Development and Validation of Vitamin D- Food Frequency Questionnaire for Moroccan Women of Reproductive Age: Use of the Sun Exposure Score and the Method of Triad’s Model
Abstract
:1. Introduction
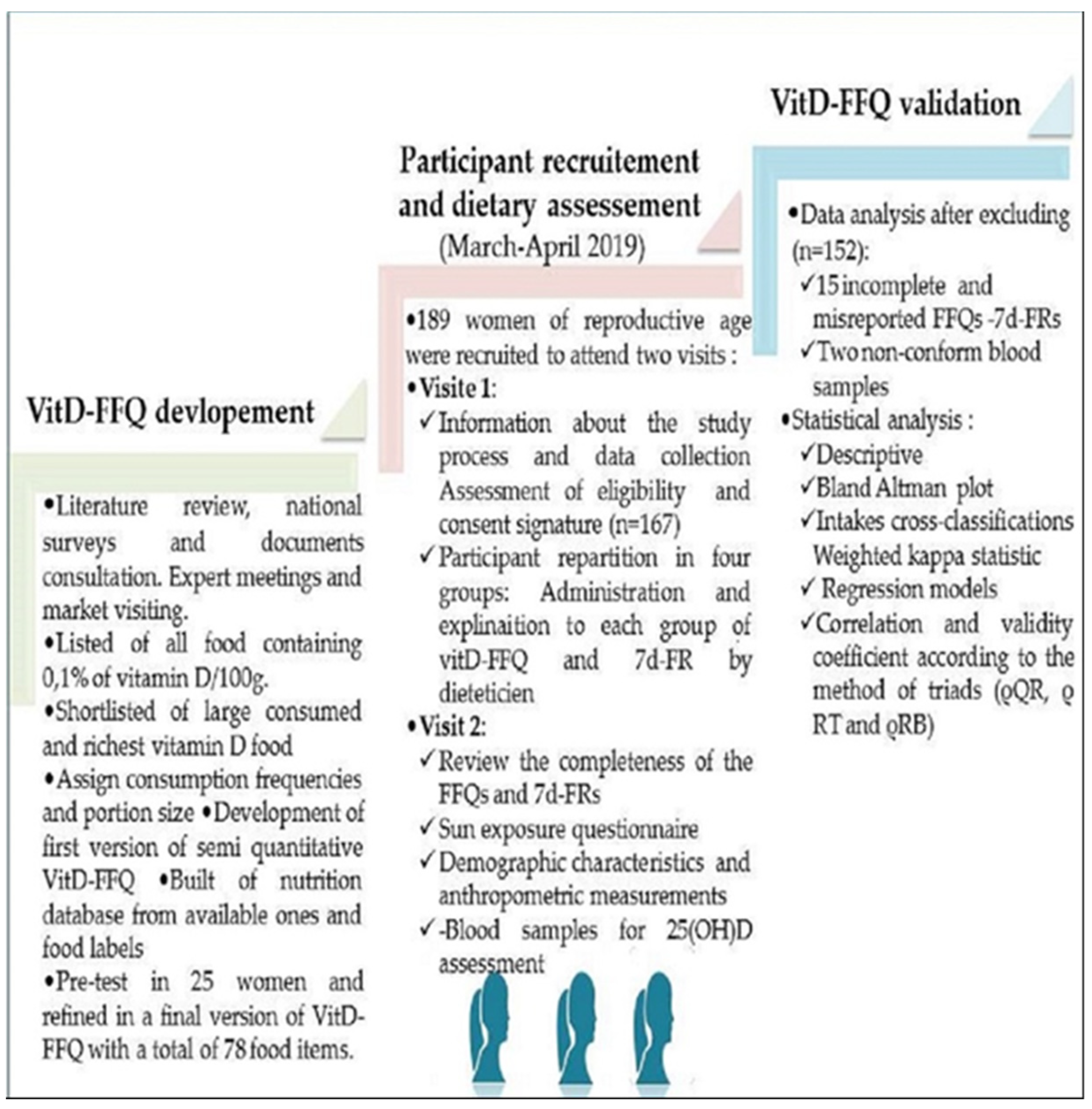
2. Materials and Methods
2.1. Study Design, Population Enrollment and Samples Size
2.2. VitD-FFQ Description and Development
2.3. Description of the 7d-FR
2.4. Sun Exposure Questionnaire (SEQ)
2.5. Vitamin D Status Biomarker Assessment (25(OH)D)
2.6. Anthropometry Measurement
2.7. Statistical Analysis
- ρ = the validity coefficient, Q = FFQ; R = 7d-DR and
- B = serum 25(OH) D concentration
3. Results
3.1. The VitD- FFQ Items Presentation
3.2. The Vit D-FFQ Validation
3.2.1. Study Participant’s Characteristics
3.2.2. Sun Exposure Scores and Related Factors
3.2.3. Vitamin D Status, Dietary Intake of Vitamin D Estimated by the VitD-FFQ and the 7d-FR
3.2.4. Association between Vitamin D Status, Dietary Intake, Sun Exposure Score, BMI and Socio-Demographic Characteristics of the Participants
3.2.5. Predictors of Serum 25(OH) D
3.3. Criterion Related Validity of the VitD-FFQ
3.3.1. Bland-Altman Analysis between VitD-FFQ and 7d-FR
3.3.2. Cross-Classification and Weighted Kappa Cross-Classification of Vitamin D Intake into Quartiles by VitD-FFQ and Validation Methods (7d-FR and Status)
3.4. Application of the Method of Triads Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleet, J.C.; Shapses, S.A. Vitamin D. In Present Knowledge in Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. 93–114. ISBN 978-0-323-66162-1. [Google Scholar]
- Binkley, N.; Bikle, D.D.; Dawson-Huμghes, B.; Plum, L.; Sempos, C.; DeLuca, H.F. Nonskeletal effects of vitamin D. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 757–774. ISBN 978-0-12-814841-9. [Google Scholar]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M. Vitamin D—Effects on Skeletal and Extraskeletal Health and the Need for Supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Tapia, G.; Mårild, K.; Dahl, S.R.; Lund-Blix, N.A.; Viken, M.K.; Lie, B.A.; Njølstad, P.R.; Joner, G.; Skrivarhauμg, T.; Cohen, A.S.; et al. Maternal and Newborn Vitamin D-Binding Protein, Vitamin D Levels, Vitamin D Receptor Genotype, and Childhood Type 1 Diabetes. Diabetes Care 2019, 42, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A.; Kerley, C.P.; Jensen, M.E.; Mauμger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D supplementation to prevent asthma exacerbations: A systematic review and meta-analysis of individual participant data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef] [PubMed]
- van Ballegooijen, A.J.; Kestenbaum, B.; Sachs, M.C.; de Boer, I.H.; Siscovick, D.S.; Hoofnagle, A.N.; Ix, J.H.; Visser, M.; Brouwer, I.A. Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2014, 63, 1214–1222. [Google Scholar] [CrossRef]
