Cross-Validation of a New General Population Resting Metabolic Rate Prediction Equation Based on Body Composition
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
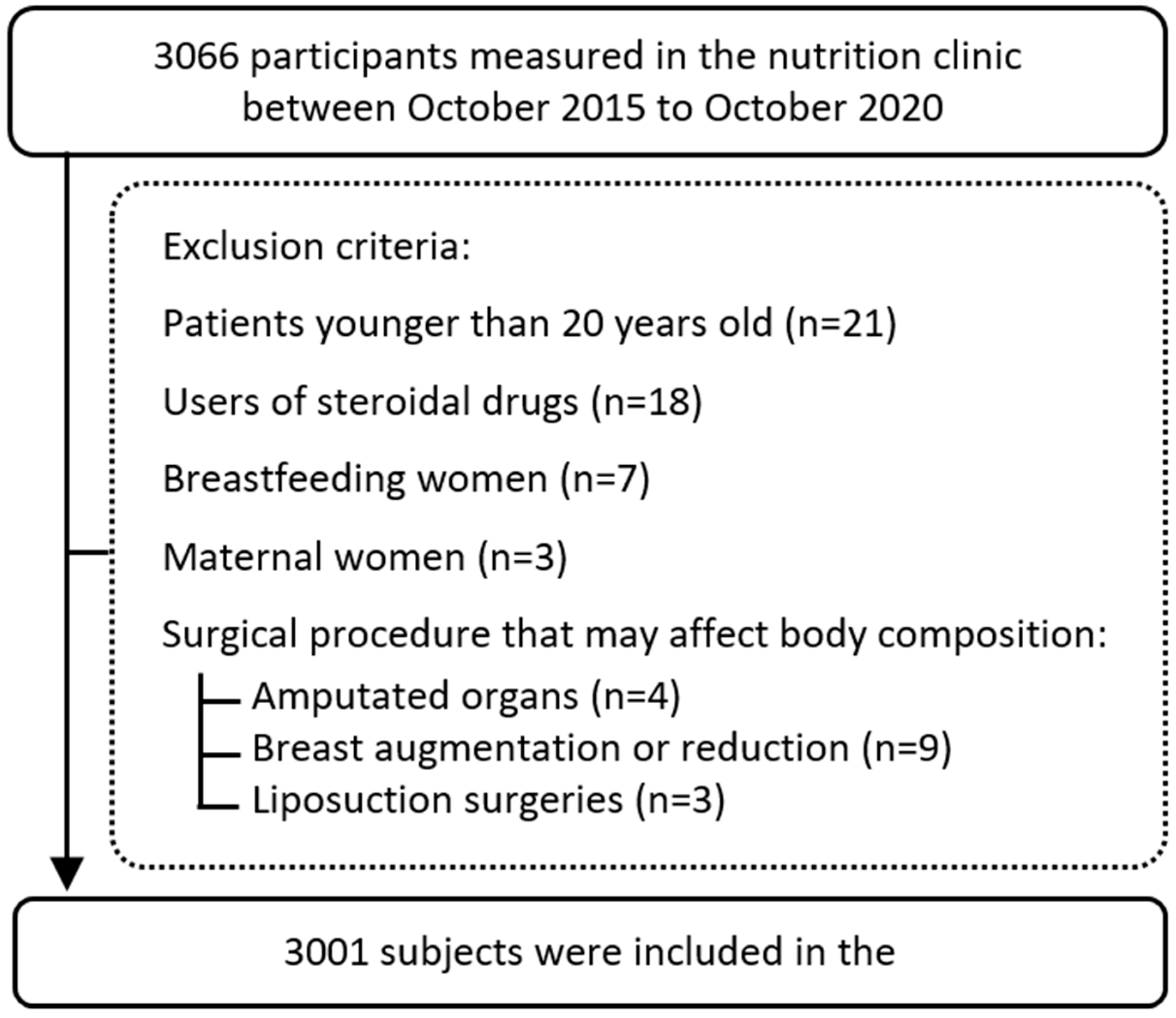
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BEE | basal energy expenditure |
| BIA | bioelectrical impedance analysis |
| BMI | body mass index |
| DXA | dual-energy X-ray absorptiometry |
| FAO | Food and Agricultural Organization |
| RMR | resting metabolic rate |
| T2DM | type 2 diabetes |
| TDEE | total daily energy expenditure |
| TEE | total energy expenditure |
| UNU | United National University |
| VIF | variance inflation factor |
| WHO | World Health Organization |
References
- Obesity and Overweighte. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 April 2020).
