Is It Time to Reconsider the U.S. Recommendations for Dietary Protein and Amino Acid Intake?
Abstract
:1. Introduction
2. Pros and Cons of Nitrogen Balance Determinations
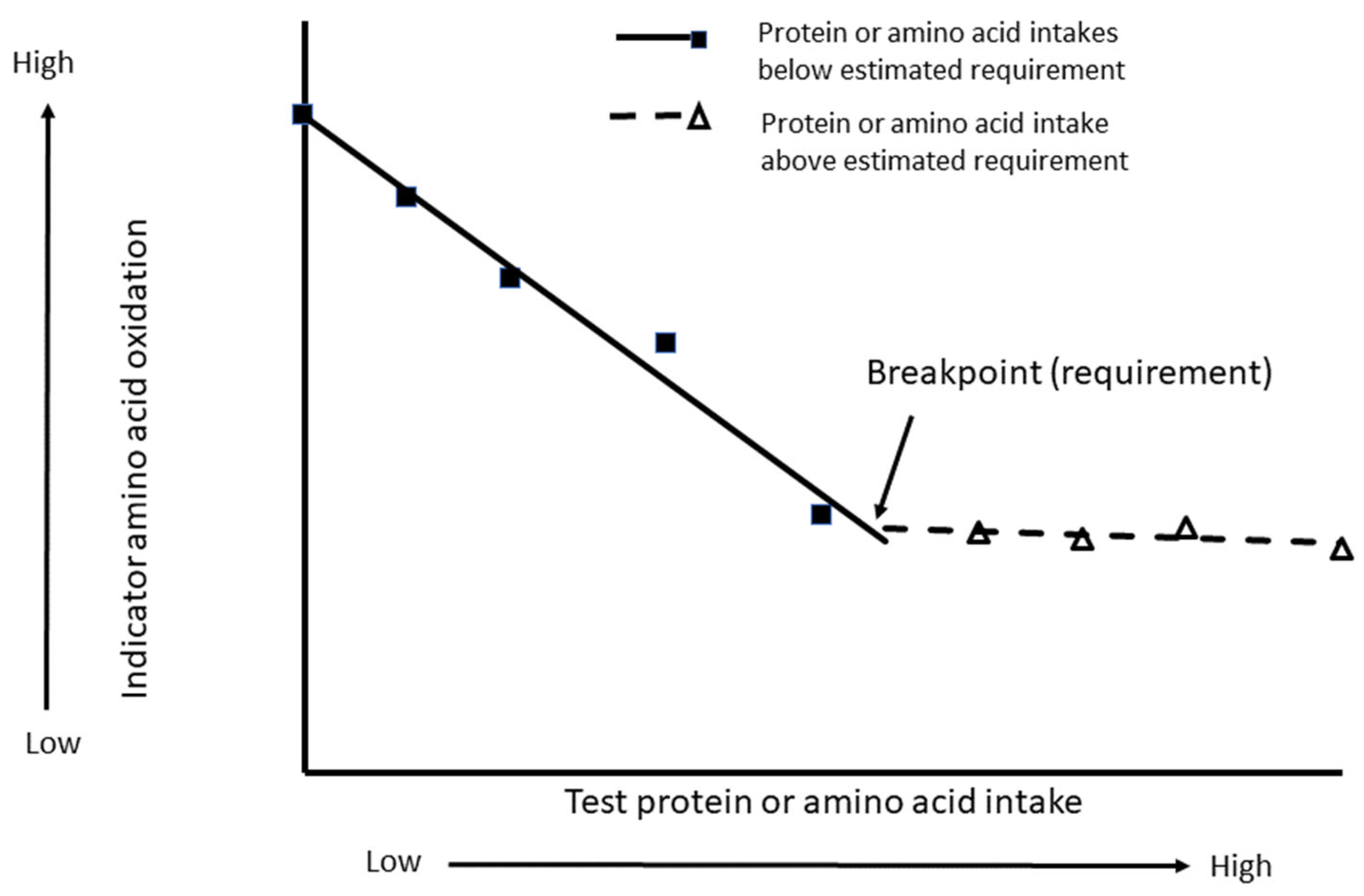
3. Results from Indicator Amino Acid Oxidation Studies for Determining Protein Requirements
4. Historical Perspectives on Higher Protein Recommendations Align with IAAO Estimates
5. Support for Higher Protein Recommendations for Older Adults (≥65 Years) Based on Evaluation of Studies Published in the Last 20 Years which Measure Health and Functional Outcomes
6. “Per Meal” Protein Requirements
7. Do Individual Amino Acid Requirements for Adults Align with Increased IAAO Estimates for Total Protein?
8. Physiological Roles and Functions of Dispensable Amino Acids Support the Need for Higher Protein Requirements
9. Support for Higher Protein Recommendations for Older Adults (≥65 Years) Based on Other Nutrition Guidelines
10. Determination of Protein Recommendations for the Pregnant and Breastfeeding Populations
11. Support for Higher Protein Recommendations for the Pregnant and Breastfeeding Populations
12. Support for Higher Protein Recommendations for the Pregnant and Breastfeeding Populations: IAAO Studies
13. New Estimates for Individual Amino Acid Requirements for the Pregnant Population, How They Compare with Increased IAAO Estimates for Total Protein and Evidence for Setting Specific Requirements for Each Trimester