- Berghout, B.P.; Fani, L.; Heshmatollah, A.; Koudstaal, P.J.; Ikram, M.A.; Zillikens, M.C.; Ikram, M.K. Vitamin D Status and Risk of Stroke: The Rotterdam Study. Stroke 2019, 50, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Triggianese, P.; Watad, A.; Cedola, F.; Perricone, C.; Amital, H.; Giambini, I.; Perricone, R.; Shoenfeld, Y.; De Carolis, C. Vitamin D deficiency in an Italian cohort of infertile women. Am. J. Reprod. Immunol. 2017, 78, e12733. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Front. Endocrinol. 2018, 9, 500. [Google Scholar] [CrossRef]
- Qin, L.-L.; Lu, F.-G.; Yang, S.-H.; Xu, H.-L.; Luo, B.-A. Does Maternal Vitamin D Deficiency Increase the Risk of Preterm Birth: A Meta-Analysis of Observational Studies. Nutrients 2016, 8, 301. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. MECHANISMS IN ENDOCRINOLOGY: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Ultraviolet B Radiation: The Vitamin D Connection. Adv. Exp. Med. Biol. 2017, 996, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, T.C.; Zhang, A.; Persons, K.S.; Kohn, N.; Berkowitz, R.; Martinello, S.; Holick, M.F. An evaluation of the vitamin D3 content in fish: Is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J. Steroid Biochem. Mol. Biol. 2007, 103, 642–644. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684. [Google Scholar] [CrossRef]
- Patterson, A.C.; Hogg, R.C.; Kishi, D.M.; Stark, K.D. Biomarker and dietary validation of a Canadian food frequency questionnaire to measure eicosapentaenoic and docosahexaenoic acid intakes from whole food, functional food, and nutraceutical sources. J. Acad. Nutr. Diet. 2012, 112, 1005–1014. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [Green Version]
- Thornton, K.; Villamor, E. Nutritional Epidemiology. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 104–107. ISBN 978-0-12-384953-3. [Google Scholar]
- Larson-Meyer, D.E.; Woolf, K.; Burke, L. Assessment of Nutrient Status in Athletes and the Need for Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Abnet, C.C.; Cross, A.J.; Sinha, R. Dietary intake and nutritional status. IARC Sci. Publ. 2011, 163, 189–198. [Google Scholar]
- Teufel, N.I. Development of culturally competent food-frequency questionnaires. Am. J. Clin. Nutr. 1997, 65, 1173S–1178S. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Monographs in Epidemiology and Biostatistics; Oxford University Press: Oxford, UK, 2013; ISBN 978-0-19-975403-8. [Google Scholar]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef]
- Ocké, M.C.; Kaaks, R.J. Biochemical markers as additional measurements in dietary validity studies: Application of the method of triads with examples from the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 1997, 65, 1240S–1245S. [Google Scholar] [CrossRef]
- Kaaks, R.; Ferrari, P.; Ciampi, A.; Plummer, M.; Riboli, E. Uses and limitations of statistical accounting for random error correlations, in the validation of dietary questionnaire assessments. Public Health Nutr. 2002, 5, 969–976. [Google Scholar] [CrossRef]
