Patient-Reported Experiences with a Low-Carbohydrate Ketogenic Diet: An International Survey in Patients with McArdle Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Part 1
2.2. Survey Part 2
2.3. Survey Part 3
2.4. Survey Distribution and Data Collection
2.5. Data Presentation
3. Results
3.1. Study Cohort
3.2. Experiences with the LCKD
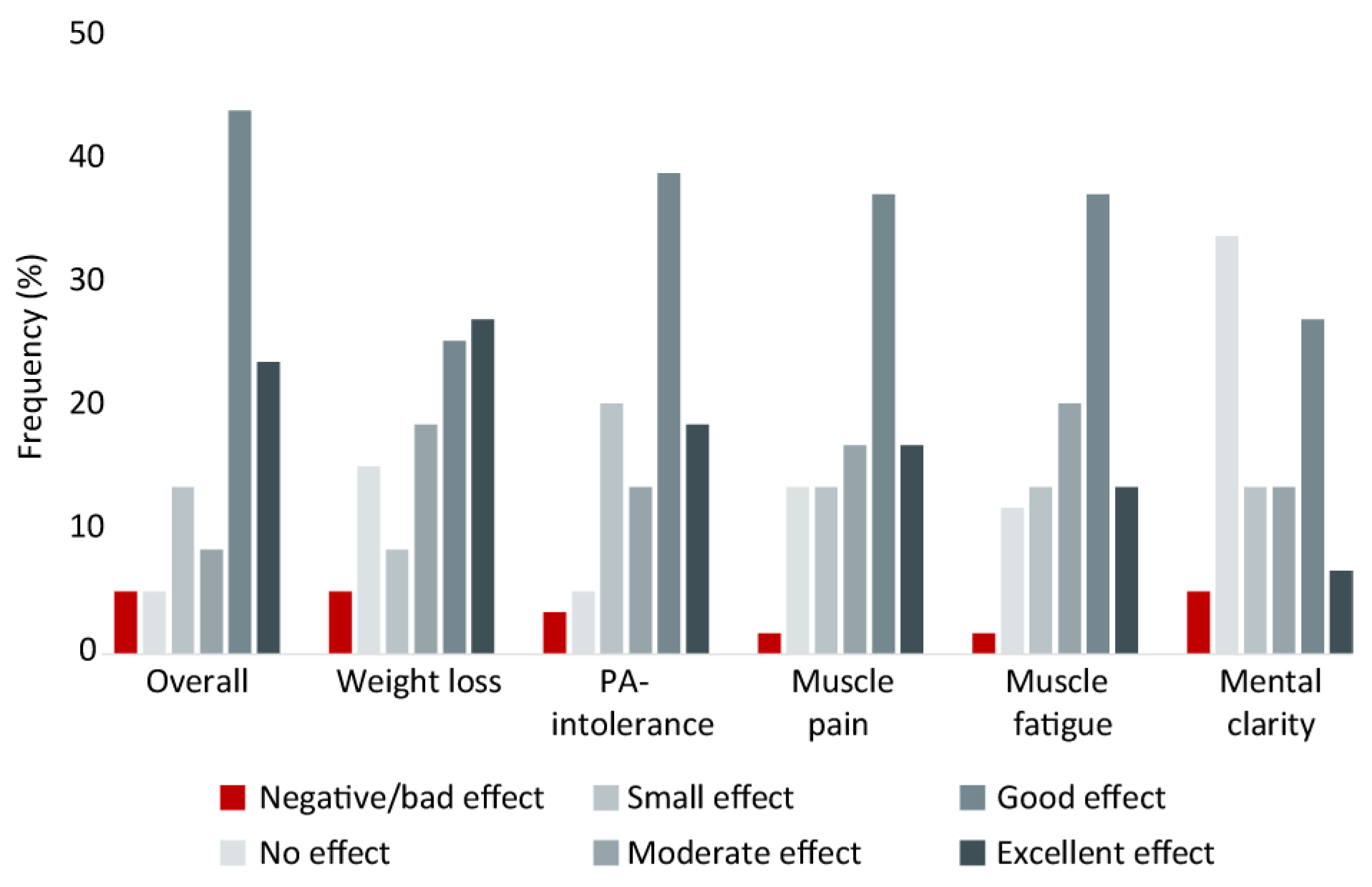