14. Support for Higher Protein Recommendations for Healthy Children to Support Growth
15. Support for Higher Protein Recommendations for Healthy Children: IAAO Studies
16. Do Individual Amino Acid Requirements for Healthy Children Align with Increased IAAO Estimates for Total Protein?
17. The Influence of Protein Quality on the Need for Higher Protein and Amino Acid Recommendations for Undernourished Children Based on Other Study Data
18. Limitations
19. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolfe, R.R.; Cifelli, A.M.; Kostas, G.; Kim, I.Y. Optimizing protein intake in adults: Interpretation and application of the Recommended Dietary Allowance compared with the Acceptable Macronutrient Distribution Range. Adv. Nutr. 2017, 8, 266–275. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Recommended Dietary Allowances; The National Academies Press: Washington, DC, USA, 1941; 197p. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Technical Report Series 935 Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Consultation; WHO: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Waterlow, J.C. The mysteries of nitrogen balance. Nutr. Res. Rev. 1999, 12, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Dalangin, R.; Kim, A.; Campbell, R.E. The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. Metabolic demands for amino acids and the human dietary requirement: Millward and Rivers 1988 revisited. J. Nutr. 1998, 128, 2563S–2576S. [Google Scholar] [CrossRef]
- Fuller, M.F.; Garlick, P.J. Human amino acid requirements Can the controversy be resolved? Annu. Rev. Nutr. 1994, 14, 217–241. [Google Scholar] [CrossRef]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Reply to DJ Millward and AA Jackson. Am. J. Clin. Nutr. 2012, 95, 1501–1502. [Google Scholar] [CrossRef]
- Bandegan, A.; Courtney-Martin, G.; Rafii, M.; Pencharz, P.B.; Lemon, P.W. Indicator Amino Acid-Derived Estimate of Dietary Protein Requirement for Male Bodybuilders on a Nontraining Day Is Several-Fold Greater than the Current Recommended Dietary Allowance. J. Nutr. 2017, 147, 850–857. [Google Scholar] [CrossRef]
- Bandegan, A.; Courtney-Martin, G.; Rafii, M.; Pencharz, P.B.; Lemon, P.W.R. Indicator amino acid oxidation protein requirement estimate in endurance-trained men 24 h postexercise exceeds both the EAR and current athlete guidelines. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E741–E748. [Google Scholar] [CrossRef]
- Kato, H.; Suzuki, K.; Bannai, M.; Moore, D.R. Protein Requirements Are Elevated in Endurance Athletes after Exercise as Determined by the Indicator Amino Acid Oxidation Method. PLoS ONE 2016, 11, e0157406. [Google Scholar] [CrossRef]
- Malowany, J.M.; West, D.W.D.; Williamson, E.; Volterman, K.A.; Abou Sawan, S.; Mazzulla, M.; Moore, D.R. Protein to Maximize Whole-Body Anabolism in Resistance-trained Females after Exercise. Med. Sci. Sports Exerc. 2019, 51, 798–804. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences Engineering and Medicine. Nutrition During Pregnancy and Lactation: Exploring New Evidence: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- Ghosh, S.; Suri, D.; Uauy, R. Assessment of protein adequacy in developing countries: Quality matters. Br. J. Nutr. 2012, 108 (Suppl. 2), S77–S87. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Evidence that protein requirements have been significantly underestimated. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Taylor, Y.S.M.; Rand, W.M.; Scrimshaw, N.S. Protein requirements of man: Efficiencies of egg protein utilization at maintenance and sub-maintenance levels in young men. J. Nutr. 1973, 103, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Rand, W.M.; Pellet, P.L.; Young, V.R. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003, 77, 109–127. [Google Scholar] [CrossRef]
- Rand, W.M.; Young, V.R. Statistical analysis of nitrogen balance data with reference to the lysine requirements in adults. J. Nutr. 1999, 129, 1920–1926. [Google Scholar] [CrossRef] [Green Version]
- Humayun, M.A.; Elango, R.; Ball, R.O.; Pencharz, P.B. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am. J. Clin. Nutr. 2007, 86, 995–1002. [Google Scholar] [CrossRef]
- Kim, K.I.; Bayley, H.S. Amino acid oxidation by young pigs receiving diets with varying levels of sulphur amino acids. Br. J. Nutr. 1983, 50, 383–390. [Google Scholar] [CrossRef]
- Kim, K.I.; Elliott, J.I.; Bayley, H.S. Oxidation of an indicator amino acid by young pigs receiving diets with varying levels of lysine or threonine, and an assessment of amino acid requirements. Br. J. Nutr. 1983, 50, 391–399. [Google Scholar] [CrossRef]
- Kim, K.I.; McMillan, I.; Bayley, H.S. Determination of amino acid requirements of young pigs using an indicator amino acid. Br. J. Nutr. 1983, 50, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Ball, R.O.; Bayley, H.S. Tryptophan requirement of the 2.5-kg piglet determined by oxidation of an indicator amino acid. J. Nutr. 1984, 114, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Zello, G.A.; Pencharz, P.B.; Ball, R.O. Dietary lysine requirement of young adult males determined by oxidation of L-[1-13C]phenylalanine. Am. J. Physiol. 1993, 264, E677–E685. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Indicator amino acid oxidation: Concept and application. J. Nutr. 2008, 138, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Recent advances in determining protein and amino acid requirements in humans. Br. J. Nutr. 2012, 108 (Suppl. 2), S22–S30. [Google Scholar] [CrossRef]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Protein requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am. J. Clin. Nutr. 2011, 94, 1545–1552. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.L.; Gou, L.Y.; Li, W.D.; Tian, Y.; Hu, Y.C.; Wang, R.; Piao, J.H.; Yang, X.G.; Zhang, Y.H. Evaluation of the protein requirement in Chinese young adults using the indicator amino acid oxidation technique. Biomed. Environ. Sci. 2013, 26, 655–662. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, J.; Zhang, Y.; Piao, J.; Gou, L.; Tian, Y.; Li, M.; Ji, Y.; Yang, X. Examination of Chinese habitual dietary protein requirements of Chinese young female adults by an indicator amino acid method. Asia Pac. J. Clin. Nutr. 2011, 20, 390–396. [Google Scholar]
- Wooding, D.J.; Packer, J.E.; Kato, H.; West, D.W.D.; Courtney-Martin, G.; Pencharz, P.B.; Moore, D.R. Increased Protein Requirements in Female Athletes after Variable-Intensity Exercise. Med. Sci. Sports Exerc. 2017, 49, 2297–2304. [Google Scholar] [CrossRef]
- Rafii, M.; Chapman, K.; Elango, R.; Campbell, W.W.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G. Dietary protein requirement of men >65 years old determined by the indicator amino acid oxidation technique is higher than the current estimated average requirement. J. Nutr. 2015, 146, 681–687. [Google Scholar] [CrossRef]
