The Effects of Dairy and Plant-Based Liquid Components on Lutein Liberation in Spinach Smoothies
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Smoothie Samples
2.2. In Vitro Digestion
2.3. Extraction of Lutein
2.4. Quantification of Lutein
2.5. Data Interpretations
2.6. Statistical Analyses
3. Results
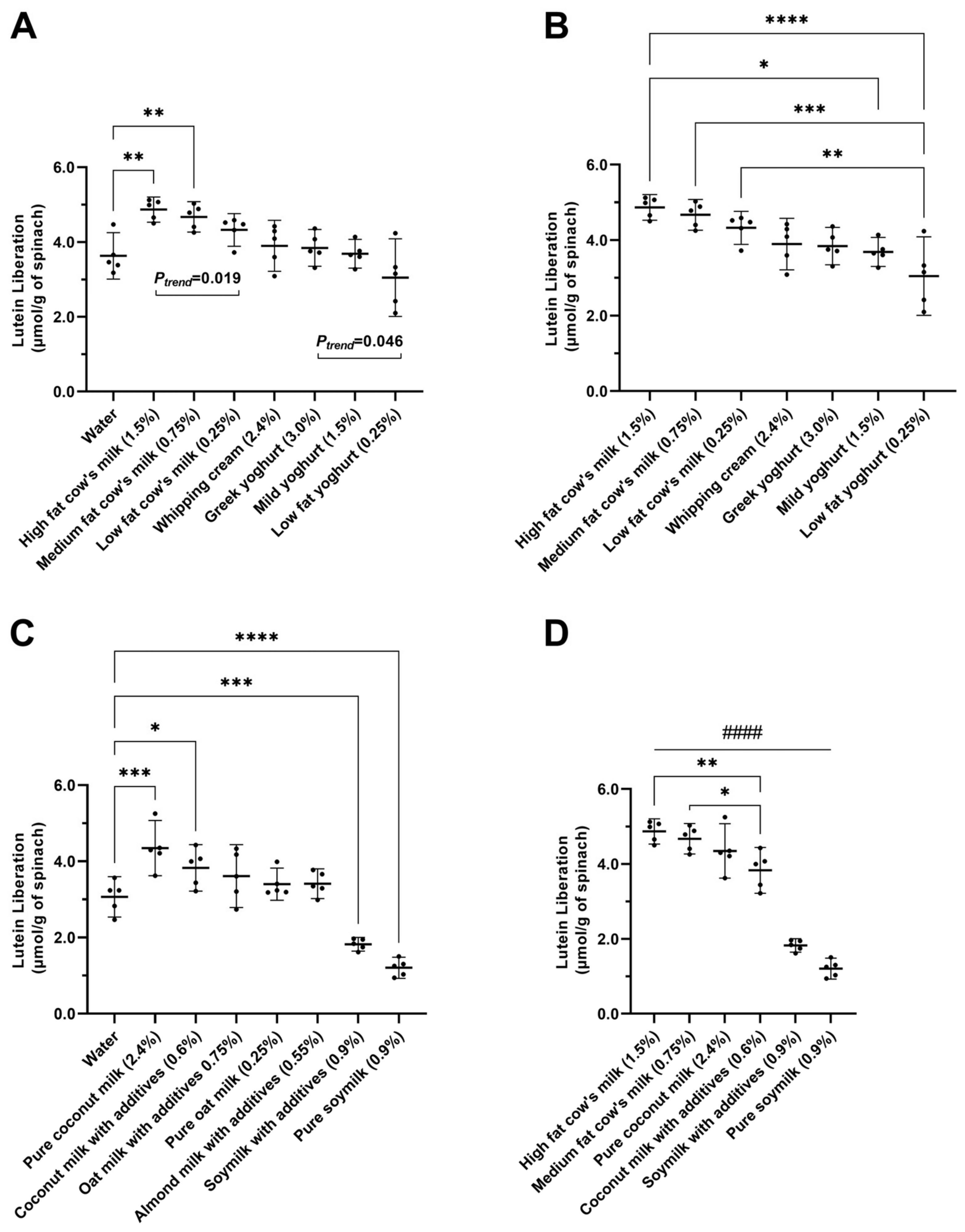
3.1. The Effects of Dairy Products on Lutein Liberation in Spinach Smoothies
3.2. The Effects of Plant-Based Products on Lutein Liberation in Spinach Smoothies
3.3. Comparing Improvers and Reducers
3.4. The Relationship between Macronutrients and Lutein Liberation
3.5. Effects of Fat on Lutein Liberation
3.6. Effects of Protein on Lutein Liberation
3.7. Effects of Fiber on Lutein Liberation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Na, H.J.; Kim, C.K.; Kim, J.Y.; Ha, K.S.; Lee, H.; Chung, H.T.; Kwon, H.J.; Kwon, Y.G.; Kim, Y.M. The non-provitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: Role of H(2)O(2) in NF-kappaB activation. Free Radic. Biol. Med. 2008, 45, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Goltz, S.R.; Campbell, W.W.; Chitchumroonchokchai, C.; Failla, M.L.; Ferruzzi, M.G. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol. Nutr. Food Res. 2012, 56, 866–877. [Google Scholar] [CrossRef]
- Chung, R.W.S.; Leanderson, P.; Gustafsson, N.; Jonasson, L. Liberation of lutein from spinach: Effects of heating time, microwave-reheating and liquefaction. Food Chem. 2019, 277, 573–578. [Google Scholar] [CrossRef]
- Conboy Stephenson, R.; Ross, R.P.; Stanton, C. Carotenoids in Milk and the Potential for Dairy Based Functional Foods. Foods 2021, 10, 1263. [Google Scholar] [CrossRef]
- Riedl, J.; Linseisen, J.; Hoffmann, J.; Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999, 129, 2170–2176. [Google Scholar] [CrossRef]
- Iddir, M.; Porras Yaruro, J.F.; Cocco, E.; Hardy, E.M.; Appenzeller, B.M.R.; Guignard, C.; Larondelle, Y.; Bohn, T. Impact of Protein-Enriched Plant Food Items on the Bioaccessibility and Cellular Uptake of Carotenoids. Antioxidants 2021, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J. Agric. Food Chem. 1999, 47, 4301–4309. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Lidebjer, C.; Leanderson, P.; Ernerudh, J.; Jonasson, L. Low plasma levels of oxygenated carotenoids in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Olmedilla-Alonso, B. Bioavailability of carotenoids and alpha-tocopherol from fruit juices in the presence of absorption modifiers: In vitro and in vivo assessment. Br. J. Nutr. 2009, 101, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.A.O.; Mercadante, A.Z.; Garrido-Fernández, J.; Pérez-Gálvez, A. Fat content affects bioaccessibility and efficiency of enzymatic hydrolysis of lutein esters added to milk and yogurt. Food Res. Int. 2014, 65, 171–176. [Google Scholar] [CrossRef]
