impact of Proanthocyanidins and Gut Microbiota. Nutrients, 15(3), 707. https://doi.org/10.3390/nu15030707