- Rafii, M.; Chapman, K.; Owens, J.; Elango, R.; Campbell, W.W.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G. Dietary protein requirement of female adults >65 years determined by the indicator amino acid oxidation technique is higher than current recommendations. J. Nutr. 2015, 145, 18–24. [Google Scholar] [CrossRef]
- Mao, D.; Chen, F.; Wang, R.; Bai, P.; Zhang, Y.; Zhao, W.; Chen, J.; Yang, L.; Yang, X.; Li, M. Protein Requirements of Elderly Chinese Adults Are Higher than Current Recommendations. J. Nutr. 2020, 150, 1208–1213. [Google Scholar] [CrossRef]
- Tang, M.; McCabe, G.P.; Elango, R.; Pencharz, P.B.; Ball, R.O.; Campbell, W.W. Assessment of protein requirement in octogenarian women with use of the indicator amino acid oxidation technique. Am. J. Clin. Nutr. 2014, 99, 891–898. [Google Scholar] [CrossRef]
- Stephens, T.V.; Payne, M.; Ball, R.O.; Pencharz, P.B.; Elango, R. Protein requirements of healthy pregnant women during early and late gestation are higher than current recommendations. J. Nutr. 2015, 145, 73–78. [Google Scholar] [CrossRef]
- Rasmussen, B.F.; Ennis, M.; Pencharz, P.; Ball, R.; Courtney-Martin, G.; Elango, R. Protein requirements of healthy lactating women are higher than current recommendations. Curr. Dev. Nutr. 2020, 4 (Suppl. 2), 653. [Google Scholar] [CrossRef]
- Millward, D.J.; Jackson, A.A. Protein requirements and the indicator amino acid oxidation method. Am. J. Clin. Nutr. 2012, 95, 1498–1501. [Google Scholar] [CrossRef]
- Kurpad, A.V.; Thomas, T. Methods to assess amino acid requirements in humans. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Scrimshaw, N.S. Shattuck Lecture-Strengths and weaknesses of the committee approach: An analysis of past and present recommended dietary allowances for protein in health and disease (first of two parts). N. Engl. J. Med. 1976, 294, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Trommelen, J.; Wardenaar, F.C.; Brinkmans, N.Y.; Versteegen, J.J.; Jonvik, K.L.; Kapp, C.; de Vries, J.; van den Borne, J.J.; Gibala, M.J.; et al. Dietary Protein Intake and Distribution Patterns of Well-Trained Dutch Athletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; de Souza, R.J.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2019, 48, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Casperson, S.L.; Dillon, E.L.; Keske, M.A.; Paddon-Jones, D.; Sanford, A.P.; Hickner, R.C.; Grady, J.J.; Sheffield-Moore, M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 2010, 24, 4117–4127. [Google Scholar] [CrossRef] [PubMed]
- Bartali, B.; Frongillo, E.A.; Stipanuk, M.H.; Bandinelli, S.; Salvini, S.; Palli, D.; Morais, J.A.; Volpato, S.; Guralnik, J.M.; Ferrucci, L. Protein intake and muscle strength in older persons: Does inflammation matter? J. Am. Geriatr. Soc. 2012, 60, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.M.; Wertheim, B.C.; LaCroix, A.Z.; Prentice, R.L.; Neuhouser, M.L.; Tinker, L.F.; Kritchevsky, S.; Shikany, J.M.; Eaton, C.; Chen, Z.; et al. Biomarker-calibrated protein intake and physical function in the Women’s Health Initiative. J. Am. Geriatr. Soc. 2013, 61, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Morais, J.A.; Payette, H.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K.; Chevalier, S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am. J. Clin. Nutr. 2016, 104, 694–703. [Google Scholar] [CrossRef]
- Geirsdottir, O.G.; Arnarson, A.; Ramel, A.; Jonsson, P.V.; Thorsdottir, I. Dietary protein intake is associated with lean body mass in community-dwelling older adults. Nutr. Res. 2013, 33, 608–612. [Google Scholar] [CrossRef]
- Granic, A.; Mendonca, N.; Sayer, A.A.; Hill, T.R.; Davies, K.; Adamson, A.; Siervo, M.; Mathers, J.C.; Jagger, C. Low protein intake, muscle strength and physical performance in the very old: The Newcastle 85+ Study. Clin. Nutr. 2018, 37, 2260–2270. [Google Scholar] [CrossRef]
- Gregorio, L.; Brindisi, J.; Kleppinger, A.; Sullivan, R.; Mangano, K.M.; Bihuniak, J.D.; Kenny, A.M.; Kerstetter, J.E.; Insogna, K.L. Adequate dietary protein is associated with better physical performance among post-menopausal women 60–90 years. J. Nutr. Health Aging 2014, 18, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Hengeveld, L.M.; Chevalier, S.; Visser, M.; Gaudreau, P.; Presse, N. Prospective associations of protein intake parameters with muscle strength and physical performance in community-dwelling older men and women from the Quebec NuAge cohort. Am. J. Clin. Nutr. 2021, 113, 972–983. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef]
- Isanejad, M.; Mursu, J.; Sirola, J.; Kroger, H.; Rikkonen, T.; Tuppurainen, M.; Erkkila, A.T. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br. J. Nutr. 2016, 115, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K. Protein quantity and quality at levels above the RDA improves adult weight loss. J. Am. Coll. Nutr. 2004, 23, 631S–636S. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Fang, A.P.; Ma, W.J.; Wu, S.L.; Li, C.L.; Chen, Y.M.; Zhu, H.L. Amount Rather than Animal vs Plant Protein Intake Is Associated with Skeletal Muscle Mass in Community-Dwelling Middle-Aged and Older Chinese Adults: Results from the Guangzhou Nutrition and Health Study. J. Acad. Nutr. Diet 2019, 119, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; Sahni, S. Dietary Protein Intake Is Protective Against Loss of Grip Strength Among Older Adults in the Framingham Offspring Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Nabuco, H.C.G.; Tomeleri, C.M.; Sugihara Junior, P.; Fernandes, R.R.; Cavalcante, E.F.; Antunes, M.; Ribeiro, A.S.; Teixeira, D.C.; Silva, A.M.; Sardinha, L.B.; et al. Effects of Whey Protein Supplementation Pre- or Post-Resistance Training on Muscle Mass, Muscular Strength, and Functional Capacity in Pre-Conditioned Older Women: A Randomized Clinical Trial. Nutrients 2018, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, S.Y.; McGlory, C.; D’Souza, L.K.; Morgan, A.K.; Saddler, N.I.; Baker, S.K.; Parise, G.; Phillips, S.M. A randomized controlled trial of the impact of protein supplementation on leg lean mass and integrated muscle protein synthesis during inactivity and energy restriction in older persons. Am. J. Clin. Nutr. 2018, 108, 1060–1068. [Google Scholar] [CrossRef]
- Park, Y.; Choi, J.E.; Hwang, H.S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef]
- Sahni, S.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; McLean, R.R. Higher Protein Intake Is Associated with Higher Lean Mass and Quadriceps Muscle Strength in Adult Men and Women. J. Nutr. 2015, 145, 1569–1575. [Google Scholar] [CrossRef] [Green Version]
- Stookey, J.D.; Adair, L.S.; Popkin, B.M. Do protein and energy intakes explain long-term changes in body composition? J. Nutr. Health Aging 2005, 9, 5–17. [Google Scholar]