- Josephs-Spaulding, J.; Beeler, E.; Singh, O.V. Human Microbiome versus Food-Borne Pathogens: Friend or Foe. Appl. Microbiol. Biotechnol. 2016, 100, 4845–4863. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- LIVESEY, G. Energy and Protein Requirements the 1985 Report of the 1981 Joint FAO/WHO/UNU Expert Consultation. Nutr. Bull. 1987, 12, 138–149. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A New Predictive Equation for Resting Energy Expenditure in Healthy Individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kavle, E.; Owen, R.S.; Polansky, M.; Caprio, S.; Mozzoli, M.A.; Kendrick, Z.V.; Bushman, M.C.; Boden, G. A Reappraisal of Caloric Requirements in Healthy Women. Am. J. Clin. Nutr. 1986, 44, 1–19. [Google Scholar] [CrossRef]
- Owen, O.E.; Holup, J.L.; D’Alessio, D.A.; Craig, E.S.; Polansky, M.; Smalley, K.J.; Kavle, E.C.; Bushman, M.C.; Owen, L.R.; Mozzoli, M.A.; et al. A Reappraisal of the Caloric Requirements of Men. Am. J. Clin. Nutr. 1987, 46, 875–885. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Thomas, D.M.; Bosy-Westphal, A.; Müller, M.J. The Anatomy of Resting Energy Expenditure: Body Composition Mechanisms. Eur. J. Clin. Nutr. 2019, 73, 166–171. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Snider, H.L. Dissecting the Energy Needs of the Body. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 143–147. [Google Scholar] [CrossRef]
- Thom, G.; Gerasimidis, K.; Rizou, E.; Alfheeaid, H.; Barwell, N.; Manthou, E.; Fatima, S.; Gill, J.M.R.; Lean, M.E.J.; Malkova, D. Validity of Predictive Equations to Estimate RMR in Females with Varying BMI. J. Nutr. Sci. 2020, 9, e17. [Google Scholar] [CrossRef]
- Lazzer, S.; Bedogni, G.; Lafortuna, C.L.; Marazzi, N.; Busti, C.; Galli, R.; De Col, A.; Agosti, F.; Sartorio, A. Relationship between Basal Metabolic Rate, Gender, Age, and Body Composition in 8,780 White Obese Subjects. Obesity 2010, 18, 71–78. [Google Scholar] [CrossRef]
- Al-Domi, H.; Al-Shorman, A. Validation of Resting Metabolic Rate Equations in Obese and Non-Obese Young Healthy Adults. Clin. Nutr. ESPEN 2018, 26, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, E.; Petridis, D.; Tolia, M.; Tsoukalas, N.; Poultsidi, A.; Fasoulas, A.; Kyrgias, G.; Giaginis, C. Estimating the Agreement between the Metabolic Rate Calculated from Prediction Equations and from a Portable Indirect Calorimetry Device: An Effort to Develop a New Equation for Predicting Resting Metabolic Rate. Nutr. Metab. 2018, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, B.A.P.; Nicoletti, C.F.; de Oliveira, C.C.; Pinhel, M.A.D.S.; Quinhoneiro, D.C.G.; Noronha, N.Y.; Marchini, J.S.; Nonino, C.B. A New Resting Metabolic Rate Equation for Women with Class III Obesity. Nutrition 2018, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McLay-Cooke, R.T.; Gray, A.R.; Jones, L.M.; Taylor, R.W.; Skidmore, P.M.L.; Brown, R.C. Prediction Equations Overestimate the Energy Requirements More for Obesity-Susceptible Individuals. Nutrients 2017, 9, 1012. [Google Scholar] [CrossRef]