- Kaaks, R.J. Biochemical markers as additional measurements in studies of the accuracy of dietary questionnaire measurements: Conceptual issues. Am. J. Clin. Nutr. 1997, 65, 1232S–1239S. [Google Scholar] [CrossRef]
- Bärebring, L.; Amberntsson, A.; Winkvist, A.; Auμgustin, H. Validation of Dietary Vitamin D Intake from Two Food Frequency Questionnaires, Using Food Records and the Biomarker 25-Hydroxyvitamin D among Pregnant Women. Nutrients 2018, 10, 745. [Google Scholar] [CrossRef]
- Djekic-Ivankovic, M.; Weiler, H.A.; Nikolic, M.; Kadvan, A.; Gurinovic, M.; Mandic, L.M.; Glibetic, M. Validity of an FFQ assessing the vitamin D intake of young Serbian women living in a region without food fortification: The method of triads model. Public Health Nutr. 2016, 19, 437–445. [Google Scholar] [CrossRef]
- Kiely, M.; Collins, A.; Lucey, A.J.; Andersen, R.; Cashman, K.D.; Hennessy, Á. Development, validation and implementation of a quantitative food frequency questionnaire to assess habitual vitamin D intake. J. Hum. Nutr. Diet. 2016, 29, 495–504. [Google Scholar] [CrossRef]
- Weir, R.R.; Carson, E.L.; Mulhern, M.S.; Laird, E.; Healy, M.; Pourshahidi, L.K. Validation of a food frequency questionnaire to determine vitamin D intakes using the method of triads. J. Hum. Nutr. Diet. 2016, 29, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Verner, D.; Treguer, D.; Redwood, J.; Christensen, J.; McDonnell, R.; Elbert, C.; Konishi, Y.; Belghazi, S. Climate Variability, Drouμght, and Drouμght Management in Morocco’s Agricultural Sector; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Bour, A.; Nejjar, B. Knowledge about vitamin D: An overview of the prevalence of hypovitaminosis D among Moroccan population. Ann. Sci. St. 2017, 1, 24–31. [Google Scholar]
- Allali, F.; El Aichaoui, S.; Khazani, H.; Benyahia, B.; Saoud, B.; El Kabbaj, S.; Bahiri, R.; Abouqal, R.; Hajjaj-Hassouni, N. High prevalence of hypovitaminosis D in Morocco: Relationship to lifestyle, physical performance, bone markers, and bone mineral density. Semin. Arthritis Rheum. 2009, 38, 444–451. [Google Scholar] [CrossRef]
- El Maghraoui, A.; Ouzzif, Z.; Mounach, A.; Rezqi, A.; Achemlal, L.; Bezza, A.; Tellal, S.; Dehhaoui, M.; Ghozlani, I. Hypovitaminosis D and prevalent asymptomatic vertebral fractures in Moroccan postmenopausal women. BMC Womens Health 2012, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Dadda, S.; Azekour, K.; Sebbari, F.; El Houate, B.; El Bouhali, B. Sun exposure, dressing habits, and vitamin D status in Morocco. E3S Web Conf. 2021, 319, 01097. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé, M. La lutte contre les troubles dus aux carences en micronutriments. Situation et perspectives. Rapport Ministère de la Santé 2003. Available online: https://www.sante.gov.ma/Publications/Guides-Manuels/Documents/Lutte%20contre%20les%20troubles[1].pdf(accessed on 20 July 2018).
