Effects of Spermidine on Mouse Gut Morphology, Metabolites, and Microbial Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Protocols
2.2. Polyamine Content Measurement
2.3. Intestinal Histo-Morphological Detection
2.4. Detection of Inflammatory Cytokines in the Colon
2.5. Metabonomic Analysis of Colonic Contents
2.6. Statistical Analysis
3. Results
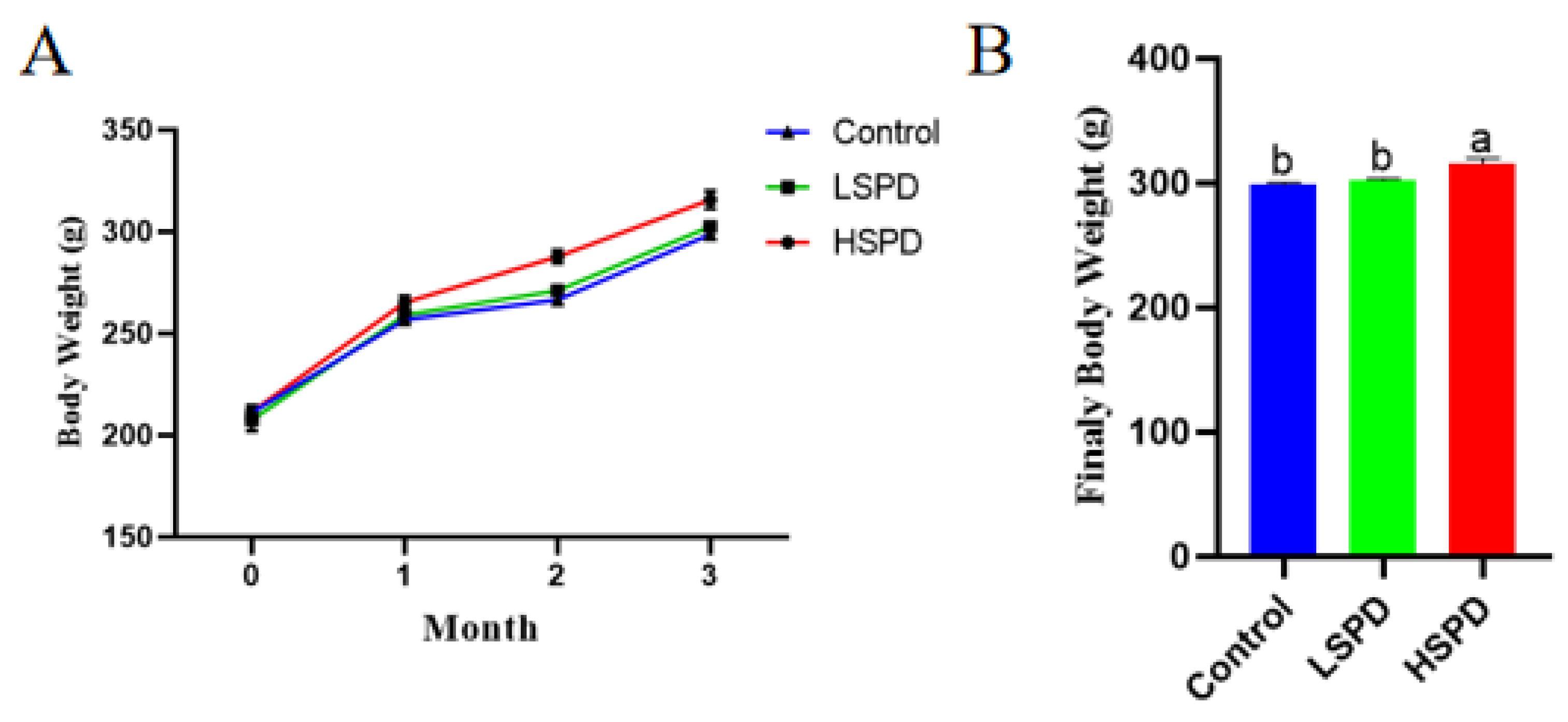
3.1. Effects of Spermidine on the Litter Weight of the Mice
3.2. Effect of Spermidine on Intestinal Morphology
3.3. Effects of Spermidine on the Intestinal Polyamine Contents of the Mice
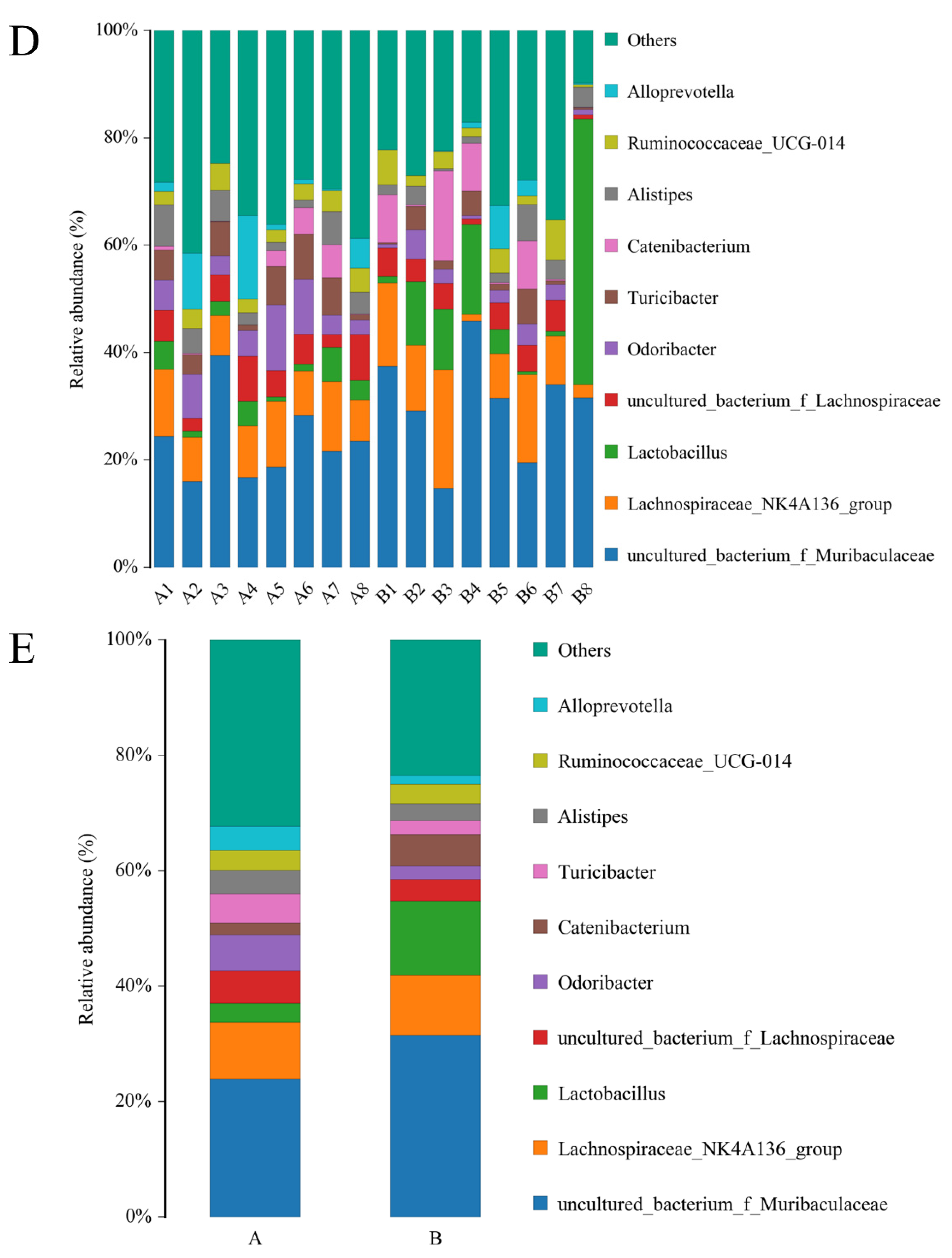
3.4. Effect of Adding SPD to Drinking Water on the Intestinal Microbiota of the Mice
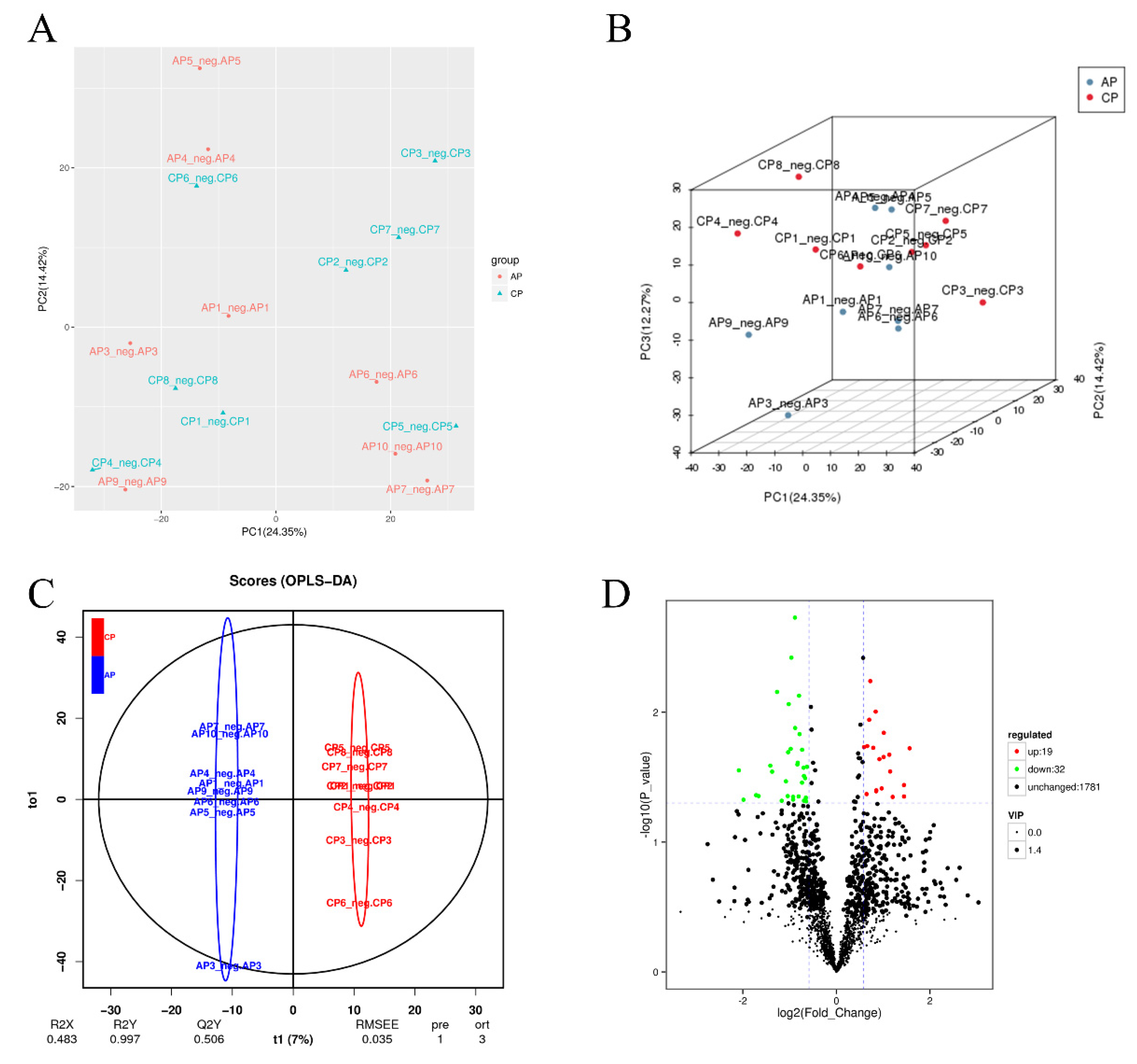
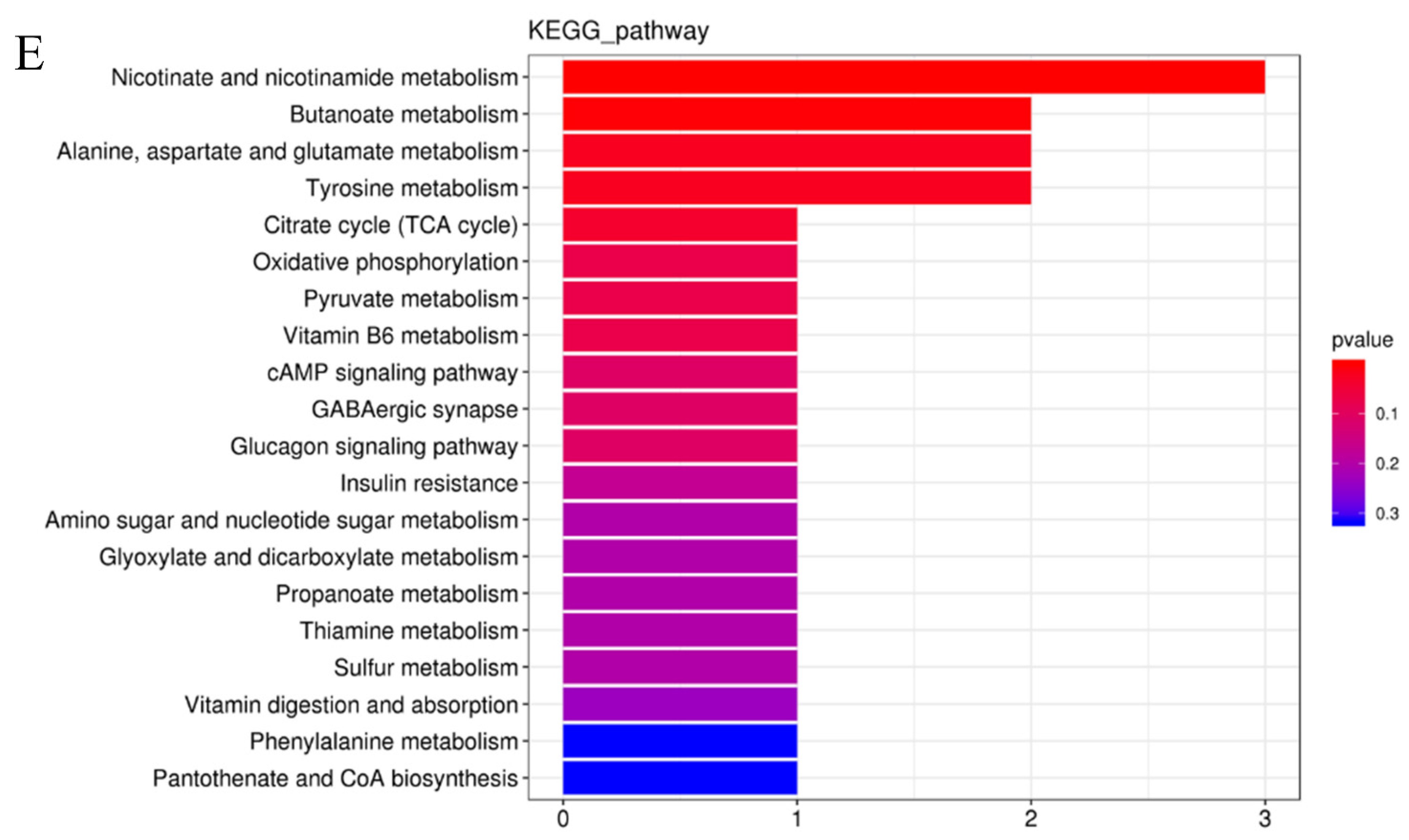
3.5. Effects of Spermidine on Intestinal Metabonomic Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy 2019, 15, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol.Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh, G.; Panse, I.; Swadling, L.; Zhang, H.; Richter, F.C.; Meyer, A.; Lord, J.; Barnes, E.; Klenerman, P.; Green, C.; et al. Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. Elife 2020, 9, e57950. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Tarachai, P. Changes in intestinal villi, cell area and intracellular autophagic vacuoles related to intestinal function in chickens. Br. Poult. Sci. 2000, 41, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Liu, G.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Cai, J. Polyamines: Regulation on Intestinal Homeostasis and Possiblie Mechanisms. Chin. J. Animal Nutrition 2016, 28, 3400–3407. [Google Scholar]
- Fang, T.; Jia, G.; Zhao, H.; Chen, X.; Tang, J.; Wang, J.; Liu, G. Effects of spermine supplementation on the morphology, digestive enzyme activities, and antioxidant capacity of intestine in weaning rats. Anim. Nutr. 2016, 2, 370–375. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, J.; Wu, X.; Xu, X.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Tian, G.; Wang, J. Putrescine enhances intestinal immune function and regulates intestinal bacteria in weaning piglets. Food Funct. 2019, 10, 4134–4142. [Google Scholar] [CrossRef]
- Zhou, B.; Yuan, Y.; Zhang, S.; Guo, C.; Li, X.; Li, G.; Xiong, W.; Zeng, Z. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, G.; Zhang, W.; Han, H. Nutritional and physiological effects of dietary putrescine supplementation in weaned piglets. Chin. J. Anim. Sci. 2011, 47, 69–72. [Google Scholar]