3.2.1. Patient-Reported LCKD Effects
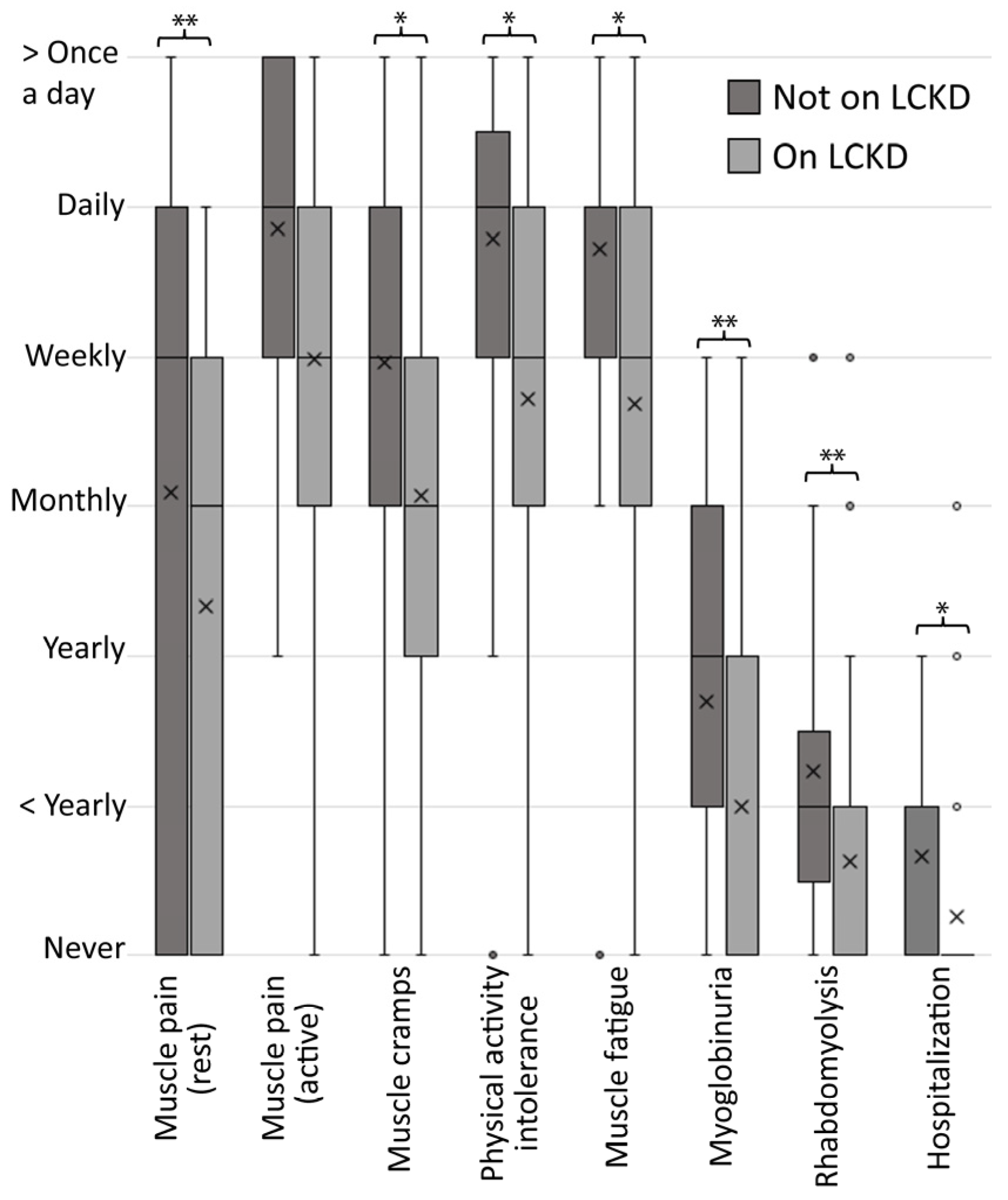
3.2.2. Consults and Self-Reported Objective Findings
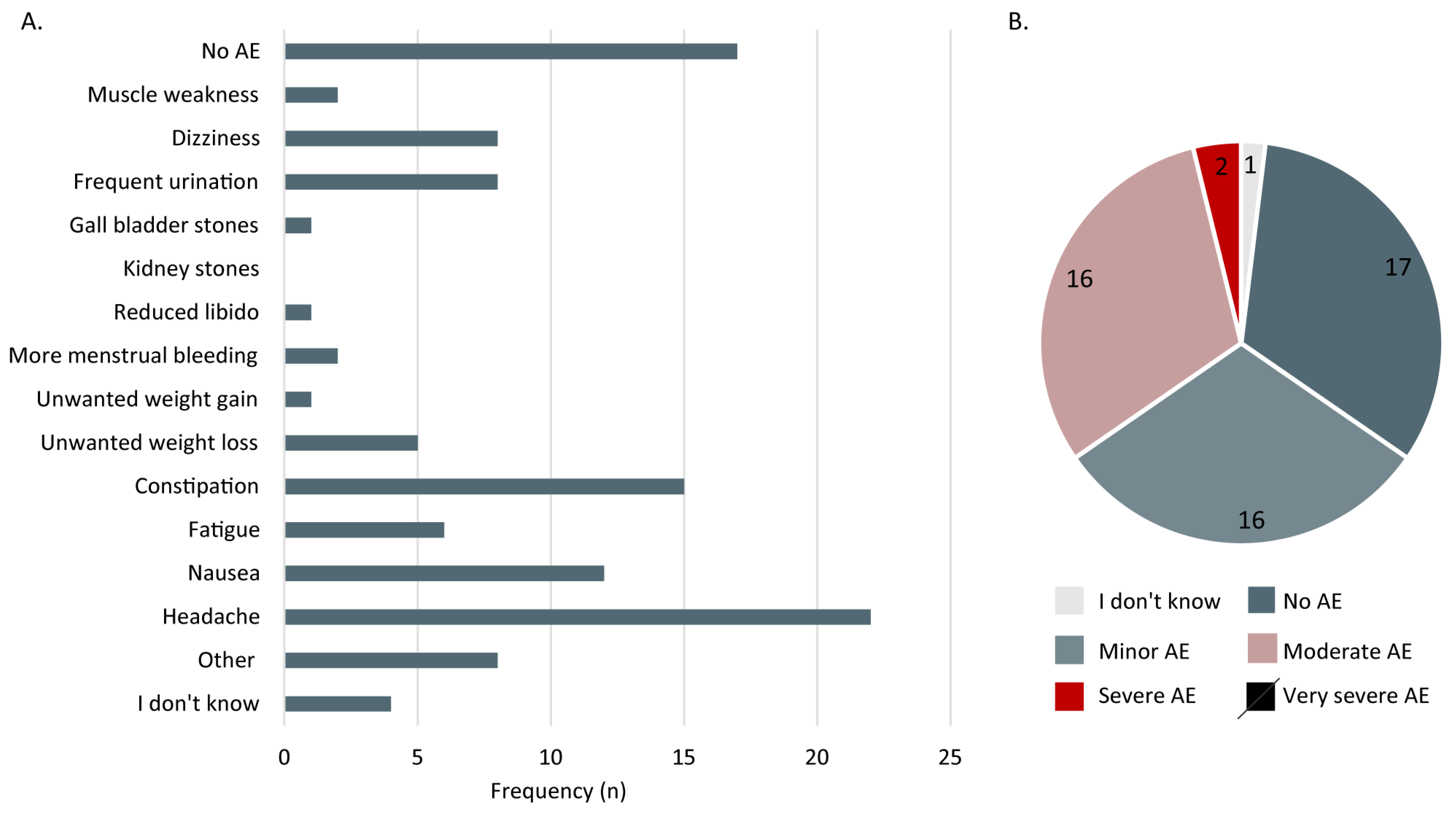
3.2.3. Adverse Effects
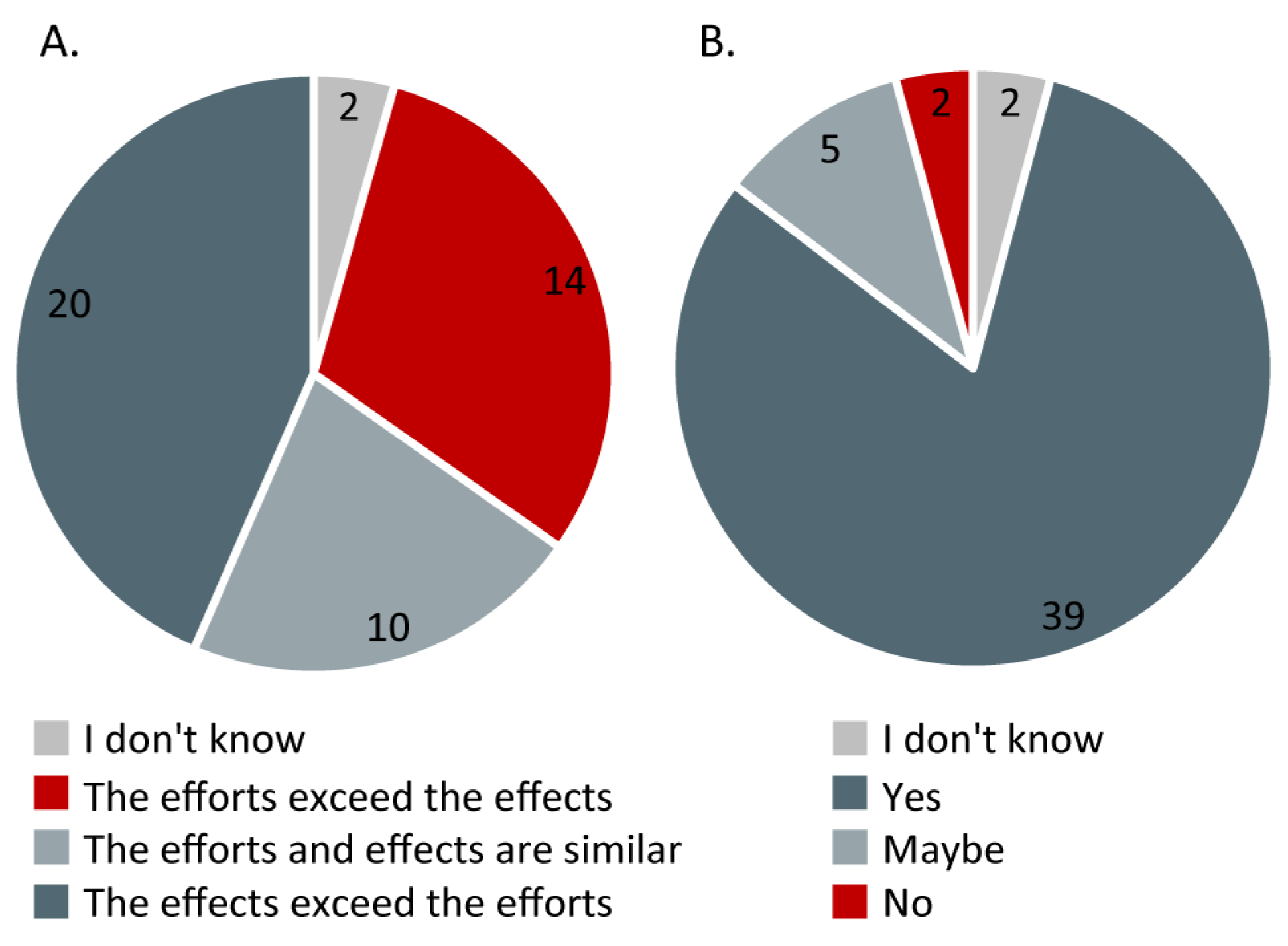
3.2.4. Effects vs. Efforts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busch, V.; Gempel, K.; Hack, A.; Müller, K.; Vorgerd, M.; Lochmüller, H.; Baumeister, F.A.M. Treatment of Glycogenosis Type V with Ketogenic Diet. Ann. Neurol. 2005, 58, 341. [Google Scholar] [CrossRef] [PubMed]
- Reason, S.L.; Westman, E.C.; Godfrey, R.; Magulre, E. Can a Low-Carbohydrate Diet Improve Exercise Tolerance in Mcardle Disease? J. Rare Disord. Diagn. Ther. 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Løkken, N.; Hansen, K.K.; Storgaard, J.H.; Ørngreen, M.C.; Quinlivan, R.; Vissing, J. Titrating a Modified Ketogenic Diet for Patients with McArdle Disease: A Pilot Study. J. Inherit. Metab. Dis. 2020, 43, 778–786. [Google Scholar] [CrossRef] [PubMed]
- IAMGSD. Research. IAMGSD Reports. Available online: https://www.iamgsd.org/iamgsd-research-papers (accessed on 7 December 2022).