- Tavera-Mendoza, L.E.; White, J.H. Cell defenses and the sunshine vitamin. Sci. Am. 2007, 297, 62–65, 68–70, 72. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Frost Andersen, L.; Henríque-Sánchez, P.; Doreste-Alonso, J.; Sánchez-Villegas, A.; Ortiz-Andrelluchi, A.; Negri, E.; La Vecchia, C. Evaluating the quality of dietary intake validation studies. Br. J. Nutr. 2009, 102 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef]
- Daurès, J.P.; Gerber, M.; Scali, J.; Astre, C.; Bonifacj, C.; Kaaks, R. Validation of a food-frequency questionnaire using multiple-day records and biochemical markers: Application of the triads method. J. Epidemiol. Biostat. 2000, 5, 109–115. [Google Scholar]
- Gröber, U.; Schmidt, J.; Kisters, K. Important druμg-micronutrient interactions: A selection for clinical practice. Crit. Rev. Food Sci. Nutr. 2020, 60, 257–275. [Google Scholar] [CrossRef]
- Czernichow, S.; Fan, T.; Nocea, G.; Sen, S.S. Calcium and vitamin D intake by postmenopausal women with osteoporosis in France. Curr. Med. Res. Opin. 2010, 26, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Secrétariat de l’Accès aux Marchés Rapport d’analyse mondiale. Agriculture et Agroalimentaire Canada. Division de l’analyse des marchés mondiaux Profil des consommateurs–Maroc 2014. 2018. Available online: http://www5.agr.gc.ca/resources/prod/Internet-Internet/MISB-DGSIM/ATS-SEA/PDF/6492-fra.pdf (accessed on 20 July 2018).
- Allali, F. Nutrition Transition in Morocco. Int. J. Med. Surg. 2017, 4, 68–71. [Google Scholar] [CrossRef]
- Benjelloun, S.H. Organisation des Nations Unies pour l’alimentation et l’agriculture. Population 1950, 5, 764. [Google Scholar] [CrossRef]
- Haut commissariat au Plan. Présentation des résultats de l’Enquête Nationale sur la Consommation et les Dépenses des Ménages 2013/2014; Haut commissariat au Plan: Casablanca, Morocco, 2016. [Google Scholar]
- Aguenaou, H.; Rahmani, M. Dossier Technique de la Fortification du Lait; ONSSA/MS/CL: Maroc, 2007. [Google Scholar]
- Benazouz, E.M.; Majdi, M.; Aguenaou, H. Référentiel législatif et réglementaire relatif à la fortification des denrées alimentaires par l’adjonction de vitamines et de minéraux; ONSSA/MS/GAIN/UNICEF: Maroc, 2006. [Google Scholar]
- Roseland, J.M.; Phillips, K.M.; Patterson, K.Y.; Pehrsson, P.R.; Taylor, C.L. Vitamin D in Foods. In Vitamin D; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–77. ISBN 978-0-12-809963-6. [Google Scholar]
- The French Agency for Food, Environmental and Occupationnal Health Safty (ANSES). Ciqual French Food Composition; ANSES: Maisons-Alfort, France, 2017. [Google Scholar]
- Pauwels, S.; Doperé, I.; Huybrechts, I.; Godderis, L.; Koppen, G.; Vansant, G. Validation of a food-frequency questionnaire assessment of methyl-group donors using estimated diet records and plasma biomarkers: The method of triads. Int. J. Food Sci. Nutr. 2014, 65, 768–773. [Google Scholar] [CrossRef]
- Neve, J. Aliments et Préparations Typiques de la Population Marocaine: Outil Pour Estimer la Consommation Alimentaire; CIRIHA: Bruxelles, Belgique, 2008. [Google Scholar]
- Jones, K.S.; Assar, S.; Harnpanich, D.; Bouillon, R.; Lambrechts, D.; Prentice, A.; Schoenmakers, I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J. Clin. Endocrinol. Metab. 2014, 99, 3373–3381. [Google Scholar] [CrossRef]
- Block, G.; Hartman, A.M.; Dresser, C.M.; Carroll, M.D.; Gannon, J.; Gardner, L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986, 124, 453–469. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. Food and Nutrient Database for Dietary Studies 2015–2016; U.S. Department of Agriculture, Agricultural Research Service: Baltimore, MD, USA, 2018.