- Liu, G.; Mo, W.; Cao, W.; Wu, X.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Wang, J. Effects of spermine on ileal physical barrier, antioxidant capacity, metabolic profile and large intestinal bacteria in piglets. RSC Adv. 2020, 10, 26709–26716. [Google Scholar] [CrossRef]
- Kang, B.; Jiang, D.; He, H.; Ma, R.; Chen, Z.; Yi, Z. Effect of Oaz1 overexpression on goose ovarian granulosa cells. Amino Acids 2017, 49, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for monitoring intestinal health in poultry: Present status and future perspectives. Vet. Res. 2018, 49, 43. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Hofer, S.J.; Pendl, T.; Bauer, M.A.; Eisenberg, T.; Carmona-Gutierrez, D.; Kroemer, G. Nutritional Aspects of Spermidine. Annu. Rev. Nutr. 2020, 40, 135–159. [Google Scholar] [CrossRef]
- Zhang, C.L.; Ren, H.J.; Liu, M.M.; Li, X.G.; Sun, D.L.; Li, N.; Ming, L. Modulation of intestinal epithelial cell proliferation, migration, and differentiation in vitro by Astragalus polysaccharides. PLoS ONE 2014, 9, e106674. [Google Scholar] [CrossRef] [PubMed]
- Sefcova, M.A.; Larrea-Alvarez, M.; Larrea-Alvarez, C.M.; Karaffova, V.; Ortega-Paredes, D.; Vinueza-Burgos, C.; Sevcikova, Z.; Levkut, M.; Herich, R.; Revajova, V. The Probiotic Lactobacillus fermentum Biocenol CCM 7514 Moderates Campylobacter jejuni-Induced Body Weight Impairment by Improving Gut Morphometry and Regulating Cecal Cytokine Abundance in Broiler Chickens. Animals 2021, 11, 235. [Google Scholar] [CrossRef]
