Nutritional Interventions during Chemotherapy for Pancreatic Cancer: A Systematic Review of Prospective Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Population: eligible patients must (i) be at least 18 years old with any nutritional status (well-nourished, at risk of malnutrition, and malnourished), (ii) have a PC diagnosis, while (iii) undergoing CHT. Due to the limited number of studies which involve PC patients only, we decided to consider also papers with PC and other gastrointestinal tumors;
- Intervention: studies with nutritional interventions including nutritional counseling, supplementary food or drink, fortified foods, oral nutrition supplements, and enteral or parenteral nutrition during CHT were considered for inclusion in this review;
- Comparison: any types of comparison were considered as possible (i.e., no nutritional intervention, isocaloric diet without specific nutrients, etc.);
- Outcomes: the outcomes considered were CIT, changes in body composition, QoL, survival, and patient’s functional capacity;
- Study designs: eligible study designs included randomized clinical trials (RCTs), prospective non-randomized studies, and other types of prospective studies.
2.2. Electronic Searches
2.3. Study Selection
2.4. Data Extraction
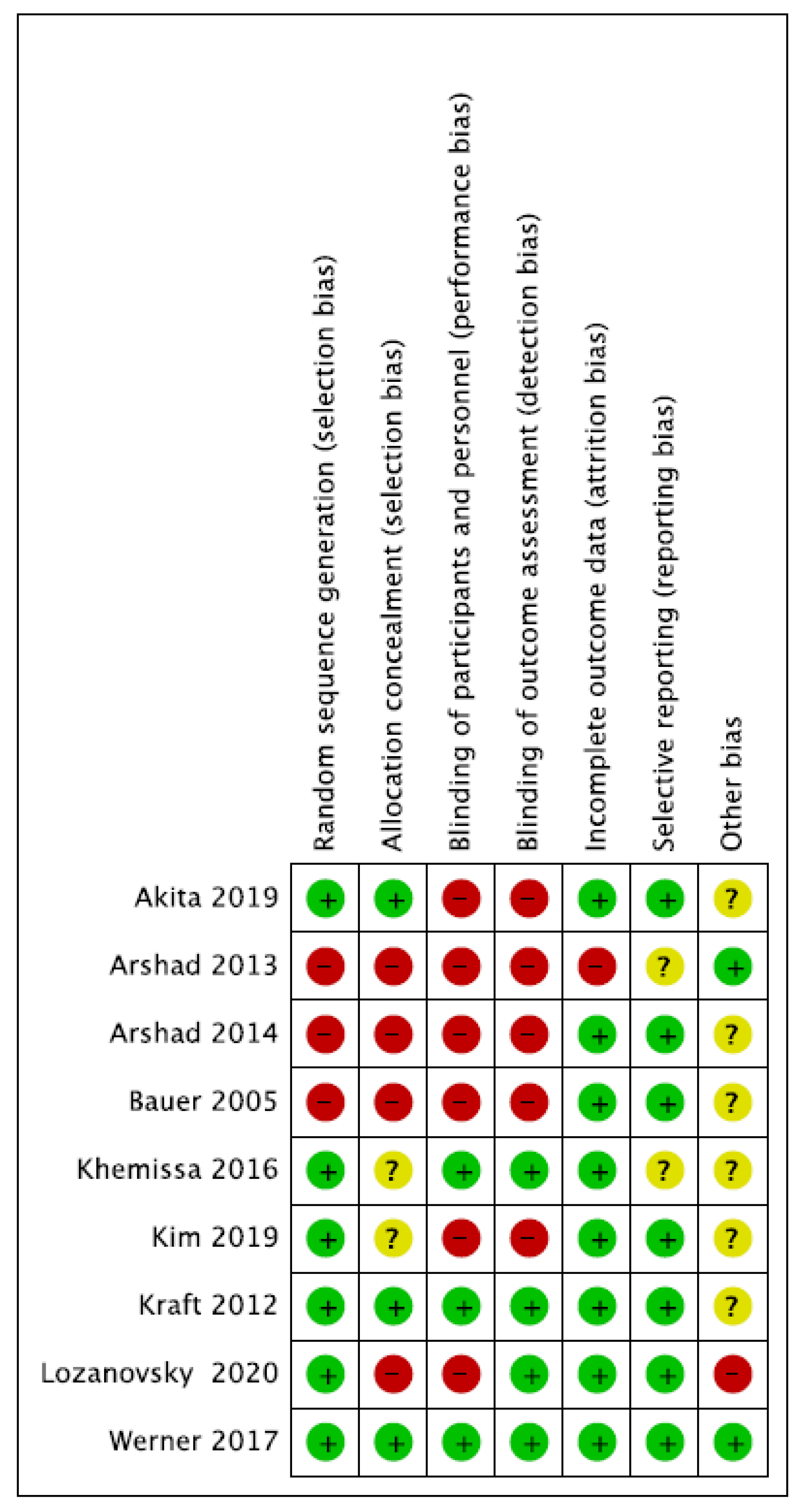
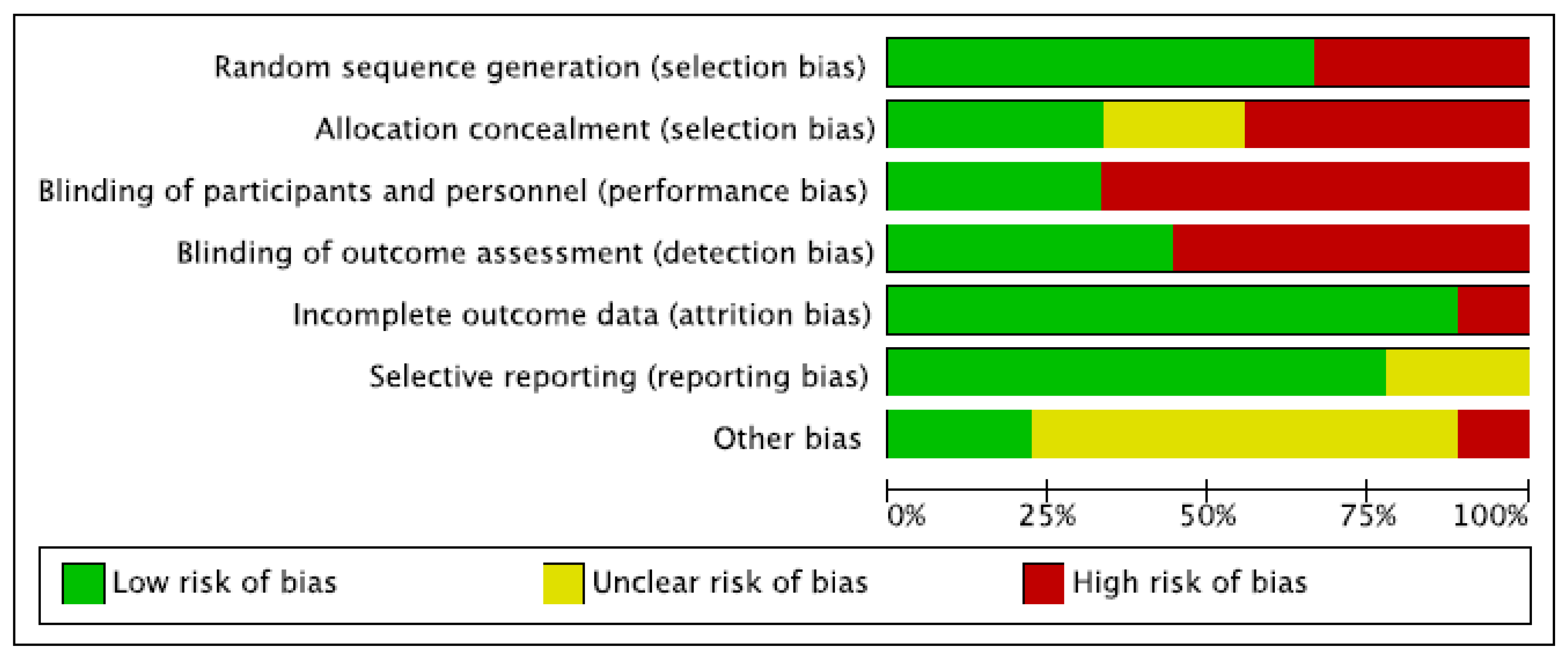
2.5. Risk of Bias and Quality Assessment
2.6. Data Synthesis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Study Quality Assessment
3.4. Summary of Results
3.4.1. Survival Analysis
3.4.2. Quality of Life
3.4.3. Chemotherapy-Induced Toxicity
3.4.4. Nutritional Status
3.4.5. Body Composition
3.4.6. Oral Intake
3.4.7. Karnofsky Performance Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.J.; Wong, M.C.S. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- National Cancer Institute. In Pancreatic Cancer Cancer Stat Facts. 2022. Available online: https://seer.cancer.gov/ (accessed on 13 March 2022).