- Loveday, S.M.; Sarkar, A.; Singh, H. Innovative yoghurts: Novel processing technologies for improving acid milk gel texture. Trends Food Sci. Technol. 2013, 33, 5–20. [Google Scholar] [CrossRef]
- Nidhi, B.; Ramaprasad, T.R.; Baskaran, V. Dietary fatty acid determines the intestinal absorption of lutein in lutein deficient mice. Food Res. Int. 2014, 64, 256–263. [Google Scholar] [CrossRef]
- Patil, U.; Benjakul, S. Coconut Milk and Coconut Oil: Their Manufacture Associated with Protein Functionality. J. Food Sci. 2018, 83, 2019–2027. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Woodruff, C.L. In vitro binding of bile acids by soy protein, pinto beans, black beans and wheat gluten. Food Chem. 2002, 79, 425–429. [Google Scholar] [CrossRef]
- Aoyama, T.; Fukui, K.; Takamatsu, K.; Hashimoto, Y.; Yamamoto, T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition 2000, 16, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Adachi, M.; Utsumi, S. Identification of the Bile Acid-binding Region in the Soy Glycinin A1aB1b Subunit. Biosci. Biotechnol. Biochem. 2002, 66, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Roodenburg, A.J.; Leenen, R.; van het Hof, K.H.; Weststrate, J.A.; Tijburg, L.B. Amount of fat in the diet affects bioavailability of lutein esters but not of α-carotene, β-carotene, and vitamin E in humans. Am. J. Clin. Nutr. 2000, 71, 1187–1193. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Olmedilla-Alonso, B.; Blanco-Navarro, I.; Pérez-Sacristán, B. Lutein bioavailability from lutein ester-fortified fermented milk: In vivo and in vitro study. J. Nutr. Biochem. 2010, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
| Categories | Liquid Components | Brands | Product Fat (g/100 g) a | Product Carbohydrate (g/100 g) a | Product Protein (g/100 g) a | Product Fiber (g/100 g) a | Notable Product Additives b | Dilution Factors c | Smoothie Fat (g/100 mL) d | Smoothie Carbohydrate (g/100 mL) d | Smoothie Protein (g/100 mL) d | Smoothie Fiber (g/100 mL) d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dairy | Low-fat yogurt | Arla f | 0.5 | 4.0 | 4.1 | 2 | 0.25 | 1.98 | 2.03 | |||
| Dairy | Mild yogurt | Arla f | 3.0 | 3.6 | 3.4 | 2 | 1.50 | 1.80 | 1.70 | |||
| Dairy | Greek yogurt | Arla f | 6.0 | 3.6 | 3.3 | 2 | 2.97 | 1.78 | 1.63 | |||
| Dairy | Low-fat cow’s milk | Arla f | 0.5 | 4.9 | 3.5 | 2 | 0.25 | 2.46 | 1.75 | |||
| Dairy | Medium-fat cow’s milk | Arla f | 1.5 | 4.9 | 3.5 | 2 | 0.75 | 2.46 | 1.76 | |||