- Xu, X.; Liu, G.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Wang, J. Effects of spermine on the proliferation and migration of porcine intestinal epithelial cells. Anim. Biotechnol. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. The function of spermine. IUBMB Life 2014, 66, 8–18. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Ferioli, M.E.; Pirona, L.; Pinotti, O. Prolactin and polyamine catabolism: Specific effect on polyamine oxidase activity in rat thymus. Mol. Cell Endocrinol. 2000, 165, 51–56. [Google Scholar] [CrossRef]
- Wang, X.; Xia, X.; Gong, M.; Yin, Y. Exogenous Polyamine: Effects on Intestinal Structure and Function in Piglet and Its Mechanism. Chin. J. Anim. Nutr. 2014, 26, 2457–2462. [Google Scholar]
- Moschen, A.R.; Gerner, R.R.; Wang, J.; Klepsch, V.; Adolph, T.E.; Reider, S.J.; Hackl, H.; Pfister, A.; Schilling, J.; Moser, P.L.; et al. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe 2016, 19, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, R.; Sun, Y.; Li, X.; He, L.; Li, Z.; Silverman, G.J.; Chen, G.; Gao, F.; Yuan, J.; et al. Lupus gut microbiota transplants cause autoimmunity and inflammation. Clin. Immunol. 2021, 233, 108892. [Google Scholar] [CrossRef]