- Ghaneh, P.; Kleeff, J.; Halloran, C.M.; Raraty, M.; Jackson, R.; Melling, J.; Jones, O.; Palmer, D.H.; Cox, T.F.; Smith, C.J.; et al. The Impact of Positive Resection Margins on Survival and Recurrence Following Resection and Adjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 520–529. [Google Scholar] [CrossRef]
- Tempero, M.A. NCCN Guidelines Updates: Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 603–605. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Kordes, M.; Larsson, L.; Engstrand, L.; Löhr, J.M. Pancreatic cancer cachexia: Three dimensions of a complex syndrome. Br. J. Cancer 2021, 124, 1623–1636. [Google Scholar] [CrossRef]
- Hendifar, A.E.; Chang, J.I.; Huang, B.Z.; Tuli, R.; Wu, B.U. Cachexia, and not obesity, prior to pancreatic cancer diagnosis worsens survival and is negated by chemotherapy. J. Gastrointest. Oncol. 2018, 9, 17–23. [Google Scholar] [CrossRef]
- Griffin, O.M.; Bashir, Y.; O’Connor, D.; Peakin, J.; McMahon, J.; Duggan, S.N.; Geoghegan, J.; Conlon, K.C. Measurement of body composition in pancreatic cancer: A systematic review, meta-analysis and recommendations for future study design. Dig. Surg. 2022, 39, 141–152. [Google Scholar] [CrossRef]
- Bundred, J.; Kamarajah, S.K.; Roberts, K.J. Body composition assessment and sarcopenia in patients with pancreatic cancer: A systematic review and meta-analysis. HPB 2019, 21, 1603–1612. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yang, Y.F.; Han, P.; Ye, P.C.; Kong, H. Protein-energy malnutrition worsens hospitalization outcomes of patients with pancreatic cancer undergoing open pancreaticoduodenectomy. Updates Surg. 2022, 74, 1627–1636. [Google Scholar] [CrossRef]
- Poulia, K.A.; Antoniadou, D.; Sarantis, P.; Karamouzis, M.V. Pancreatic Cancer Prognosis, Malnutrition Risk, and Quality of Life: A Cross-Sectional Study. Nutrients 2022, 14, 442. [Google Scholar] [CrossRef]
- Trestini, I.; Carbognin, L.; Sperduti, I.; Bonaiuto, C.; Auriemma, A.; Melisi, D.; Salvatore, L.; Bria, E.; Tortora, G. Prognostic impact of early nutritional support in patients affected by locally advanced and metastatic pancreatic ductal adenocarcinoma undergoing chemotherapy. Eur. J. Clin. Nutr. 2018, 72, 772–779. [Google Scholar] [CrossRef]
- Trestini, I.; Cintoni, M.; Rinninella, E.; Grassi, F.; Paiella, S.; Salvia, R.; Bria, E.; Pozzo, C.; Alfieri, S.; Gasbarrini, A.; et al. Neoadjuvant treatment: A window of opportunity for nutritional prehabilitation in patients with pancreatic ductal adenocarcinoma. World J. Gastrointest. Surg. 2021, 13, 885–903. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 [Updated February 2022]. Available online: www.cochrane-handbook.org (accessed on 12 January 2023).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Mele, M.C.; Rinninella, E.; Grassi, F. Nutritional Interventions during Chemotherapy for Pancreatic Cancer: A Systematic Review of Prospective Studies. PROSPERO 2020 CRD42020185706. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020185706 (accessed on 12 January 2023).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Bauer, J.D.; Capra, S. Nutrition intervention improves outcomes in patients with cancer cachexia receiving chemotherapy--a pilot study. Support Care Cancer 2005, 13, 270–274. [Google Scholar] [CrossRef]
- Kraft, M.; Kraft, K.; Gärtner, S.; Mayerle, J.; Simon, P.; Weber, E.; Schütte, K.; Stieler, J.; Koula-Jenik, H.; Holzhauer, P.; et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)—A randomized multicentre trial. Nutr. J. 2012, 11, 52. [Google Scholar] [CrossRef]