| Dairy | High-fat cow’s milk | Arla f | 3.0 | 4.8 | 3.4 | 2 | 1.51 | 2.41 | 1.71 | |||
| Dairy | Whipping cream | ICA g | 36.0 e | 3.2 e | 2.3 e | Stabilizer (carrageenan) | 15 | 2.40 | 0.21 | 0.15 | ||
| Plant-based | Pure soymilk | Alpro h | 1.8 e | 2.3 e | 3.0 e | 0.5 e | 2 | 0.90 | 1.15 | 1.50 | 0.25 | |
| Plant-based | Soymilk with additives | Alpro h | 1.8 e | 0.0 e | 3.3 e | 0.6 e | Acidity regulators (Potassium phosphates). Calcium carbonate. Stabilizer (gellan gum). | 2 | 0.90 | 0 | 1.65 | 0.30 |
| Plant-based | Pure oat milk | Oatly i | 0.5 e | 6.7 e | 1.0 e | 0.8 e | 2 | 0.25 | 3.35 | 0.50 | 0.40 | |
| Plant-based | Oat milk with additives | Oatly i | 1.5 e | 6.7 e | 1.0 e | 0.8 e | Rapeseed oil. Calcium carbonate. Calcium phosphates. Potassium iodide. | 2 | 0.75 | 3.35 | 0.50 | 0.40 |
| Plant-based | Almond milk with additives | Alpro h | 1.1 e | 0.0 e | 0.4 e | 0.3 e | Tri-calcium phosphate. Sea salt. Stabilizers (locust bean gum. gellan gum). Emulsifier (lecithins (sunflower)). | 2 | 0.55 | 0 | 0.20 | 0.15 |
| Plant-based | Pure coconut milk | Santa Maria j | 18.0 | 2.7 | 1.9 | 7.5 | 2.45 | 0.37 | 0.26 | |||
| Plant-based | Coconut milk with additives | Alpro h | 1.2 e | 0.0 e | 0.1 e | Tri-calcium phosphate. Stabilizers (guar gum. xanthan gum. gellan gum). Sea salt. | 2 | 0.6 | 0 | 0.05 |
| Fat Content | Protein Content | Carbohydrate Content | Fiber Content | |
|---|---|---|---|---|
| Lutein liberation/g of spinach | −0.004 (ns) (n = 14) | 0.165 (ns) (n = 14) | 0.468 (<0.001) (n = 11) | 0.302 (ns) (n = 5) |
| Fat content | −0.187 (ns) (n = 14) | −0.171 (ns) (n = 11) | −0.263 (ns) (n = 5) | |
| Protein content | 0.888 (<0.001) (n = 11) | 0.158 (ns) (n = 5) | ||
| Carbohydrate content | 1.000 (<0.001) (n = 3) |
| Unadjusted Model (r2 = 0.952, p < 0.001) | Adjusted Model (r2 = 0.939, p < 0.001) | |||||
|---|---|---|---|---|---|---|
| Liquid Components | Mean (%) | Confidence Interval | Estimated Mean (%) | Confidence Interval | ||
| Lower | Upper | Lower | Upper | |||
| Medium-fat cow’s milk | 135.0 | 36.7 | 233.3 | 163.4 | 124.7 | 202.1 |
| High-fat cow´s milk | 79.6 | 25.4 | 133.8 | 106.8 | 68.5 | 145.1 |
| Coconut milk with additives | 140.6 | 77.1 | 204.0 | 14.0 | −74.2 | 102.2 |
| Pure coconut milk | 57.2 | 44.1 | 70.3 | 39.8 | 3.9 | 75.7 |
| Soymilk with additives | −148.3 | −172.1 | −124.6 | −106.7 | −150.1 | −63.4 |
| Pure soymilk | −225.4 | −235.2 | −215.5 | −178.6 | −2424.0 | −133.2 |
| Unadjusted Model (r2 = 0.921, p < 0.001) | Adjusted Model (r2 = 0.901, p < 0.001) | |||||
|---|---|---|---|---|---|---|