- Flack, K.D.; Siders, W.A.; Johnson, L.A.; Roemmich, J.N. Cross-Validation of Resting Metabolic Rate Prediction Equations. J. Acad. Nutr. Diet. 2016, 116, 1413–1422. [Google Scholar] [CrossRef]
- Reneau, J.; Obi, B.; Moosreiner, A.; Kidambi, S. Do We Need Race-Specific Resting Metabolic Rate Prediction Equations? Nutr. Diabetes 2019, 9, 21. [Google Scholar] [CrossRef]
- Zanella, P.B.; Ávila, C.C.; de Souza, C.G. Estimating Resting Energy Expenditure by Different Methods as Compared With Indirect Calorimetry for Patients With Pulmonary Hypertension. Nutr. Clin. Pract. 2018, 33, 217–223. [Google Scholar] [CrossRef]
- Miller, S.; Milliron, B.J.; Woolf, K. Common Prediction Equations Overestimate Measured Resting Metabolic Rate in Young Hispanic Women. Top. Clin. Nutr. 2013, 28, 120–135. [Google Scholar] [CrossRef]
- Cunningham, J.J. A Reanalysis of the Factors Influencing Basal Metabolic Rate in Normal Adults. Am. J. Clin. Nutr. 1980, 33, 2372–2374. [Google Scholar] [CrossRef]
- Bi, X.; Forde, C.G.; Goh, A.T.; Henry, C.J. Basal Metabolic Rate and Body Composition Predict Habitual Food and Macronutrient Intakes: Gender Differences. Nutrients 2019, 11, 2653. [Google Scholar] [CrossRef] [Green Version]
- Broad, E.M.; Newsome, L.J.; Dew, D.A.; Barfield, J.P. Measured and Predicted Resting Energy Expenditure in Wheelchair Rugby Athletes. J. Spinal Cord Med. 2020, 43, 388–397. [Google Scholar] [CrossRef]
- Ten Haaf, T.; Weijs, P.J.M. Resting Energy Expenditure Prediction in Recreational Athletes of 18-35 Years: Confirmation of Cunningham Equation and an Improved Weight-Based Alternative. PLoS ONE 2014, 9, e108460. [Google Scholar] [CrossRef] [PubMed]
- Devrim-Lanpir, A.; Kocahan, T.; Deliceoğlu, G.; Tortu, E.; Bilgic, P. Is There Any Predictive Equation to Determine Resting Metabolic Rate in Ultra-Endurance Athletes? Prog. Nutr. 2019, 21, 25–33. [Google Scholar] [CrossRef]
- Calton, E.K.; Pathak, K.; Soares, M.J.; Alfonso, H.; Keane, K.N.; Newsholme, P.; Cummings, N.K.; Chan She Ping-Delfos, W.; Hamidi, A. Vitamin D Status and Insulin Sensitivity Are Novel Predictors of Resting Metabolic Rate: A Cross-Sectional Analysis in Australian Adults. Eur. J. Nutr. 2016, 55, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R.; Meijer, G.A.L.; Kester, A.D.M.; Wouters, L.; Ten Hoor, F. Fat-Free Mass as a Function of Fat Mass and Habitual Activity Level. Int. J. Sports Med. 1992, 13, 163–166. [Google Scholar] [CrossRef]
- Fullmer, S.; Benson-Davies, S.; Earthman, C.P.; Frankenfield, D.C.; Gradwell, E.; Lee, P.S.P.; Piemonte, T.; Trabulsi, J. Evidence Analysis Library Review of Best Practices for Performing Indirect Calorimetry in Healthy and Non-Critically Ill Individuals. J. Acad. Nutr. Diet. 2015, 115, 1417–1446. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best Practice Methods to Apply to Measurement of Resting Metabolic Rate in Adults: A Systematic Review. J. Am. Diet. Assoc. 2006, 115, 1417–1446. [Google Scholar] [CrossRef]
- Mendis, S.; Armstrong, T.; Bettcher, D.; Branca, F.; Lauer, J.; Mace, C.; Poznyak, V.; Riley, L.; Da Costa, E.; Silva, V.; et al. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014.