- Wu, X. Effects of Putrescine Supplementation on Intestinal Development, Antioxidant Function, Immunity, and Microbes in Weaned Piglets. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2018. [Google Scholar]
- Jung, I.L.; Kim, I.G. Polyamines and glutamate decarboxylase-based acid resistance in Escherichia coli. J. Biol. Chem. 2003, 278, 22846–22852. [Google Scholar] [CrossRef] [PubMed]
- Mangifesta, M.; Mancabelli, L.; Milani, C.; Gaiani, F.; de’Angelis, N.; de’Angelis, G.L.; van Sinderen, D.; Ventura, M.; Turroni, F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018, 8, 13974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zou, G.; Li, B.; Du, X.; Sun, Z.; Sun, Y.; Jiang, X. Fecal Microbiota Transplantation (FMT) Alleviates Experimental Colitis in Mice by Gut Microbiota Regulation. J. Microbiol. Biotechnol. 2020, 30, 1132–1141. [Google Scholar] [CrossRef]
- Guillon, A.; Brea-Diakite, D.; Cezard, A.; Wacquiez, A.; Baranek, T.; Bourgeais, J.; Picou, F.; Vasseur, V.; Meyer, L.; Chevalier, C.; et al. Host succinate inhibits influenza virus infection through succinylation and nuclear retention of the viral nucleoprotein. EMBO J. 2022, 41, e108306. [Google Scholar] [CrossRef]