- Miranda, M.J.; Turner, Z.; Magrath, G. Alternative Diets to the Classical Ketogenic Diet—Can We Be More Liberal? Epilepsy Res. 2012, 100, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Reason, S.L.; Løkken, N.; Voermans, N. International Patient Group Harnesses Social Media to Help Inform Rare Disease Research: Use of a Low Carbohydrate Ketogenic Diet in McArdle Disease. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Martinuzzi, A.; Nogales-Gadea, G.; Quinlivan, R.; Reason, S.; Bali, D.; Godfrey, R.; Haller, R.; Kishnani, P.; Laforêt, P.; et al. Clinical Practice Guidelines for Glycogen Storage Disease V & VII (McArdle Disease and Tarui Disease) from an International Study Group. Neuromuscul. Disord. 2021, 31, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, R.; Buckley, J.; James, M.; Twist, A.; Ball, S.; Duno, M.; Vissing, J.; Bruno, C.; Cassandrini, D.; Roberts, M.; et al. McArdle Disease: A Clinical Review. J. Neurol. Neurosurg. Psychiatry Lond. 2010, 81, 1182. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; Lucia, A. Perspective: Ketone Supplementation in Sports—Does It Work? Adv. Nutr. 2021, 12, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Løkken, N.; Storgaard, J.H.; Revsbech, K.L.; Voermans, N.C.; Van Hall, G.; Vissing, J.; Ørngreen, M.C. No Effect of Oral Ketone Ester Supplementation on Exercise Capacity in Patients with McArdle Disease and Healthy Controls: A Randomized Placebo-Controlled Cross-over Study. J. Inherit. Metab. Dis. 2022, 45, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Mitchell, G.; Sass, J.O.; Hori, T.; Orii, K.; Aoyama, Y. Ketone Body Metabolism and Its Defects. J. Inherit. Metab. Dis. 2014, 37, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Martinuzzi, A.; Sartori, E.; Fanin, M.; Nascimbeni, A.; Valente, L.; Angelini, C.; Siciliano, G.; Mongini, T.; Tonin, P.; Tomelleri, G.; et al. Phenotype Modulators in Myophosphorylase Deficiency. Ann. Neurol. 2003, 53, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Downloadable Questionnaires—International Physical Activity Questionnaire. Available online: https://sites.google.com/site/theipaq/questionnaire_links (accessed on 7 November 2022).
- A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 7 December 2022).
- Stierman, B.; Mishra, S. Special Diets Among Adults: United States, 2015–2018. NCHS Data Brief 2020, 8, 1–8. [Google Scholar]
- Løkken, N. Odified Ketogenic Diet in Patients with McArdle Disease Part B—A Placebo-Controlled, Cross-Over Study. 2019. Available online: clinicaltrials.gov (accessed on 7 December 2022).