- Vellas, B.J.; Hunt, W.C.; Romero, L.J.; Koehler, K.M.; Baumgartner, R.N.; Garry, P.J. Changes in nutritional status and patterns of morbidity among free-living elderly persons: A 10-year longitudinal study. Nutrition 1997, 13, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.L.; Wang, Y.; Bergia Iii, R.E.; Campbell, W.W. Protein Intake Greater than the RDA Differentially Influences Whole-Body Lean Mass Responses to Purposeful Catabolic and Anabolic Stressors: A Systematic Review and Meta-analysis. Adv. Nutr. 2020, 11, 548–558. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Milan, A.M.; Mitchell, S.M.; Zeng, N.; Ramzan, F.; Sharma, P.; Knowles, S.O.; Roy, N.C.; Sjodin, A.; Wagner, K.H.; et al. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: A 10-wk randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Apovian, C.M.; Travison, T.G.; Pencina, K.; Moore, L.L.; Huang, G.; Campbell, W.W.; Li, Z.; Howland, A.S.; Chen, R.; et al. Effect of Protein Intake on Lean Body Mass in Functionally Limited Older Men: A Randomized Clinical Trial. JAMA Intern. Med. 2018, 178, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; van Baak, M.A.; Monsheimer, S.; Saris, W.H. The effect of a low-fat, high-protein or high-carbohydrate ad libitum diet on weight loss maintenance and metabolic risk factors. Int. J. Obes. 2009, 33, 296–304. [Google Scholar] [CrossRef]
- Reimer, R.A.; Willis, H.J.; Tunnicliffe, J.M.; Park, H.; Madsen, K.L.; Soto-Vaca, A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1700484. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ellis, V.; Dhaliwal, S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br. J. Nutr. 2010, 104, 716–723. [Google Scholar] [CrossRef]
- Murphy, C.H.; Oikawa, S.Y.; Phillips, S.M. Dietary Protein to Maintain Muscle Mass in Aging: A Case for Per-meal Protein Recommendations. J. Frailty Aging 2016, 5, 49–58. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol. Rep. 2016, 4, e12893. [Google Scholar] [CrossRef]
- Layman, D.K.; Anthony, T.G.; Rasmussen, B.B.; Adams, S.H.; Lynch, C.J.; Brinkworth, G.D.; Davis, T.A. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am. J. Clin. Nutr. 2015, 101, 1330S–1338S. [Google Scholar] [CrossRef] [PubMed]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Schutzler, S.; Schrader, A.; Spencer, H.; Kortebein, P.; Deutz, N.E.; Wolfe, R.R.; Ferrando, A.A. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E21–E28. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.; Toomey, C.; McCormack, W.G.; Francis, P.; Saunders, J.; Kerin, E.; Jakeman, P. Protein Supplementation at Breakfast and Lunch for 24 Weeks beyond Habitual Intakes Increases Whole-Body Lean Tissue Mass in Healthy Older Adults. J. Nutr. 2016, 146, 65–69. [Google Scholar] [CrossRef]
- National Research Council. Recommended Dietary Allowances, 10th ed.; National Academies Press: Washington, DC, USA, 1989. [Google Scholar] [CrossRef]
- World Health Organization. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- Szwiega, S.; Pencharz, P.B.; Rafii, M.; Lebarron, M.; Chang, J.; Ball, R.O.; Kong, D.; Xu, L.; Elango, R.; Courtney-Martin, G. Dietary leucine requirement of older men and women is higher than current recommendations. Am. J. Clin. Nutr. 2021, 113, 410–419. [Google Scholar] [CrossRef]
- Tian, Y.; Peng, J.; Chen, Y.; Gong, J.; Xu, H. Examination of lysine requirement of healthy young male adults on a Chinese habitual diet by the modified indicator amino acid oxidation method. Nutr. Res. Pract. 2014, 8, 59–65. [Google Scholar] [CrossRef]
- Holt, L.E.; Albanese, A.A. Observations on amino acid deficiencies in man. Trans. Assoc. Am. Physicians 1944, 58, 143–156. [Google Scholar]
- Hou, Y.; Yin, Y.; Wu, G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp. Biol. Med. 2015, 240, 997–1007. [Google Scholar] [CrossRef]
- Rose, W.C. The sequence of events leading to the establishment of the amino acid needs of man. Am. J. Public Health 1968, 58, 2020–2027. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Meininger, C.J.; Knabe, D.A.; Bazer, F.W.; Rhoads, J.M. Arginine nutrition in development, health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 59–66. [Google Scholar] [CrossRef]
- Tessari, P. Are there dietary requirements for dispensable amino acids and if so, how do we assess requirements? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.; Ball, R.O.; Pencharz, P.B.; Sakai, R.; Elango, R. Dispensable Amino Acids, except Glutamine and Proline, Are Ideal Nitrogen Sources for Protein Synthesis in the Presence of Adequate Indispensable Amino Acids in Adult Men. J. Nutr. 2020, 150, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Heger, J.; Frydych, Z.; Fronek, P. The effect of nonessential nitrogen on the utilization of dietary protein in the growing rat. J. Anim. Physiol. Anim. Nutr. 1987, 57, 130–139. [Google Scholar] [CrossRef]
- Lenis, N.P.; van Diepen, H.T.M.; Bikker, P.; Jongbloed, A.W.; van der Meulen, J. Effect of the ratio between essential and nonessential amino acids in the diet on utilization of nitrogen and amino acids by growing pigs. J. Anim. Sci. 1999, 77, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wu, G. Nutritionally Nonessential Amino Acids: A Misnomer in Nutritional Sciences. Adv. Nutr. 2017, 8, 137–139. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxid. Med. Cell Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef]
- Gibson, N.R.; Jahoor, F.; Ware, L.; Jackson, A.A. Endogenous glycine and tyrosine production is maintained consuming a marginal-protein diet. Am. J. Clin. Nutr. 2002, 75, 511–518. [Google Scholar] [CrossRef]
- Melendez-Hevia, E.; De Paz-Lugo, P. Branch-point stoichiometry can generate weak links in metabolism: The case of glycine biosynthesis. J. Biosci. 2008, 33, 771–780. [Google Scholar] [CrossRef]
- Rasmussen, B.F.; Ennis, M.A.; Dyer, R.A.; Lim, K.; Elango, R. Glycine, a Dispensable Amino Acid, Is Conditionally Indispensable in Late Stages of Human Pregnancy. J. Nutr. 2021, 151, 361–369. [Google Scholar] [CrossRef]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary glycine is rate-limiting for glutathione synthesis and may have broad potential for health protection. Ochsner J. 2018, 18, 81–87. [Google Scholar]
- Campbell, W.W.; Trappe, T.A.; Wolfe, R.R.; Evans, W.J. The Recommended Dietary Allowance for protein may not be adequate for older people to maintain skeletal muscle. J. Gerontol. 2001, 56A, M373–M380. [Google Scholar] [CrossRef] [PubMed]
- Pencharz, P.B.; Elango, R.; Wolfe, R.R. Recent developments in understanding protein needs—How much and what kind should we eat? Appl. Physiol. Nutr. Metab. 2016, 41, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Courtney-Martin, G.; Ball, R.O.; Pencharz, P.B.; Elango, R. Protein Requirements during Aging. Nutrients 2016, 8, 492. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Your MyPlate Plan—2600 Calories, Ages 14+ Years. Available online: https://www.myplate.gov/myplate-plan/results/2600-calories-ages-14-plus (accessed on 29 November 2021).