- Jiang, K.; Fu, X.; Huang, R.; Fan, X.; Ji, L.; Cai, D.; Liu, X.; Fu, Y.; Sun, A.; Feng, C. Production of Prebiotic Xylooligosaccharides via Dilute Maleic Acid-Mediated Xylan Hydrolysis Using an RSM-Model-Based Optimization Strategy. Front. Nutr. 2022, 9, 909283. [Google Scholar] [CrossRef]
- Kasbo, J.; Saleem, M.; Perwaiz, S.; Mignault, D.; Lamireau, T.; Tuchweber, B.; Yousef, I. Biliary, fecal and plasma deoxycholic acid in rabbit, hamster, guinea pig, and rat: Comparative study and implication in colon cancer. Biol. Pharm. Bull. 2002, 25, 1381–1384. [Google Scholar] [CrossRef]
- Ma, Y.; Bao, Y.; Wang, S.; Li, T.; Chang, X.; Yang, G.; Meng, X. Anti-Inflammation Effects and Potential Mechanism of Saikosaponins by Regulating Nicotinate and Nicotinamide Metabolism and Arachidonic Acid Metabolism. Inflammation 2016, 39, 1453–1461. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- de Vries, L.E.; Lunghi, M.; Krishnan, A.; Kooij, T.W.A.; Soldati-Favre, D. Pantothenate and CoA biosynthesis in Apicomplexa and their promise as antiparasitic drug targets. PLoS Pathog. 2021, 17, e1010124. [Google Scholar] [CrossRef] [PubMed]
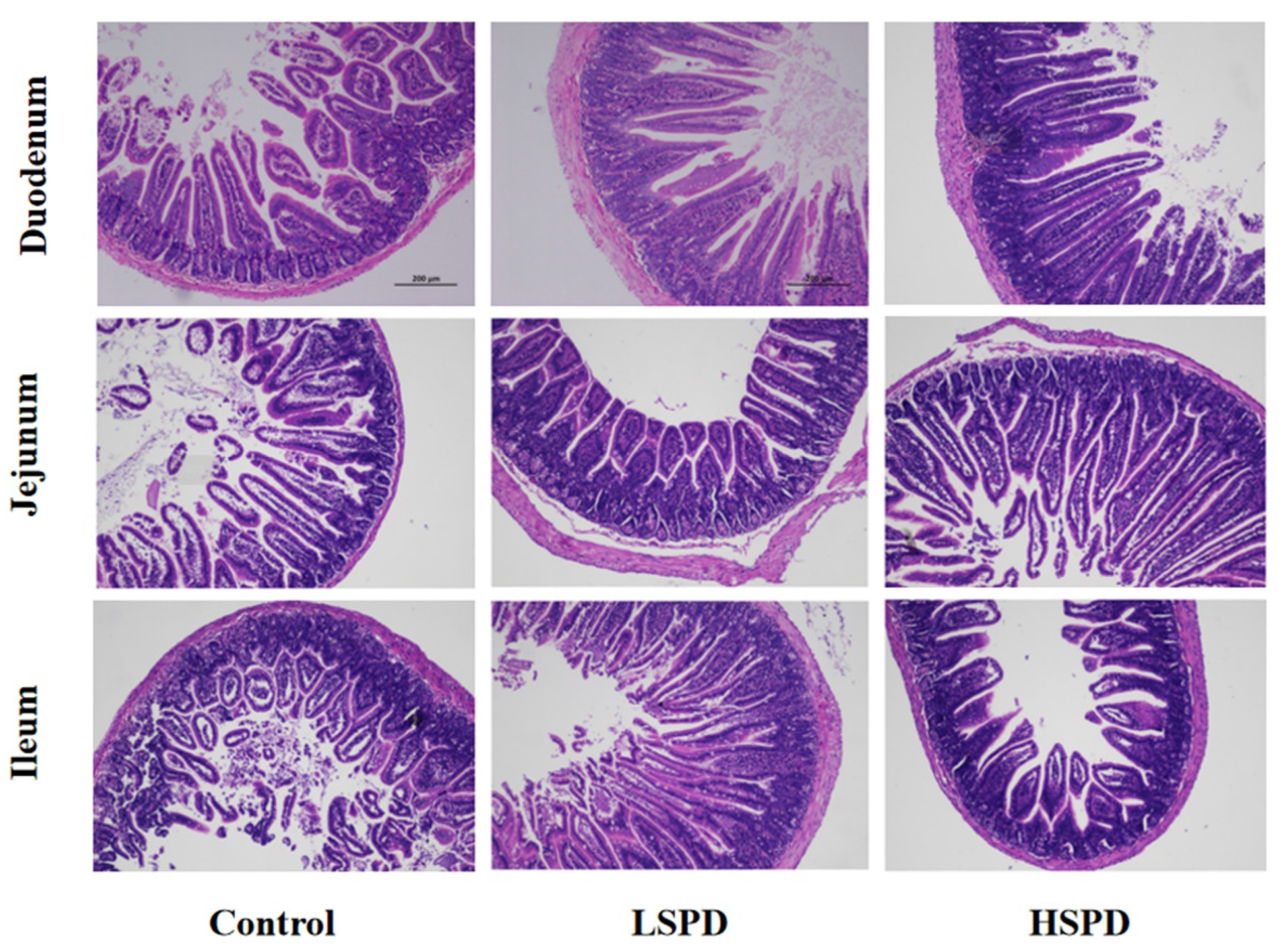



| Items | Control | LSPD | HSPD |
|---|---|---|---|
| Duodenum | |||
| Villus height, μm | 0.31 ± 0.07 | 0.38 ± 0.01 | 0.35 ± 0.07 |