- Sham, L.; Yeh, E.A.; Magalhaes, S.; Parra, E.J.; Gozdzik, A.; Banwell, B.; Hanwell, H.E. Evaluation of fall Sun Exposure Score in predicting vitamin D status in young Canadian adults, and the influence of ancestry. J. Photochem. Photobiol. B Biol. 2015, 145, 25–29. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I Throuμgh VI. Arch. Dermatol. 1988, 124, 869. [Google Scholar] [CrossRef]
- Hanwell, H.E.C.; Vieth, R.; Cole, D.E.C.; Scillitani, A.; Modoni, S.; Frusciante, V.; Ritrovato, G.; Chiodini, I.; Minisola, S.; Carnevale, V. Sun exposure questionnaire predicts circulating 25-hydroxyvitamin D concentrations in Caucasian hospital workers in southern Italy. J. Steroid Biochem. Mol. Biol. 2010, 121, 334–337. [Google Scholar] [CrossRef]
- Nikolaou, V.; Stratigos, A.J.; Antoniou, C.; Sypsa, V.; Avgerinou, G.; Danopoulou, I.; Nicolaidou, E.; Katsambas, A.D. Sun exposure behavior and protection practices in a Mediterranean population: A questionnaire-based study. Photodermatol. Photoimmunol. Photomed. 2009, 25, 132–137. [Google Scholar] [CrossRef]
- Yu, M.G.; Castillo-Carandang, N.; Sison, M.E.G.; Uy, A.B.; Villarante, K.L.; Maningat, P.; Paz-Pacheco, E.; Abesamis-Cubillan, E. Development and validation of a sunlight exposure questionnaire for urban adult Filipinos. Epidemiol. Health 2018, 40, e2018050. [Google Scholar] [CrossRef] [PubMed]
- Køster, B.; Søndergaard, J.; Nielsen, J.B.; Allen, M.; Olsen, A.; Bentzen, J. The validated sun exposure questionnaire: Association of objective and subjective measures of sun exposure in a Danish population-based sample. Br. J. Dermatol. 2017, 176, 446–456. [Google Scholar] [CrossRef]
- Serrano, M.-A. Contribution of sun exposure to the vitamin D dose received by various groups of the Spanish population. Sci. Total Environ. 2018, 619–620, 545–551. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Cronbach’s alpha. BMJ 1997, 314, 572. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Cannell, J.J.; Hollis, B.W.; Zasloff, M.; Heaney, R.P. Diagnosis and treatment of vitamin D deficiency. Expert Opin. Pharmacother. 2008, 9, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- WHO Consultation on Obesity (1997: Geneva, Switzerland); World Health Organization, Division of Noncommunicable Diseases; World Health Organization, Programme of Nutrition, Family and Reproductive Health. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity, Geneva, 3–5 June 1997; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect si. Trends Sport Sci. 2014, 21, 19–25. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Cui, J. Using the Bland-Altman method to measure agreement with repeated measures. Br. J. Anaesth. 2007, 99, 309–311. [Google Scholar] [CrossRef]
- Masson, L.F.; McNeill, G.; Tomany, J.O.; Simpson, J.A.; Peace, H.S.; Wei, L.; Grubb, D.A.; Bolton-Smith, C. Statistical approaches for assessing the relative validity of a food-frequency questionnaire: Use of correlation coefficients and the kappa statistic. Public Health Nutr. 2003, 6, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.W.; Waters, E. Four assumptions of multiple regression that researchers should always test. Pract. Assess. Res. Eval. 2002, 8, 2. [Google Scholar] [CrossRef]
- Lombard, M.J.; Steyn, N.P.; Charlton, K.E.; Senekal, M. Application and interpretation of multiple statistical tests to evaluate validity of dietary intake assessment methods. Nutr. J. 2015, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, C.; Rutishauser, I.; Conn, J.; O’Dea, K. Reproducibility of a meal-based food frequency questionnaire. The influence of format and time interval between questionnaires. Eur. J. Clin. Nutr. 1994, 48, 795–809. [Google Scholar]
- Ganji, V.; Abu-Dbaa, R.; Othman, H.; Zewein, M.; Al-Abdi, T.; Shi, Z. Validation of Vitamin D-Specific Food Frequency Questionnaire against Food Records for Qatari Women. Foods 2020, 9, 195. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.M.; Seechurn, T.; Atkinson, S.A. A Food Frequency Questionnaire for the Assessment of Calcium, Vitamin D and Vitamin K: A Pilot Validation Study. Nutrients 2010, 2, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, S.-H.; Lim, Y.-T.; Ha, Y.-C.; Chang, J.-S.; Kim, I.-S.; Min, Y.-K.; Chung, H.-Y. Validation of a new food frequency questionnaire for assessment of calcium and vitamin d intake in korean women. J. Bone Metab. 2013, 20, 67–74. [Google Scholar] [CrossRef]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hosp. 2015, 31 (Suppl. S3), 38–45. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Douμglas, C.S.; Thomas, J.J.; Johnson, E.C.; Barcal, J.N.; Heller, J.E.; Hollis, B.W.; Halliday, T.M. Validation of a Vitamin D Specific Questionnaire to Determine Vitamin D Status in Athletes. Nutrients 2019, 11, 2732. [Google Scholar] [CrossRef]
- Faid, F.; Nikolic, M.; Milesevic, J.; Zekovic, M.; Kadvan, A.; Gurinovic, M.; Glibetic, M. Assessment of vitamin D intake among Libyan women—Adaptation and validation of specific food frequency questionnaire. Libyan J. Med. 2018, 13, 1502028. [Google Scholar] [CrossRef]
- Wilkens, L.R.; Hankin, J.H.; Yoshizawa, C.N.; Kolonel, L.N.; Lee, J. Comparison of long-term dietary recall between cancer cases and noncases. Am. J. Epidemiol. 1992, 136, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Baki, S.; El Mghari, G.; El Ansari, N.; Harkati, I.; Tali, A.; Chabaa, L. Statut de la vitamine de la vitamine D chez les femmes marocaines vivant a Marrakech. Ann. D’endocrinologie 2015, 76, 490. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Rahme, M.; Chamoun, N.; El-Hajj Fuleihan, G. Vitamin D in the Middle East and North Africa. Bone Rep. 2018, 8, 135–146. [Google Scholar] [CrossRef]
- Organisation des nations unies pour l’alimentation et l’agriculture Profil Nutritionnel du Maroc—Division de la nutrition et de la protection des consommateurs. 2011. Available online: https://www.fao.org/3/ap843f/ap843f.pdf (accessed on 21 June 2018).