- Academy of Nutrition and Dietetics; American Diabetes Association. Choose Your Foods: Food Lists for Diabetes; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2019; pp. 1–62. [Google Scholar]
- Berryman, C.E.; Lieberman, H.R.; Fulgoni, V.L., 3rd; Pasiakos, S.M. Protein intake trends and conformity with the Dietary Reference Intakes in the United States: Analysis of the National Health and Nutrition Examination Survey, 2001–2014. Am. J. Clin. Nutr. 2018, 108, 405–413. [Google Scholar] [CrossRef]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [CrossRef]
- Thompson, G.N.; Halliday, D. Protein turnover in pregnancy. Eur. J. Clin. Nutr. 1992, 46, 411–417. [Google Scholar]
- Burke, B.S.; Vickers, V.V.; Stuart, H.C. Nutrition studies during pregnancy IV. Relation of protein content of mother’s diet to birth length, birth weight, and condition of infant at birth. J. Pediatr. 1943, 23, 506–515. [Google Scholar] [CrossRef]
- Higgins, A.C. Nutritional status and the outcome of pregnancy. J. Can. Diet Assoc. 1976, 37, 17–35. [Google Scholar]
- Cuco, G.; Fernandez-Ballart, J.; Sala, J.; Viladrich, C.; Iranzo, R.; Vila, J.; Arija, V. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur. J. Clin. Nutr. 2006, 60, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, M.S.; Kakuma, R.; Kramer, M.S. Energy and protein intake in pregnancy. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Blackwell, R.Q.; Chow, B.F.; Chinn, K.S.K.; Blackwell, B.N.; Hsu, S.C. Prospective maternal nutrition study in Taiwan: Rationale, study design, feasibility, and preliminary findings. Nutr. Rep. Int. 1973, 7, 517–532. [Google Scholar]
- Imdad, A.; Bhutta, Z.A. Effect of balanced protein energy supplementation during pregnancy on birth outcomes. BMC Public Health 2011, 11 (Suppl. 3), S17. [Google Scholar] [CrossRef]
- Brion, M.J.; Ness, A.R.; Rogers, I.; Emmett, P.; Cribb, V.; Davey Smith, G.; Lawlor, D.A. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: Exploring parental comparisons and prenatal effects. Am. J. Clin. Nutr. 2010, 91, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Rush, D.; Stein, Z.; Susser, M. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics 1980, 65, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Crume, T.L.; Brinton, J.T.; Shapiro, A.; Kaar, J.; Glueck, D.H.; Siega-Riz, A.M.; Dabelea, D. Maternal dietary intake during pregnancy and offspring body composition: The Healthy Start Study. Am. J. Obstet. Gynecol. 2016, 215, 609.e1–609.e8. [Google Scholar] [CrossRef]
- Sloan, N.L.; Lederman, S.A.; Leighton, J.; Himes, J.H.; Rush, D. The effect of prenatal dietary protein intake on birth weight. Nutr. Res. 2001, 21, 129–139. [Google Scholar] [CrossRef]
- Morisaki, N.; Nagata, C.; Yasuo, S.; Morokuma, S.; Kato, K.; Sanefuji, M.; Shibata, E.; Tsuji, M.; Senju, A.; Kawamoto, T.; et al. Optimal protein intake during pregnancy for reducing the risk of fetal growth restriction: The Japan Environment and Children’s Study. Br. J. Nutr. 2018, 120, 1432–1440. [Google Scholar] [CrossRef]
- Switkowski, K.M.; Jacques, P.F.; Must, A.; Kleinman, K.P.; Gillman, M.W.; Oken, E. Maternal protein intake during pregnancy and linear growth in the offspring. Am. J. Clin. Nutr. 2016, 104, 1128–1136. [Google Scholar] [CrossRef]
- Tahir, M.J.; Haapala, J.L.; Foster, L.P.; Duncan, K.M.; Teague, A.M.; Kharbanda, E.O.; McGovern, P.M.; Whitaker, K.M.; Rasmussen, K.M.; Fields, D.A.; et al. Higher Maternal Diet Quality during Pregnancy and Lactation Is Associated with Lower Infant Weight-For-Length, Body Fat Percent, and Fat Mass in Early Postnatal Life. Nutrients 2019, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Stephens, T.V.; Woo, H.; Innis, S.M.; Elango, R. Healthy pregnant women in Canada are consuming more dietary protein at 16- and 36-week gestation than currently recommended by the Dietary Reference Intakes, primarily from dairy food sources. Nutr. Res. 2014, 34, 569–576. [Google Scholar] [CrossRef] [PubMed]
- United Nations Population Division. World Population Prospects. 2022. Available online: https://population.un.org/wpp/ (accessed on 28 July 2022).
- Centers for Disease Control and Prevention; National Center for Health Statistics. Body Measurements. Available online: https://www.cdc.gov/nchs/fastats/body-measurements.htm (accessed on 14 October 2022).
- Levesque, C.L.; Moehn, S.; Pencharz, P.B.; Ball, R.O. The threonine requirement of sows increases in late gestation. J. Anim. Sci. 2011, 89, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.S.; Moehn, S.; Pencharz, P.B.; Ball, R.O. Dietary lysine requirement of sows increases in late gestation. J. Anim. Sci. 2012, 90, 4896–4904. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.J.; Josephson, J.K.; Moehn, S.; Pencharz, P.B.; Ball, R.O. Isoleucine requirement of pregnant sows. J. Anim. Sci. 2013, 91, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.J.; Josephson, J.K.; Moehn, S.; Pencharz, P.B.; Ball, R.O. Tryptophan requirement of pregnant sows. J. Anim. Sci. 2014, 92, 4457–4465. [Google Scholar] [CrossRef]
- Payne, M.; Stephens, T.; Lim, K.; Ball, R.O.; Pencharz, P.B.; Elango, R. Lysine Requirements of Healthy Pregnant Women are Higher During Late Stages of Gestation Compared to Early Gestation. J. Nutr. 2018, 148, 94–99. [Google Scholar] [CrossRef]
- Ennis, M.A.; Rasmussen, B.F.; Lim, K.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G.; Elango, R. Dietary phenylalanine requirements during early and late gestation in healthy pregnant women. Am. J. Clin. Nutr. 2020, 111, 351–359. [Google Scholar] [CrossRef]
- Wu, G. Dietary requirements of synthesizable amino acids by animals: A paradigm shift in protein nutrition. J. Anim. Sci. Biotechnol. 2014, 5, 34. [Google Scholar] [CrossRef]
- Tessari, P. Nonessential amino acid usage for protein replenishment in humans: A method of estimation. Am. J. Clin. Nutr. 2019, 110, 255–264. [Google Scholar] [CrossRef]