- Arshad, A.; Chung, W.Y.; Steward, W.; Metcalfe, M.S.; Dennison, A.R. Reduction in circulating pro-angiogenic and pro-inflammatory factors is related to improved outcomes in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega-3 fish oil. HPB 2013, 15, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Arshad, A.; Chung, W.Y.; Isherwood, J.; Mann, C.D.; Al-Leswas, D.; Steward, W.P.; Metcalfe, M.S.; Dennison, A.R. Cellular and plasma uptake of parenteral omega-3 rich lipid emulsion fatty acids in patients with advanced pancreatic cancer. Clin. Nutr. 2014, 33, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Khemissa, F.; Mineur, L.; Amsellem, C.; Assenat, E.; Ramdani, M.; Bachmann, P.; Janiszewski, C.; Cristiani, I.; Collin, F.; Courraud, J.; et al. A phase III study evaluating oral glutamine and transforming growth factor-beta 2 on chemotherapy-induced toxicity in patients with digestive neoplasm. Dig. Liver Dis. 2016, 48, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Werner, K.; de Gaudry, D.K.; Taylor, L.A.; Keck, T.; Unger, C.; Hopt, U.T.; Massing, U. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: Marine phospholipids versus fish oil—A randomized controlled double-blind trial. Lipids Health Dis. 2017, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Takahashi, H.; Asukai, K.; Tomokuni, A.; Wada, H.; Marukawa, S.; Yamasaki, T.; Yanagimoto, Y.; Takahashi, Y.; Sugimura, K.; et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: Prospective randomized control study. Clin. Nutr. ESPEN 2019, 33, 148–153. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M.; Jeung, H.C.; Lee, I.J.; Park, J.S.; Song, M.; Lee, D.K.; Lee, S.M. The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients 2019, 11, 1145. [Google Scholar] [CrossRef]
- Lozanovski, V.J.; Polychronidis, G.; Gross, W.; Gharabaghi, N.; Mehrabi, A.; Hackert, T.; Schemmer, P.; Herr, I. Broccoli sprout supplementation in patients with advanced pancreatic cancer is difficult despite positive effects-results from the POUDER pilot study. Investig. New Drugs 2020, 38, 776–784. [Google Scholar] [CrossRef]
- Cañamares-Orbís, P.; García-Rayado, G.; Alfaro-Almajano, E. Nutritional Support in Pancreatic Diseases. Nutrients 2022, 14, 4570. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Vitaloni, M.; Caccialanza, R.; Ravasco, P.; Carrato, A.; Kapala, A.; de van der Schueren, M.; Constantinides, D.; Backman, E.; Chuter, D.; Santangelo, C.; et al. The impact of nutrition on the lives of patients with digestive cancers: A position paper. Support Care Cancer 2022, 30, 7991–7996. [Google Scholar] [CrossRef]
- Nayak, M.G.; George, A.; Vidyasagar, M.S.; Mathew, S.; Nayak, S.; Nayak, B.S.; Shashidhara, Y.N.; Kamath, A. Quality of Life among Cancer Patients. Indian J. Palliat. Care 2017, 23, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Lluch Taltavull, J.I.; Mercadal Orfila, G.; Afonzo Gobbi, Y.S. Improvement of the nutritional status and quality of life of cancer patients through a protocol of evaluation and nutritional intervention. Nutr. Hosp. 2018, 35, 606–611. [Google Scholar] [CrossRef]
- Souza, A.P.S.; Silva, L.C.D.; Fayh, A.P.T. Nutritional Intervention Contributes to the Improvement of Symptoms Related to Quality of Life in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: A Randomized Clinical Trial. Nutrients 2021, 13, 589. [Google Scholar] [CrossRef]
- Karnofsky, D.A.; Abelmann, W.H.; Craver, L.F.; Burchenal, J.H. The Use of the Nitrogen Mustards in the Palliative Treatment of Carcinoma—With Particular Reference to Bronchogenic Carcinoma. Cancer 1948, 1, 634–656. [Google Scholar] [CrossRef]