- Frankenfield, D.; Roth-Yousey, L.; Compher, C. Comparison of Predictive Equations for Resting Metabolic Rate in Healthy Nonobese and Obese Adults: A Systematic Review. J. Am. Diet. Assoc. 2005, 105, 775–789. [Google Scholar] [CrossRef]
- Frankenfield, D.C.; Rowe, W.A.; Smith, J.S.; Cooney, R.N. Validation of Several Established Equations for Resting Metabolic Rate in Obese and Nonobese People. J. Am. Diet. Assoc. 2003, 105, 775–789. [Google Scholar] [CrossRef]
- Feurer, I.D.; Crosby, L.O.; Buzby, G.P.; Rosato, E.F.; Mullen, J.L. Resting Energy Expenditure in Morbid Obesity. Ann. Surg. 1983, 197, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Koehler, K.; De Souza, M.J.; Williams, N.I. Less-than-Expected Weight Loss in Normal-Weight Women Undergoing Caloric Restriction and Exercise Is Accompanied by Preservation of Fat-Free Mass and Metabolic Adaptations. Eur. J. Clin. Nutr. 2017, 71, 365–371. [Google Scholar] [CrossRef]
- Johannsen, D.L.; Knuth, N.D.; Huizenga, R.; Rood, J.C.; Ravussin, E.; Hall, K.D. Metabolic Slowing with Massive Weight Loss despite Preservation of Fat-Free Mass. J. Clin. Endocrinol. Metab. 2012, 97, 2489–2496. [Google Scholar] [CrossRef]
- Hopkins, M.; Gibbons, C.; Caudwell, P.; Hellström, P.M.; Näslund, E.; King, N.A.; Finlayson, G.; Blundell, J.E. The Adaptive Metabolic Response to Exercise-Induced Weight Loss Influences Both Energy Expenditure and Energy Intake. Eur. J. Clin. Nutr. 2014, 68, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Knuth, N.D.; Johannsen, D.L.; Tamboli, R.A.; Marks-Shulman, P.A.; Huizenga, R.; Chen, K.Y.; Abumrad, N.N.; Ravussin, E.; Hall, K.D. Metabolic Adaptation Following Massive Weight Loss Is Related to the Degree of Energy Imbalance and Changes in Circulating Leptin. Obesity 2014, 22, 2563–2569. [Google Scholar] [CrossRef]
- Nymo, S.; Coutinho, S.R.; Torgersen, L.C.H.; Bomo, O.J.; Haugvaldstad, I.; Truby, H.; Kulseng, B.; Martins, C. Timeline of Changes in Adaptive Physiological Responses, at the Level of Energy Expenditure, with Progressive Weight Loss. Br. J. Nutr. 2018, 120, 141–149. [Google Scholar] [CrossRef]
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.Y.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent Metabolic Adaptation 6 Years after “The Biggest Loser” Competition. Obesity 2016, 24, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Madigan, C.D.; Pavey, T.; Daley, A.J.; Jolly, K.; Brown, W.J. Is Weight Cycling Associated with Adverse Health Outcomes? A Cohort Study. Prev. Med. 2018, 108, 47–52. [Google Scholar] [CrossRef]
- Mason, C.; Foster-Schubert, K.E.; Imayama, I.; Xiao, L.; Kong, A.; Campbell, K.L.; Duggan, C.R.; Wang, C.Y.; Alfano, C.M.; Ulrich, C.M.; et al. History of Weight Cycling Does Not Impede Future Weight Loss or Metabolic Improvements in Postmenopausal Women. Metabolism. 2013, 62, 127–136. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J.; Wildman, R.; Kleiner, S.; VanDusseldorp, T.; Taylor, L.; Earnest, C.P.; Arciero, P.J.; Wilborn, C.; Kalman, D.S.; et al. International Society of Sports Nutrition Position Stand: Diets and Body Composition. J. Int. Soc. Sports Nutr. 2017, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Kahlhöfer, J.; Lagerpusch, M.; Skurk, T.; Müller, M.J. Deep Body Composition Phenotyping during Weight Cycling: Relevance to Metabolic Efficiency and Metabolic Risk. Obes. Rev. 2015, 16, 36–44. [Google Scholar] [CrossRef]