- Thame, M.M.; Fletcher, H.M.; Baker, T.M.; Marini, J.C.; Kao, C.C.; Jahoor, F. Arginine flux, but not nitric oxide synthesis, decreases in adolescent girls compared with adult women during pregnancy. J. Nutr. 2011, 141, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Thame, M.; Fletcher, H.; Baker, T.; Jahoor, F. Comparing the in vivo glycine fluxes of adolescent girls and adult women during early and late pregnancy. Br. J. Nutr. 2010, 104, 498–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paz-Lugo, P.; Lupianez, J.A.; Melendez-Hevia, E. High glycine concentration increases collagen synthesis by articular chondrocytes in vitro: Acute glycine deficiency could be an important cause of osteoarthritis. Amino Acids 2018, 50, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Kurpad, A.V.; Dwarkanath, P.; Thomas, T.; Mhaskar, A.; Thomas, A.; Mhaskar, R.; Jahoor, F. Comparison of leucine and dispensable amino acid kinetics between Indian women with low or normal body mass indexes during pregnancy. Am. J. Clin. Nutr. 2010, 92, 320–329. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Thornburg, K.L.; Prentice, A.M.; Campisi, S.; Lassi, Z.S.; Koletzko, B.; Bhutta, Z.A. Nutrition in adolescents: Physiology, metabolism, and nutritional needs. Ann. N. Y. Acad. Sci. 2017, 1393, 21–33. [Google Scholar] [CrossRef]
- Gattas, V.; Barrera, G.A.; Riumallo, J.S.; Uauy, R. Protein-energy requirements of prepubertal school-age boys determined by using the nitrogen-balance response to a mixed-protein diet. Am. J. Clin. Nutr. 1990, 52, 1037–1042. [Google Scholar] [CrossRef]
- Hudson, J.L.; Baum, J.I.; Diaz, E.C.; Borsheim, E. Dietary Protein Requirements in Children: Methods for Consideration. Nutrients 2021, 13, 1554. [Google Scholar] [CrossRef]
- Bolster, D.R.; Pikosky, M.A.; McCarthy, L.M.; Rodriguez, N.R. Exercise affects protein utilization in healthy children. J. Nutr. 2001, 131, 2659–2663. [Google Scholar] [CrossRef]
- Pikosky, M.; Faigenbaum, A.; Westcott, W.; Rodriguez, N. Effects of resistance training on protein utilization in healthy children. Med. Sci. Sports Exerc. 2002, 34, 820–827. [Google Scholar] [CrossRef]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Lysine requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am. J. Clin. Nutr. 2007, 86, 360–365. [Google Scholar] [CrossRef]
- Pillai, R.R.; Elango, R.; Muthayya, S.; Ball, R.O.; Kurpad, A.V.; Pencharz, P.B. Lysine requirement of healthy, school-aged Indian children determined by the indicator amino acid oxidation technique. J. Nutr. 2010, 140, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.R.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Branched-chain amino acid requirements in school-aged children determined by indicator amino acid oxidation (IAAO). J. Nutr. 2003, 133, 3540–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.M.; Humayn, M.A.; Elango, R.; Rafii, M.; Langos, V.; Ball, R.O.; Pencharz, P.B. Total sulfur amino acid requirement of healthy school-age children as determined by indicator amino acid oxidation technique. Am. J. Clin. Nutr. 2006, 83, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.W.; Ball, R.O.; Pencharz, P.B. Evidence that phenylalanine may not provide the full needs for aromatic amino acids in children. Pediatr. Res. 2007, 61, 361–365. [Google Scholar] [CrossRef]
- Hsu, J.W.; Goonewarde, L.A.; Rafii, M.; Ball, R.O.; Pencharz, P.B. Aromatic amino acid requirements in healthy men measured by indicator amino acid oxidation. Am. J. Clin. Nutr. 2006, 83, 82–88. [Google Scholar] [CrossRef]
- Di Buono, M.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Total sulfur amino acid requirement in young men as determined by indicator amino acid oxidation with L-[1-13C]phenylalanine. Am. J. Clin. Nutr. 2001, 74, 756–760. [Google Scholar] [CrossRef]
- Riazi, R.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. The total branched-chain amino acid requirement in young healthy adult men determined by indicator amino acid oxidation by use of L-[1-13C]phenylalanine. J. Nutr. 2003, 133, 1383–1389. [Google Scholar] [CrossRef]
- Pillai, R.R.; Elango, R.; Ball, R.O.; Kurpad, A.V.; Pencharz, P.B. Lysine requirements of moderately undernourished school-aged Indian children are reduced by treatment for intestinal parasites as measured by the indicator amino acid oxidation technique. J. Nutr. 2015, 145, 954–959. [Google Scholar] [CrossRef]
- Lazaris-Brumner, G.; Rafii, M.; Ball, R.O.; Pencharz, P.B. Tryptophan requirement in young adult women as determined by indicator amino acid oxidation with L-[13C]phenylalanine. Am. J. Clin. Nutr. 1998, 68, 303–310. [Google Scholar] [CrossRef]
- Al-Mokbel, A.; Courtney-Martin, G.; Elango, R.; Ball, R.O.; Pencharz, P.B.; Tomlinson, C. Tryptophan requirement in school-age children determined by the indicator amino acid oxidation method is similar to current recommendations. J. Nutr. 2019, 149, 280–285. [Google Scholar] [CrossRef]
- Kriengsinyos, W.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Oral and intravenous tracer protocols of the indicator amino acid oxidation method provide the same estimate of the lysine requirement in healthy men. J. Nutr. 2002, 132, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- United Nations Children’s Fund (UNICEF). Stop Stunting Regional Conference. Available online: https://www.unicef.org/rosa/stop-stunting-regional-conference-2014 (accessed on 1 August 2022).
- FAO. Understanding the True Cost of Malnutrition. Available online: https://www.fao.org/zhc/detail-events/en/c/238389/ (accessed on 1 August 2022).
- Arsenault, J.E.; Brown, K.H. Effects of protein or amino-acid supplementation on the physical growth of young children in low-income countries. Nutr. Rev. 2017, 75, 699–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Shardell, M.; Ashour, F.A.S.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Ordiz, M.I.; Kraemer, K.; Khadeer, M.A.; Ferrucci, L.; et al. Child stunting is associated with low circulating essential amino acids. EBioMedicine 2016, 6, 246–252. [Google Scholar] [CrossRef]
- Totzauer, M.; Luque, V.; Escribano, J.; Closa-Monasterolo, R.; Verduci, E.; ReDionigi, A.; Hoyos, J.; Langhendries, J.P.; Gruszfeld, D.; Socha, P.; et al. European Childhood Obesity Trial Study Group. Effect of Lower Versus Higher Protein Content in Infant Formula Through the First Year on Body Composition from 1 to 6 Years: Follow-Up of a Randomized Clinical Trial. Obesity 2018, 26, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Nuss, E.T.; Tanumihardjo, S.A. Quality protein maize for Africa: Closing the protein inadequacy gap in vulnerable populations. Adv. Nutr. 2011, 2, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.C.; Kurpad, A.V.; Duggan, C.P.; Ghosh, S.; Maxwell, D.G. Dietary intake of sulfur amino acids and risk of kwashiorkor malnutrition in eastern Democratic Republic of the Congo. Am. J. Clin. Nutr. 2021, 114, 925–933. [Google Scholar] [CrossRef]
- Kurpad, A.K.; Optimizing Nutrition for Maternal, Newborn, and Child Health. Amino Acid Digestibility [Speech audio recording]. Virtual Keystone Symposia. Available online: https://virtual.keystonesymposia.org/ks/live/537/page/3777 (accessed on 14 October 2022).