- Plyta, M.; Patel, P.S.; Fragkos, K.C.; Kumagai, T.; Mehta, S.; Rahman, F.; Di Caro, S. Nutritional Status and Quality of Life in Hospitalised Cancer Patients Who Develop Intestinal Failure and Require Parenteral Nutrition: An Observational Study. Nutrients 2020, 12, 2357. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, S.A.; Wang, K.; Chen, C.; Wang, J.; Chen, X.; Yan, J. Impact of Nutrition Counseling in Head and Neck Cancer Sufferers Undergoing Antineoplastic Therapy: A Randomized Controlled Pilot Study. Curr. Oncol. 2022, 29, 6947–6955. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Rotolo, S.; Cintoni, M.; Rinninella, E.; Pulcini, G.; Schena, C.A.; Ferracci, F.; Grassi, F.; Raoul, P.; Moroni, R.; et al. The prognostic value of skeletal muscle index on clinical and survival outcomes after cytoreduction and HIPEC for peritoneal metastases from colorectal cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2022, 48, 649–656. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Ponziani, F.R.; Pompili, M.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; et al. Prognostic value of skeletal muscle mass during tyrosine kinase inhibitor (TKI) therapy in cancer patients: A systematic review and meta-analysis. Intern. Emerg. Med. 2021, 16, 1341–1356. [Google Scholar] [CrossRef]
- Rinninella, E.; Fagotti, A.; Cintoni, M.; Raoul, P.; Scaletta, G.; Scambia, G.; Gasbarrini, A.; Mele, M.C. Skeletal muscle mass as a prognostic indicator of outcomes in ovarian cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2020, 30, 654–663. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2045–2054. [Google Scholar] [CrossRef]
- Rizzo, S.; Scala, I.; Robayo, A.R.; Cefalì, M.; De Dosso, S.; Cappio, S.; Xhepa, G.; Del Grande, F. Body composition as a predictor of chemotherapy-related toxicity in pancreatic cancer patients: A systematic review. Front. Oncol. 2022, 12, 974116. [Google Scholar] [CrossRef]
- Emori, T.; Itonaga, M.; Ashida, R.; Tamura, T.; Kawaji, Y.; Hatamaru, K.; Yamashita, Y.; Shimokawa, T.; Koike, M.; Sonomura, T.; et al. Impact of sarcopenia on prediction of progression-free survival and overall survival of patients with pancreatic ductal adenocarcinoma receiving first-line gemcitabine and nab-paclitaxel chemotherapy. Pancreatology 2022, 22, 277–285. [Google Scholar] [CrossRef]
- Bicakli, D.H.; Uslu, R.; Güney, S.C.; Coker, A. The Relationship Between Nutritional Status, Performance Status, and Survival Among Pancreatic Cancer Patients. Nutr. Cancer 2020, 72, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Wang, M.; Zhu, F.; Qin, T.; Qin, R. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with pancreatic cancer undergoing radical surgery. Asian J. Surg. 2022, 45, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- de van der Schueren, M.A.E.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Orsso, C.E.; Pereira, S.L.; Atherton, P.J.; Deutz, N.E.P. Effects of β-hydroxy β-methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Effects of nutritional interventions on nutritional status in patients with gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Reece, L.; Hogan, S.; Allman-Farinelli, M.; Carey, S. Oral nutrition interventions in patients undergoing gastrointestinal surgery for cancer: A systematic literature review. Support Care Cancer 2020, 28, 5673–5691. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Shimokawa, T.; Harada, K.; Yoshida, K. Effectiveness of elemental diets to prevent oral mucositis associated with cancer therapy: A meta-analysis. Clin. Nutr. ESPEN 2022, 49, 172–180. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ijichi, H.; Saito, K.; Ishigaki, K.; Takami, M.; Sekine, R.; Usami, S.; Nakai, Y.; Koike, K.; Kubota, N. Protein intake after the initiation of chemotherapy is an independent prognostic factor for overall survival in patients with unresectable pancreatic cancer: A prospective cohort study. Clin. Nutr. 2021, 40, 4792–4798. [Google Scholar] [CrossRef]