- Chee, W.S.S.; Suriah, A.R.; Chan, S.P.; Zaitun, Y.; Chan, Y.M. The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporos. Int. 2003, 14, 828–834. [Google Scholar] [CrossRef]
- Nikooyeh, B.; Neyestani, T.R.; Farvid, M.; Alavi-Majd, H.; Houshiarrad, A.; Kalayi, A.; Shariatzadeh, N.; Gharavi, A.; Heravifard, S.; Tayebinejad, N.; et al. Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: A randomized clinical trial. Am. J. Clin. Nutr. 2011, 93, 764–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mahony, L.; Stepien, M.; Gibney, M.J.; Nuμgent, A.P.; Brennan, L. The potential role of vitamin D enhanced foods in improving vitamin D status. Nutrients 2011, 3, 1023–1041. [Google Scholar] [CrossRef]
- Mansibang, N.M.M.; Yu, M.G.Y.; Jimeno, C.A.; Lantion-Ang, F.L. Association of sunlight exposure with 25-hydroxyvitamin D levels among working urban adult Filipinos. Osteoporos. Sarcopenia 2020, 6, 133–138. [Google Scholar] [CrossRef]
- Humayun, Q.; Iqbal, R.; Azam, I.; Khan, A.H.; Siddiqui, A.R.; Baig-Ansari, N. Development and validation of sunlight exposure measurement questionnaire (SEM-Q) for use in adult population residing in Pakistan. BMC Public Health 2012, 12, 421. [Google Scholar] [CrossRef]
- Johnson, M.A.; Davey, A.; Park, S.; Hausman, D.B.; Poon, L.W. Georgia Centenarian Study Age, race and season predict vitamin D status in African American and white octogenarians and centenarians. J. Nutr. Health Aging 2008, 12, 690–695. [Google Scholar] [CrossRef]
- McCarty, C.A. Sunlight exposure assessment: Can we accurately assess vitamin D exposure from sunlight questionnaires? Am. J. Clin. Nutr. 2008, 87, 1097S–1101S. [Google Scholar] [CrossRef]
- Taylor, C.; Lamparello, B.; Kruczek, K.; Anderson, E.J.; Hubbard, J.; Misra, M. Validation of a Food Frequency Questionnaire for Determining Calcium and Vitamin D Intake by Adolescent Girls with Anorexia Nervosa. J. Am. Diet. Assoc. 2009, 109, 479–485.e3. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Sidor, P.; Włodarek, D. Vitamin D Dietary Intake Questionnaire Validation Conducted among Young Polish Women. Nutrients 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M.; Fernandez, E. Vitamin D receptor polymorphisms and diseases. Clin. Chim. Acta 2006, 371, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Henauw, S.; Brants, H.A.M.; Becker, W.; Kaic-Rak, A.; Ruprich, J.; Sekula, W.; Mensink, G.B.M.; Koenig, J.S.; EFCOSUM Group. Operationalization of food consumption surveys in Europe: Recommendations from the European Food Consumption Survey Methods (EFCOSUM) Project. Eur. J. Clin. Nutr. 2002, 56 (Suppl. S2), S75–S88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, R.M.; Requejo, A.M.; López-Sobaler, A. Models of questionnaires for dietary studies, in the assessment of nutritional status. In Nutriguía. Manual of Clinical Nutrition in Primary Care; Ortega, R.M., Requejo, A.M., Eds.; Complutense: Madrid, Spain, 2006; pp. 456–467. [Google Scholar]



| Food Groups | Items | Number of Items |
|---|---|---|