- Uauy, R.; Suri, D.J.; Ghosh, S.; Kurpad, A.; Rosenberg, I.H. Low circulating amino acids and protein quality: An interesting piece in the puzzle of early childhood stunting. EBioMedicine 2016, 8, 28–29. [Google Scholar] [CrossRef] [Green Version]
- FAO Expert Consultation. Dietary Protein Quality Evaluation in Human Nutrition: FAO Food and Nutrition Paper 92; Food and Agricultural Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
| Population | Mean Age or Age Range (y) | Proposed EAR (g/kg BW/d) | Current EAR (g/kg BW/d) | Proposed Population Safe Intake (e.g., RDA or RNI, g/kg BW/d) | Current Population Safe Intake (e.g., RDA, RNI, g/kg BW/d) | Reference |
|---|---|---|---|---|---|---|
| Bodybuilders, male | 22.5 | 1.70 | 0.66 | 2.20 | 0.80 | [10] |
| Endurance trained males, 24 h post exercise | 26.6 | 2.10 | 0.66 | 2.60 | 0.80 | [11] |
| Endurance athletes, male | 28 | 1.65 | 0.66 | 1.83 | 0.80 | [12] |
| Resistance-trained females | 23 | 1.49 | 0.66 | 1.93 | 0.80 | [13] |
| Young adult males | ~27 | 0.93 | 0.66 | 1.20 | 0.80 | [22] |
| Children | 6–11 | 1.30 | 0.76 | 1.55 | 0.95 | [30] |
| Young adults, China | 21 | 0.87 | 0.92 | 0.98 | 1.16 | [31] |
| Young female adults, China | 21 | 0.91 | 0.92 | 1.09 | 1.16 | [32] |
| Female athletes, variable intensity exercise | 21.2 | 1.41 | 0.66 | 1.71 | 0.80 | [33] |
| Older males | >65 | 0.94 | 0.66 | 1.24 | 0.80 | [34] |
| Older females | >65 | 0.96 | 0.66 | 1.29 | 0.80 | [35] |
| Older adults, China | >65 | 0.91 | 0.88 | 1.17 | 0.98 | [36] |
| Octogenarian females | 82 | 0.85 | 0.66 | 1.15 | 0.80 | [37] |
| Pregnant women | 24–37 | [38] | ||||
| Early gestation (11–20 wks) | 1.22 | 0.88 | 1.66 (upper end of 95% CI) | 1.10 | ||
| Late gestation (31–38 wks) | 1.52 | 0.88 | 1.77 (upper end of 95% CI) | 1.10 | ||
| Lactating Women (3–6 mo. Postpartum) | 1.7–1.9 | 1.05 | NA | 1.30 | [39] |
| Study | Population (n) | Study Details | Results |
|---|---|---|---|
| Bartali et al., 2012 [47] | Community-dwelling men and women ≥ 65 y (598) | Mean protein intake 77 g/d (48.5 g animal protein/d); mean energy intake 1999 kcal/d | Main effect of protein on muscle strength was not significant Lower protein intake was associated with greater decline in muscle strength in those with higher inflammatory markers (CRP *, IL-6 *, TNF-α *) |
| Beasley et al., 2013 [48] | Postmenopausal women 65–79 y (5346) | Calibrated energy and protein intake and physical function assessed | Calibrated protein intake in quintile 5 (15–22.3% energy) compared with quintile 1 (6.6–13.1% energy) was associated with higher self-reported physical function at baseline, slower rate of functional decline, higher GS * at baseline, slower declines in GS *, and more chair stands at baseline |
| Farsijani et al., 2016 [49] | Healthy community-dwelling men and women 67–84 y (712) | Protein quantity and distribution at meals at baseline and 2-year follow-up association with body composition | Men and women with evenly distributed protein intakes and men with high protein intakes showed higher LM or aLM throughout the entire follow-up period |
| Geirsdottir et al., 2013 [50] | Healthy community-dwelling men and women 65–92 y (237) | The association between dietary protein intake and body composition was measured | Mean protein intake was 0.98 ± 0.28 and 0.95 ± 0.29 g/kg body weight in male and female participants, respectively Dietary protein intake higher than RDA, was positively associated with LM |
| Granic et al., 2018 [51] | Community-dwelling men and women ≥ 85 y (722) | Evaluated associations between low protein intake (<1 g/kg aBW *) and changes in GS * and TUG * | Low protein intake associated with 1.62 kg lower baseline GS *, especially women, but rate of decline over 5 y not affected by protein status Women with low protein intake had worse baseline TUG, but rate of decline in TUG not affected by protein status |
| Gregorio et al., 2014 [52] | Healthy women 60–90 y (387) | Cross-sectional analysis of body composition and physical performance tests compared for those with protein intake below vs. at or above the RDA for protein (0.8 g/kg/d) | High protein group had lower total, fat, and lean mass and fat-to-lean ratio vs. lower protein group Upper and lower extremity function was impaired in low protein vs. high protein group |
| Hengeveld et al., 2021 [53] | Community-dwelling healthy men and women 67–84 y (1098) | Outcome measures included GS *, KES *, and physical performance (TUG *) Protein intake assessed via nine 24-h food records collected over 3 y | Higher daily protein intake was associated with better KES * and physical performance at 3 years in both genders and there was less physical performance decline in women In men, more uneven protein distribution was associated with better TUG * at 3 years and less GS * decline In women, higher number of protein snacks was associated with better GS * and KES * at 3 years and less GS * decline |
| Houston et al., 2008 [54] | Community-dwelling healthy men and women 70–79 y (2066) | The association between dietary protein intake and body composition was measured for 3-year changes. Quintiles for protein intake in g/d (Q1: 56.9 ± 18.6, Q2: 53.6 ± 19.8, Q3: 59.2 ± 18.2, Q4: 67.1 ± 19.2, Q5: 91.0 ± 27.1 | Participants in the highest quintile of protein intake lost ~40% less LM and aLM than did those in the lowest quintile of protein intake |
| Isanejad et al., 2016 [55] | Women 65.3 to 71.6 y (554) | Cross-sectional and prospective study that assessed body composition and physical function Protein intake was grouped into lower (≤0.80 g/kg BM */d), moderate (PROT-AGE study group recommendation of 0.81–1.19 g/kg BM */d) or higher (≥1.2 g/kg BM */d) | At baseline, the higher protein group had better performance in the GS/BM *, KES/BM *, one-leg stance, chair rise, squat, squat to the ground, and had faster walking speed for 10 m and higher short physical performance battery vs. those with moderate and lower protein intakes At 3 y follow up, higher protein intake was associated with less decline in GS/BM *, one leg stance, and tandem walk for 6 m |