- Dou, S.; Ding, H.; Jiang, W.; Li, R.; Qian, Y.; Wu, S.; Ling, Y.; Zhu, G. Effect of oral supplements on the nutritional status of nasopharyngeal carcinoma patients undergoing concurrent chemotherapy: A randomized controlled Phase II trial. J. Cancer Res. Ther. 2020, 16, 1678–1685. [Google Scholar]
- Grupińska, J.; Budzyń, M.; Maćkowiak, K.; Brzeziński, J.J.; Kycler, W.; Leporowska, E.; Gryszczyńska, B.; Kasprzak, M.P.; Iskra, M.; Formanowicz, D. Beneficial Effects of Oral Nutritional Supplements on Body Composition and Biochemical Parameters in Women with Breast Cancer Undergoing Postoperative Chemotherapy: A Propensity Score Matching Analysis. Nutrients 2021, 13, 3549. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Valente, R.; Del Chiaro, M.; Permert, J.; Löhr, J.M. Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, D.; Avci, B.; Kiriukova, M.; Panic, N.; Bozhychko, M.; Sandru, V.; de-Madaria, E.; Capurso, G. Pancreatic exocrine insufficiency and pancreatic enzyme replacement therapy in patients with advanced pancreatic cancer: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2020, 8, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Caccialanza, R.; Capurso, G.; Brunetti, O.; Milella, M.; Falconi, M. Pancreatic Enzyme Replacement Therapy in Pancreatic Cancer. Cancers 2020, 12, 275. [Google Scholar] [CrossRef]
- Domínguez-Muñoz, J.E.; Nieto-Garcia, L.; López-Díaz, J.; Lariño-Noia, J.; Abdulkader, I.; Iglesias-Garcia, J. Impact of the treatment of pancreatic exocrine insufficiency on survival of patients with unresectable pancreatic cancer: A retrospective analysis. BMC Cancer 2018, 18, 534. [Google Scholar] [CrossRef]
- Layer, P.; Kashirskaya, N.; Gubergrits, N. Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency. World J. Gastroenterol. 2019, 25, 2430–2441. [Google Scholar] [CrossRef] [PubMed]

| Refs | Author and Year | Study Design | % Pancreatic Cancer | Sample Size (IG/CG) | Time of Intervention | Type of Nutritional Intervention | Comparison | Results |
|---|---|---|---|---|---|---|---|---|
| [21] | Bauer JD 2005 | Single-arm trial | 71.4 | 7 (7/0) | 8 weeks | ONS | - | - ↑ protein (p = 0.011), energy (p = 0.011), and fiber (p = 0.006) intake ↑ nutritional status (p = 0.019) ↑ KPS (p = 0.01) ↑ QoL(p = 0.019) Not statistically significant improvements in: - BW (p = 0.368) - LBM (p = 0.225) |
| [22] | Kraft M et al. 2012 | Prospective, multi-center, placebo-controlled, randomized, and double-blinded trial | 100 | 72 (38/34) | 12 weeks | Oral liquid formulation of L-Carnitine | Placebo | in the IG group vs. CG: ↑ BCM after 6 weeks (p = 0.013) ↑ BF after 12 weeks (p = 0.041) ↑ BMI after 12 weeks (p < 0.018) ↑ cognitive function after 6 weeks (p < 0.034) ↑ global health status after 12 weeks (p < 0.041) ↓ gastrointestinal symptoms after 12 weeks (p < 0.033) No significant differences between the two groups in survival |
| [23] | Arshad A et al. 2013 | Single-arm phase II clinical trial | 100 | 32 (32/0) | Weekly for 3 weeks followed by a rest week during the CHT period | Parenteral supplement n-3FA-rich lipid emulsion | - | ↓ OS in high expressors of IL-6 (p = 0.009) and IL-8 (p = 0.02) ↓ PFS in high expressors of IL-8 (p = 0.002) |
| [24] | Arshad A et al. 2014 | Single-arm phase II clinical trial | 100 | 21 (21/0) | Weekly for 3 weeks followed by a rest week for up to six months | Parenteral supplement n-3FA-rich lipid emulsion | - | Over the entire treatment course of up to six months: ↑ ECM pellet uptake of EPA (p = 0.005) and DHA (p < 0.001) ↓ n6:n3 ratio (p < 0.001) |