| 1-Dairy Products and beverages | Cow milk: Vit D3 fortified (pasteurized, whole fat, 3.5% fat) Ultra high temperature whole cow milk vit D fortifed Semi skimmed milk (Milk, cow, partly skimmed, 1.5% fat) Whole fat powdered Milk , reconstituted with water Farm-fresh cow’s milk Yogurt (whole fat, nature). Light yogurt (0% fat) enriched with vitamin D3 Yogurt flavored or with fruit, sweetened, non-reduced fat Yoghurt flavoured, sweetened, with cream Milk drink, or drinking yogurt, flavored or with fruit, sweetened. Soy drinks: vitamin D3 and calcium fortified Processed cheese: Hard cheese (Emmental, Cheddar, Comte, Abondance) Hard cheese (Gruyere, Masdam) Hard cheese (Parmeson, Requefort) Semi-hard cheese (Gouda, Edam, Munster) Soft cheese (Camembert, Brie) feta- cheese (in oil or aromatic) Cottage cheese natural or aromatic Fresh cheese (ricotta, Mozarelle,) Melted cheese in portions or spreadable cubes | 21 |
| 2-Eggs | Pan-fried egg with added fat Pan-fried egg without added fat Crambled eggs Boiled egg Poached egg. | 5 |
| 3-Fish and Sea Products | Fatty fish fresh and frozen (Sardin, truit, salamon, hareng-ranga, mackerel-cabaila, swordfish-chbada, halibut, chinchar–chren, eel –salbah, carp, perch, tuna…) White fish fresh and frozen (sole, whiting, sea bream…) Code liver Canned fish, smoked fish, and salted fish Shellfish (calmer, scampi, shrimps, mussels) | 15 |
| 4-Meats Products | Red meat (Beef, veal, sheep..),eaten with or without fat Poultry (chicken and turkey) Organ meats (liver, kidney) Sausage or merguez Smoked meats or charcuterie (chorizo, mortadella, salami, ham, casheer, etc.) | 15 |
| 5-Fat Products | Margarine vit D3 fortified Normal margarine (more than 70% fat) light margarine (40 to 60% fat) Butter Sweet butter pasteurized at 80% or 82% fat Light pasteurized unsalted butter (40 to 60% fat) Unsalted butter or low-fat fortified salt Semn Vegetable oil vitamin D3 fortified: Mixed table oil with 3 seeds: sunflower, soya, colza Soya oil | 10 |
| 6-Breakfast cereals | Cereal vit D fortified Other non-fortified Muesli (normal and crispy) and oatmeal vit D fortified. | 3 |
| 7-Backery and Moroccan biscuit | Ordinary homemade cake or prepackaged Madeleine Viennoiserie (krachel, chocolate bread, raisin bread, croissant, brioche) Moroccan biscuits (thee dry biscuit, Fekkas, date biscuit) | 3 |
| 8-Chocolate and cacao | Milk chocolate, bar Dark chocolate with 70% cocoa minimum, extra, tasting, bar Dark chocolate with dried fruits (hazelnuts, almonds, raisins, praline), bar Cocoa unsweetened, soluble powder for drinks Sweet cocoa or chocolate powder for drinks, enriched with vitamins and minerals | 4 |
| Other vitamin D3 fortified product if applicable | Type, quantity and frequency | 1 |
| Food supplements(in vitamin D) used if applicable | Type, dosage and frequency | 1 |
| TOTAL | 78 |
| Unit/Category | Study Participants (n = 152) | |
|---|---|---|
| Age (Years) | Median (IQR) Range | 25.00 (±11.00) 20.00–44.00 |
| Employment: Unemployed: Students Housewives Employed: Professor Liberal profession | % | 90.78 72.46 18.31 9.22 7.90 1.32 |
| Education degree Primary secondary University or higher | % | 9.88 16.44 73.68 |
| Localization Urban Rural | % | 61.84 38.15 |
| Length (m) | Median (IQR) | 1.64 (±0.04) |
| weight (kg) | Median (IQR) | 70.00 (±17.00) |
| BMI (kg/m2) | Median (IQR) | 26.45 (±7.67) |
| BMI classification by kg/m2 Underweight (<18.5) Normal weight (18.5–24.99) Overweight (25–29.99) Obese (≥30) Sever obesity (30–34.9) | % | 1.30 23.80 45.00 20.00 10.00 |
| SEQ Domains Factors | Exposure Estimated Scores (n = 152) | Exposure Categories (Insufficient, Moderat, Sufficient, High) b | |
|---|---|---|---|
| Mean ± SD | Median ± IQR | p Value c | |
| 1.Indoor sun exposure | 7.60 (±4.10) | 9.00 (±2.00) | <0.001 |