| Crypt depth, μm | 0.08 ± 0.01 | 0.1 ± 0.03 | 0.10 ± 0.01 |
| Villus height: crypt depth | 3.88 ± 1.09 | 3.40 ± 0.99 | 3.45 ± 0.42 |
| Jejunum | |||
| Villus height, μm | 0.34 ± 0.03 b | 0.28 ± 0.06 c | 0.42 ± 0.01 a |
| Crypt depth, μm | 0.072 ± 0.02 b | 0.10 ± 0.01 a | 0.10 ± 0.01 a |
| Villus height: crypt depth | 4.56 ± 0.84 a | 3.07 ± 0.80 b | 3.98 ± 0.37 a |
| Ileum | |||
| Villus height, μm | 0.22 ± 0.02 b | 0.30 ± 0.07 a | 0.21 ± 0.02 b |
| Crypt depth, μm | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 |
| Villus height: crypt depth | 2.28 ± 0.42 | 3.55 ± 0.79 | 3.00 ± 0.69 |
| Group Name | All Difference | Down-Regulated | Up-Regulated |
|---|---|---|---|
| Control vs. spermidine-treated | 51 | 32 | 19 |
| ID | Metabolite Names | p-Value | VIP | Regulated |
|---|---|---|---|---|
| 321 | Succinate | 0.022117 | 0.323393 | Up |
| 504 | Maleic acid | 0.018546 | 2.256601 | Up |
| 4350 | Pantetheine | 0.018791 | 2.261522 | Up |
| 1120 | N1-Methyl-2-pyridone-5-carboxamide | 0.044214 | 2.036004 | Down |
| 3192 | Pyridoxal 5′-phosphate | 0.047058 | 1.961114 | Down |
| 3351 | Ribulose 5-phosphate | 0.006978 | 2.50337 | Down |
| 4439 | D-glucosamine 6-phosphate | 0.025789 | 2.29682 | Down |
| 7282 | 6k-PGFlalpha-d4 | 0.034865 | 2.04618 | Down |