| [25] | Khemissa F et al. 2016 | Double-blind, randomized, controlled, and multicenter trial | 7 | 201 (99/102) | Five days before the start of each CHT cycle | ONS | Isocaloric ONS | No significant differences between the two groups in term of compliance and toxicities |
| [26] | Werner K et al. 2017 | Randomized, double-blind, controlled trial | 100 | 60 (31/29) | 6 weeks | FO capsules | MPL capsules | in both groups: BW stabilization (p = 0.001 in FO group; p = 0.003 in MPL group) ↑ meal portions (p = 0.02 in FO group; p = 0.05 in MPL group) No significant changes in both groups in QoL, and food intake. |
| [27] | Akita H et al. 2019 | RCT | 100 | 62 (31/31) | 5 weeks | ONS | Normal diet | in CG group: ↓ Post/pre ratio of SMM (p = 0.014) in both groups: ↓ PMA (IG p = 0.002; CG p < 0.001) ↓ BMI (IG p = 0.011; CG p = 0.001) in IG group: ↑ Post/pre ratio of PMA (p = 0.001) ↑ Post/pre ratio of SMM (p = 0.042) ↑ Post/pre ratio of PMA (p < 0.001) No significant difference between the two groups in NACRT-related toxicity |
| [28] | Kim SH et al. 2019 | Prospective randomized study | 29.4 | 58 enrolled (36/22) | 8 weeks | ONS | Nutritional care only | No significant difference between the two groups in BW, FFM, SMM, BCM, QoL, and biochemical tests (all patients) (dividing population based on CHT cycles) In IG vs. CG: ↑ dietary intake ↓ reduction of fatigue (p = 0.041) ↑ PG-SGA grade ratio (p < 0.05) ↑ BW (p = 0.049) ↑ FFM (p = 0.034) ↑ SMM (p = 0.049) ↑ BCM (p = 0.049) |
| [29] | Lozanovski et al. 2020 | Prospective, placebo-controlled trial | 100 | 40 (29/11) | 12 months | Daily intake of broccoli sprouts containing 90 mg sulforaphane and 180 mg glucoraphanin or methylcellulose a | Placebo | In IG: Drop out: 72% IG vs. CG: ↑ Survival at 180 days (p = 0.291) |
| Refs | Author | ONS Type | ONS Quantity | Amount (per Day) | Energy (kcal per Day) | Protein (g per Day) | Other |
|---|---|---|---|---|---|---|---|
| [21] | Bauer JD et al. | L | Not reported | At least 1 | 310 | 16 | 1.1 g EPA |
| [25] | Khemissa F et al. | P | 75 g | 2 | 691 | 45.75 | 13.5 g glutamine + TGF-β2 20 mg |
| [27] | Akita H et al. | L | 220 mL | 2 | 560 | 29.3 | 1.98 g EPA |
| [28] | Kim SH et al. | L | 150 mL | 2 | 400 | 18 | 2.5 g fiber |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cintoni, M.; Grassi, F.; Palombaro, M.; Rinninella, E.; Pulcini, G.; Di Donato, A.; Salvatore, L.; Quero, G.; Tortora, G.; Alfieri, S.; et al. Nutritional Interventions during Chemotherapy for Pancreatic Cancer: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 727. https://doi.org/10.3390/nu15030727
Cintoni M, Grassi F, Palombaro M, Rinninella E, Pulcini G, Di Donato A, Salvatore L, Quero G, Tortora G, Alfieri S, et al. Nutritional Interventions during Chemotherapy for Pancreatic Cancer: A Systematic Review of Prospective Studies. Nutrients. 2023; 15(3):727. https://doi.org/10.3390/nu15030727
Chicago/Turabian StyleCintoni, Marco, Futura Grassi, Marta Palombaro, Emanuele Rinninella, Gabriele Pulcini, Agnese Di Donato, Lisa Salvatore, Giuseppe Quero, Giampaolo Tortora, Sergio Alfieri, and et al. 2023. "Nutritional Interventions during Chemotherapy for Pancreatic Cancer: A Systematic Review of Prospective Studies" Nutrients 15, no. 3: 727. https://doi.org/10.3390/nu15030727
APA StyleCintoni, M., Grassi, F., Palombaro, M., Rinninella, E., Pulcini, G., Di Donato, A., Salvatore, L., Quero, G., Tortora, G., Alfieri, S., Gasbarrini, A., & Mele, M. C. (2023). Nutritional Interventions during Chemotherapy for Pancreatic Cancer: A Systematic Review of Prospective Studies. Nutrients, 15(3), 727. https://doi.org/10.3390/nu15030727