| Layman et al., 2004 [56] | Women 45–56 y with BMI * > 26 kg/m2 (24) | 10-wk, 1700 kcal weight loss diet with either a carbohydrate/protein ratio of 3.5 (68 g protein/d; CHO group) or 1.4 (125 g protein/d; PRO group) with body composition and blood lipids measured | The PRO group lost 7.53 ± 1.44 kg body mass, while the CHO group lost 6.96 ± 1.36 kg body mass Weight loss in the PRO group had a higher proportion of fat/lean (6.3 ± 1.2 g/g) vs. the CHO group (3.8 ± 0.9 g/g) (p < 0.05) |
| Li et al., 2019 [57] | Men and women 40–80 y (3213) | Cross-sectional analysis in which dietary protein intake and body composition were obtained. Quintiles of protein intake were established (Q1: ≤0.96; Q2: 0.97–1.16; Q3: 1.17–1.38; Q4: 1.39–1.67; Q5: ≥1.68 g/kg/d). | The SMI * increased stepwise across percentiles in the fully adjusted model for relative total protein intake, relative animal protein intake, and relative plant protein intake (Ptrend < 0.001 in all cases) The odds of an individual having LMM * steadily decreased with each increase in total protein intake above Q1. |
| McLean et al., 2016 [58] | Men and women 29–85 y (1746) | Relationship between dietary protein (total, animal, and plant) and GS * was determined over 6 y follow up | Greater protein intake was associated with less decrease in GS *; ranging from lowest to highest quartiles of total protein intake, change in GS * (% per y) were −0.27, −0.15, 0.07, and 0.52). The trends for GS maintenance/improvement with higher protein intake were stronger for ages 60 + y vs. <60 y. |
| Nabuco et al., 2018 [59] | Healthy women ≥ 60 y (70) | Women resistance trained 3 days per wk for 12 wk. Women were assigned to: (1) 35 g hydrolyzed whey protein before each training session and carbohydrate placebo after (n = 24); (2) Carbohydrate placebo before and 35 g hydrolyzed whey protein after each training session (n = 23); or (3) Carbohydrate placebo before and after training (n = 23) | Protein supplementation increased total protein intake to 1.38 to 1.49 g/kg/d and each supplementation regimen equally increased energy intake from 22–23 kcal/kg to 26–28 kcal/kg Supplement timing relative to exercise did not affect the results, but whey protein hydrolysate supplementation improved SMM *, LLLST *, CP *, KES *, TS* and 10-m walk time vs. placebo only group |
| Oikawa et al., 2018 [60] | Healthy Men and women 68–69 y (31) | 4-phase protocol: EB (1 wk): energy balance; 0.8 g/kg/d protein ER (1 wk): −500 kcal/d energy restriction; 1.6 g/kg/d protein (60 g/d whey or collagen peptides) ER + SR (2 wk): ER plus step reduction to <750/d RC (1 wk): Recovery of normal activity plus 1.6 g/kg/d protein (60 g/d whey or collagen peptides) | Higher protein intake did not protect against loss of leg lean mass from energy restriction or step reduction During RC, whey but not collagen: -Increased leg lean mass from ER + SR -Restored integrated muscle protein synthesis that had declined in ER and ER + SR |
| Park et al., 2018 [61] | Frail men and women 70–85 y (99) | In a 12-wk study, three protein intake groups: (1) 0.8 g/kg/d; maltodextrin powder; (2) 1.2 g/kg/d; combination of maltodextrin and whey protein powder; (3) 1.5 g/kg/d; combination of maltodextrin and whey protein powder | The 1.5 g/kg protein group, compared with the 0.8 g/kg protein group, had higher ASM *, ASM */weight, ASM */BMI *, ASM */fat ratio, and SMI *. Compared with the 0.8 g/kg protein group, the 1.5 g/kg protein group had improved gait speed. |
| Sahni et al., 2015 [62] | Healthy men and women 29–86 y (2675) | Protein intake, leg lean mass and isometric quadriceps strength were measured. Protein intake in g/d was split into quartiles for men and women, respectively—Q1: 64.9, 57.8; Q2: 70.8, 63.1; Q3: 79.2, 73.5; Q4: 101.1, 93.4 | In both men and women, leg lean mass was higher in participants in the highest quartiles of total protein intake compared with those in the lowest quartiles of protein intake |
| Stookey et al., 2005 [63] | Healthy men and women 50–69 y (608) | Regression models used to determine if 3-day mean protein (% of energy) predicted changes in MAMA | Higher protein intake was associated with less loss of MAMA for both sexes |
| Vellas et al., 1997 [64] | Healthy men and women > 60 y (304) | Subjects were recruited into a 10-y longitudinal study to assess the relationships between nutrition and morbidity and mortality. | Women with protein intakes greater than the midrange of 0.8–1.2 g/kg body weight (1.20–1.76 g/kg) tended to have fewer health problems than those with protein intakes <0.8 g/kg |
| Amino Acid | Current Population Safe Intake for Adults (e.g., RDA or RNI, mg/kg BW/d) [3] | Current Population Safe Intake for Children (Boys & Girls 4–13 Years) (e.g., RDA or RNI, mg/kg BW/d) [3] | IAAO Proposed Population Safe Intake for Adults (e.g., RDA or RNI, mg/kg BW/d) | IAAO Proposed Population Safe Intake for Healthy Children 6–10 y (e.g., RDA or RNI, mg/kg BW/d) |
|---|---|---|---|---|
| Tryptophan, mg | 5 | 6 | 5.0 [150] | 6.1 [151] |
| Total Aromatic Amino Acids (TAA), mg | 33 | 41 | 44–52 [146] | 28 [145] |
| Total Sulfur Amino Acids (SAA), mg | 19 | 22 | 21 [147] | 17.9 [144] |
| Total Branched-chain Amino Acids (BCAA), mg | 85 | 99 | 210 [148] | 192 [143] |
| Lysine, mg | 38 | 46 | 52.5 [152] 58.2 [27] | 58 [141] 46.6 [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiler, M.; Hertzler, S.R.; Dvoretskiy, S. Is It Time to Reconsider the U.S. Recommendations for Dietary Protein and Amino Acid Intake? Nutrients 2023, 15, 838. https://doi.org/10.3390/nu15040838
Weiler M, Hertzler SR, Dvoretskiy S. Is It Time to Reconsider the U.S. Recommendations for Dietary Protein and Amino Acid Intake? Nutrients. 2023; 15(4):838. https://doi.org/10.3390/nu15040838
Chicago/Turabian StyleWeiler, Mary, Steven R. Hertzler, and Svyatoslav Dvoretskiy. 2023. "Is It Time to Reconsider the U.S. Recommendations for Dietary Protein and Amino Acid Intake?" Nutrients 15, no. 4: 838. https://doi.org/10.3390/nu15040838
APA StyleWeiler, M., Hertzler, S. R., & Dvoretskiy, S. (2023). Is It Time to Reconsider the U.S. Recommendations for Dietary Protein and Amino Acid Intake? Nutrients, 15(4), 838. https://doi.org/10.3390/nu15040838