- Gallagher, D.; Kelley, D.E.; Thornton, J.; Boxt, L.; Pi-Sunyer, X.; Lipkin, E.; Nyenwe, E.; Janumala, I.; Heshka, S. Changes in Skeletal Muscle and Organ Size after a Weight-Loss Intervention in Overweight and Obese Type 2 Diabetic Patients. Am. J. Clin. Nutr. 2017, 105, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Lahav, Y.; Goldstein, N.; Gepner, Y. Comparison of Body Composition Assessment across Body Mass Index Categories by Two Multifrequency Bioelectrical Impedance Analysis Devices and Dual-Energy X-Ray Absorptiometry in Clinical Settings. Eur. J. Clin. Nutr. 2021, 75, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Merrigan, J.; Stute, N.; Eckerle, J.; Mackowski, N.; Walters, J.; O’Connor, M.; Barrett, K.; Robert, R.; Strang, A.; Hagen, J. Reliability and Validity of Contemporary Bioelectrical Impedance Analysis Devices for Body Composition Assessment. J. Exerc. Nutr. 2022, 5, 103133. [Google Scholar] [CrossRef]


| Females n = 1559 | Males n = 1442 | p | Total n = 3001 | |
|---|---|---|---|---|
| Age (years) | 40.3 ± 13.06 | 41.7 ± 13.01 | p = 0.007 | 41 ± 13.01 |
| Body weight (kg) | 72.4 ± 15.4 | 91.1 ± 18.2 | p < 0.001 | 81.3 ± 19.2 |
| Height (m) | 1.64 ± 0.06 | 1.77 ± 0.07 | p < 0.001 | 1.7 ± 0.09 |
| BMI (kg/m2) | 27 ± 5.6 | 29 ± 5.3 | p < 0.001 | 28.5 ± 5.5 |
| FFM (kg) | 43.4 ± 6.4 | 64.1 ± 9.1 | p < 0.001 | 53.3 ± 13 |
| FM (kg) | 28.1 ± 11.7 | 26.5 ± 12.2 | p < 0.001 | 27.3 ± 12 |
| FM (%) | 39.5 ± 9.1 | 29.2 ± 9.3 | p < 0.001 | 34.5 ± 10.5 |
| RMR (kcal/24 h) | 1615 ± 236 | 2075 ± 325 | p < 0.001 | 1841 ± 365 |
| Abdominal circumference (cm) | 96.1 ± 13.1 | 102 ± 14 | p < 0.001 | 98.9 ± 13.9 |
| Neck circumference (cm) | 32.9 ± 2.6 | 40 ± 3.5 | p < 0.001 | 36.5 ± 4.7 |
| Diabetes | 37 (2.4%) | 78 (5.4%) | p < 0.001 | 115 (3.8%) |
| Hypothyroidism | 109 (7%) | 27 (1.9%) | p < 0.001 | 136 (4.5%) |
| Bariatric surgery, yes (%) | 26 (1.7%) | 20 (1.4%) | p = 0.53 | 46 (1.5%) |
| BMI Group * | ||||
| Underweight | 20 (1.3%) | 8 (0.6%) | p = 0.038 | 28 (0.9%) |
| Normal weight | 642 (41%) | 326 (22.6%) | p < 0.001 | 968 (32.3%) |
| Overweight | 507 (32.5%) | 546 (37%) | p = 0.002 | 1053 (35.1%) |
| Obesity class 1 | 247 (15.8%) | 385 (26.7) | p < 0.001 | 632 (21.1%) |
| Obesity class 2 | 101 (6.5%) | 129 (8.9%) | p = 0.011 | 230 (7.7%) |
| Obesity class 3 | 41 (2.6%) | 48 (3.3%) | p = 0.26 | 89 (3%) |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Unstandardized β | Standardized Coefficient β | p | Unstandardized β | Standardized Coefficient β | p | |
| Constant | 709 | - | p < 0.001 | 824 | - | p < 0.001 |
| Age (years) | −5 | −0.18 | p < 0.001 | −5 | −0.18 | p < 0.001 |
| sex (male) | 66.8 | 0.09 | p < 0.001 | 67.7 | 0.09 | p < 0.001 |
| FFM (kg) | 20.5 | 0.73 | p < 0.001 | 18.5 | 0.65 | p < 0.001 |
| FM (kg) | 7.7 | 0.25 | p < 0.001 | 3.5 | 0.11 | p = 0.016 |
| FFM (kg) × FM (kg) | - | - | p < 0.001 | 0.07 | 0.16 | p = 0.003 |
| R2 | 0.745 | 0.746 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kfir, A.; Lahav, Y.; Gepner, Y. Cross-Validation of a New General Population Resting Metabolic Rate Prediction Equation Based on Body Composition. Nutrients 2023, 15, 805. https://doi.org/10.3390/nu15040805
Kfir A, Lahav Y, Gepner Y. Cross-Validation of a New General Population Resting Metabolic Rate Prediction Equation Based on Body Composition. Nutrients. 2023; 15(4):805. https://doi.org/10.3390/nu15040805
Chicago/Turabian StyleKfir, Aviv, Yair Lahav, and Yftach Gepner. 2023. "Cross-Validation of a New General Population Resting Metabolic Rate Prediction Equation Based on Body Composition" Nutrients 15, no. 4: 805. https://doi.org/10.3390/nu15040805