| Liquid Components | Mean (%) | Confidence Interval | Estimated Mean (%) | Confidence Interval | ||
| Lower | Upper | Lower | Upper | |||
| Medium-fat cow’s milk | 57.5 | 15.6 | 99.4 | −362.0 | −697.5 | −26.4 |
| High-fat cow´s milk | 70.3 | 22.4 | 118.1 | −168.4 | −420.3 | 83.4 |
| Coconut milk with additives | 1686.6 | 925.4 | 2447.9 | 1248.9 | 903.9 | 1593.9 |
| Pure coconut milk | 539.0 | 416.0 | 662.1 | 373.4 | 147.0 | 599.7 |
| Soymilk with additives | −80.9 | −93.9 | −67.9 | 424.2 | 43.0 | 805.4 |
| Pure soymilk | −135.2 | −141.1 | −129.3 | 621.3 | 95.6 | 1147.0 |
| Unadjusted Model (r2 = 0.708, p < 0.001) | Adjusted Model (r2 = 0.856, p < 0.001) | |||||
|---|---|---|---|---|---|---|
| Liquid Components | Mean (%) | Confidence Interval | Estimated Mean (%) | Confidence Interval | ||
| Lower | Upper | Lower | Upper | |||
| Oat milk with additives | 77.5 | 24.9 | 130.0 | 47.5 | −223.1 | 318.1 |
| Pure Oat milk | 162.1 | −79.0 | 403.3 | −18.8 | −307.9 | 270.4 |
| Almond milk with additives | 74.1 | −6.1 | 154.3 | 158.5 | −114.6 | 431.6 |
| Soymilk with additives | −148.3 | −172.1 | −124.6 | −82.8 | −293.2 | 127.5 |
| Pure soymilk | −225.4 | −235.2 | −215.5 | −164.3 | −777.6 | 449.0 |
| Unadjusted Model | Adjusted Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Pure Oat Milk | Almond Milk with Additives | Soymilk with Additives | Pure Soymilk | Pure Oat Milk | Almond Milk with Additives | Soymilk with Additives | Pure Soymilk | |
| Oat milk with additives | ns | ns | 0.011 | <0.001 | ns | ns | ns | ns |
| Pure Oat milk | ns | <0.001 | <0.001 | 0.012 | ns | ns | ||
| Almond milk with additives | 0.013 | <0.001 | ns | ns | ||||
| Soymilk with additives | ns | ns | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neelissen, J.; Leanderson, P.; Jonasson, L.; Chung, R.W.S. The Effects of Dairy and Plant-Based Liquid Components on Lutein Liberation in Spinach Smoothies. Nutrients 2023, 15, 779. https://doi.org/10.3390/nu15030779
Neelissen J, Leanderson P, Jonasson L, Chung RWS. The Effects of Dairy and Plant-Based Liquid Components on Lutein Liberation in Spinach Smoothies. Nutrients. 2023; 15(3):779. https://doi.org/10.3390/nu15030779
Chicago/Turabian StyleNeelissen, Jan, Per Leanderson, Lena Jonasson, and Rosanna W. S. Chung. 2023. "The Effects of Dairy and Plant-Based Liquid Components on Lutein Liberation in Spinach Smoothies" Nutrients 15, no. 3: 779. https://doi.org/10.3390/nu15030779
APA StyleNeelissen, J., Leanderson, P., Jonasson, L., & Chung, R. W. S. (2023). The Effects of Dairy and Plant-Based Liquid Components on Lutein Liberation in Spinach Smoothies. Nutrients, 15(3), 779. https://doi.org/10.3390/nu15030779