| 2.Outdoor sun exposure | 16.28 (±5.39) | 16.50 (±10.75) | <0.001 |
| 3.Sun protection practices | 6.71 (±2.64) | 6.00 (±5.00) | <0.001 |
| Sun Exposure Score (SES) a | 16.07 (±5.62) | 15.75 (±8.25) | <0.001 |
| Mean ± SD | Rang | Median ± IQR | MD a | Diffrence % (IQR) b | p Value * | |
|---|---|---|---|---|---|---|
| Vitamin D intake(µg/d) | 8.77 (±4.98) | 3.28–21.60 | 7.10 ± 6.95 | 0.77 | 10.84 (−0.27–4.36) | 0.12 |
| VitD-FFQ | ||||||
| 7d-FR | 8.16 (±4.78) | 3.01–25.96 | 6.33 ± 5.02 | |||
| 25(OH)D (ng/mL) | 9.53 (±5.38) | 3–29.29 | 8.48 ± 6.55 |
| VitD-FFQ | 7d-FR | Sun Exposure Score | Age | Employment | Education Degree | Localization | BMI | |
|---|---|---|---|---|---|---|---|---|
| 25(OH) D (ng/mL) | 0.463 ** | 0.36 ** | 0.36 ** | 0.13 | −0.21 | 0.036 | −0.15 * | −0.17 * |
| Predicators Variables | Coefficient B (SE) | (97.5% CI) | p Value | ||
|---|---|---|---|---|---|
| Lower Limite | Upper Limite | ||||
| Model I (R = 0.47, p < 0.001) | Vitamin D intake (VitD-FFQ) | 0.56 (0.08) | 0.39 | 0.73 | p < 0.001 |
| SES | 0.32 (0.04) | 0.23 | 0.41 | p < 0.001 | |
| Localization | 3.17(0.86) | 1.45 | 4.89 | p < 0.001 | |
| Model II (R = 0.47, p < 0.001) | Vitamin D intake (7d-FR) | 0.36 | 0.27 | 0.55 | p < 0.001 |
| SES | 0.47 | 0.25 | 0.43 | p < 0.001 | |
| BMI | −0.16 | −0.31 | −0.04 | p < 0.001 | |
| Vitamin D Intake/Status Assessed by | Vitamin D Intake Assessed by VitD-FFQ | Weighted Kappa b (95 % Lower, Upper CI) | ||
|---|---|---|---|---|
| Classified into Same Quartile (%) | Classified into Same ± 1 Quartile (%) | Classified into Opposite Quartile a (%) | ||
| 7d-FR | 59.21 | 35.52 | 1.31 | 0.37 (0.27–0.46) |
| 25(OH)D | 45.39 | 34.21 | 3.94 | 0.28 (0.18–0.38) |
| Serum 25(OH)D Concentration (n = 152) | ||
|---|---|---|
| Simple Correlation | Adjusted for Covariate a | |
| Correlation coefficients | ||
| rQR (95%CI) | 0.64 ** (0.53–0.73) | 0.53 (0.38–0.68) |
| rQB (95%CI) | 0.46 ** (0.32–0.59) | 0.46 (0.31–0.59) |
| rBR (95%CI) | 0.36 ** (0.21–0.50) | 0.46 (0.29–0.60) |
| Validity coefficients | ||
| ρQT (95%CI) range | 0.90 (0.89–0.92) (0.64–0.90) | 0.72 (0.63–0.81) |
| ρRT (95%CI) range | 0.70 (0.58–0.78) (0.46–0.70) | 0.72 (0.59–0.83) |
| ρBT (95%CI) range | 0.53 (0.35–0.63) (0.36–0.53) | 0.63 (0.48 0.72) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouine, N.; Lhilali, I.; Menouni, A.; Godderis, L.; El Midaoui, A.; El Jaafari, S.; Zegzouti Filali, Y. Development and Validation of Vitamin D- Food Frequency Questionnaire for Moroccan Women of Reproductive Age: Use of the Sun Exposure Score and the Method of Triad’s Model. Nutrients 2023, 15, 796. https://doi.org/10.3390/nu15040796
Zouine N, Lhilali I, Menouni A, Godderis L, El Midaoui A, El Jaafari S, Zegzouti Filali Y. Development and Validation of Vitamin D- Food Frequency Questionnaire for Moroccan Women of Reproductive Age: Use of the Sun Exposure Score and the Method of Triad’s Model. Nutrients. 2023; 15(4):796. https://doi.org/10.3390/nu15040796
Chicago/Turabian StyleZouine, Noura, Ilham Lhilali, Aziza Menouni, Lode Godderis, Adil El Midaoui, Samir El Jaafari, and Younes Zegzouti Filali. 2023. "Development and Validation of Vitamin D- Food Frequency Questionnaire for Moroccan Women of Reproductive Age: Use of the Sun Exposure Score and the Method of Triad’s Model" Nutrients 15, no. 4: 796. https://doi.org/10.3390/nu15040796