| 7761 | Deoxycholic-acid | 0.030936 | 2.062915 | Down |
| 10518 | Adynerin | 0.007462 | 2.55801 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.-M.; Wang, Z.-L.; Yang, J.-D.; Wang, X.; Niu, C.-Y.; Ji, C.-W.; Ling, W.-K.; An, X.-G.; Guo, Y.-N.; Sun, Q.; et al. Effects of Spermidine on Mouse Gut Morphology, Metabolites, and Microbial Diversity. Nutrients 2023, 15, 744. https://doi.org/10.3390/nu15030744
Jiang D-M, Wang Z-L, Yang J-D, Wang X, Niu C-Y, Ji C-W, Ling W-K, An X-G, Guo Y-N, Sun Q, et al. Effects of Spermidine on Mouse Gut Morphology, Metabolites, and Microbial Diversity. Nutrients. 2023; 15(3):744. https://doi.org/10.3390/nu15030744
Chicago/Turabian StyleJiang, Dong-Mei, Ze-Long Wang, Jia-Di Yang, Xin Wang, Chun-Yang Niu, Cheng-Weng Ji, Wei-Kang Ling, Xiao-Guang An, Yong-Ni Guo, Qian Sun, and et al. 2023. "Effects of Spermidine on Mouse Gut Morphology, Metabolites, and Microbial Diversity" Nutrients 15, no. 3: 744. https://doi.org/10.3390/nu15030744
APA StyleJiang, D.-M., Wang, Z.-L., Yang, J.-D., Wang, X., Niu, C.-Y., Ji, C.-W., Ling, W.-K., An, X.-G., Guo, Y.-N., Sun, Q., Bai, L., Li, D.-B., Si, X.-H., & Kang, B. (2023). Effects of Spermidine on Mouse Gut Morphology, Metabolites, and Microbial Diversity. Nutrients, 15(3), 744. https://doi.org/10.3390/nu15030744