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Santalla, A.; Nogales-Gadea, G.; Encinar, A.B.; Vieitez, I.; González-Quintana, A.; Serrano-Lorenzo, P.; Consuegra, I.G.; Asensio, S.; Ballester-Lopez, A.; Pintos-Morell, G.; et al. Genotypic and Phenotypic Features of All Spanish Patients with McArdle Disease: A 2016 Update. BMC Genom. 2017, 18, 819. [Google Scholar] [CrossRef] [PubMed]
- Scalco, R.S.; Lucia, A.; Santalla, A.; Martinuzzi, A.; Vavla, M.; Reni, G.; Toscano, A.; Musumeci, O.; Voermans, N.C.; Kouwenberg, C.V.; et al. Data from the European Registry for Patients with McArdle Disease and Other Muscle Glycogenoses (EUROMAC). Orphanet J. Rare Dis. 2020, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Crutzen, R.; Göritz, A.S. Does Social Desirability Compromise Self-Reports of Physical Activity in Web-Based Research? Int. J. Behav. Nutr. Phys. Act. 2011, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; McAuley, E.; DiStefano, C. Is Social Desirability Associated with Self-Reported Physical Activity? Prev. Med. 2005, 40, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, G.; Chen, J.; Chen, Z.; Xu, F.; Zhu, F.; Zhang, J.; Yuan, S. The Effect of Periodic Ketogenic Diet on Newly Diagnosed Overweight or Obese Patients with Type 2 Diabetes. BMC Endocr. Disord. 2022, 22, 34. [Google Scholar] [CrossRef] [PubMed]


| Overall Sample n = 183 | Subgroup: No LKCD n = 123 (67.2%) | Subgroup: LCKD n = 60 (32.8%) | |
|---|---|---|---|
| Age, years (median (IQR)) | n = 178 51 (37–63) | n = 123 51 (37–65) | n = 57 52 (38–60) |
| Sex (n (%)) | n = 183 | n = 123 | n = 60 |
| Female | 108 (59.0%) | 73 (59.3%) | 35 (58.3%) |
| Male | 74 (40.4%) | 50 (40.7%) | 24 (40.0%) |
| Other | 1 (0.5%) | 1 (1.7%) | |
| BMI, kg/m2 (median (IQR)) | n = 181 25.9 (22.8–30.2) | n = 121 25.5 (22.2–30.1) | n = 60 27.0 (23.2–30.6) |
| McArdle symptom score (median (range; IQR)) | n = 183 18.0 (7–25; 15–20) | n = 123 18.0 (7–25; 15–20) | n = 60 18.0 (9–25; 15–20) |
| Country (n (%subgroup)(%country)) | n = 183 | n = 123 | n = 60 |
| DK + FO + S | 14 (7.7%) | 3 (2.4%) (21.4%) | 11 (18.3%) (78.6%) |
| ES | 33 (18.0%) | 32 (26.0%) (97.0%) | 1 (1.7%) (3.0%) |
| NL | 28 (15.3%) | 23 (18.7%) (82.1%) | 5 (8.3%) (17.9%) |
| USA + CAN | 47 (25.7%) | 24 (19.5%) (51.1%) | 23 (38.3%) (48.9%) |
| UK + IRL | 30 (16.3%) | 24 (19.5%) (80.0%) | 6 (10.0%) (20.0%) |
| AUS + NZ | 14 (7.7%) | 8 (6.5%) (57.1%) | 6 (10.0%) (42.9%) |
| Other countries | 17 (9.3%) | 9 (7.3%) (52.9%) | 8 (13.3%) (47.1%) |
| IPAQ-SF, MET-min/week (median (ICR)) | n = 168 2560 (1188–4617) | n = 111 2880 (1356–5295) | n = 57 2079 (922–3795) |
| Comorbidities (n (%)) | n = 183 | n = 123 | n = 60 |
| Anxiety/depression | 74 (40.4%) | 49 (39.8%) | 25 (41.7%) |
| Chronic pain | 44 (24.0%) | 29 (23.6%) | 15 (25.0%) |
| Rheumatoid arthritis | 7 (3.8%) | 5 (4.1%) | 2 (3.3%) |
| Osteoarthritis | 12 (6.6%) | 4 (3.3%) | 8 (13.3%) |
| Chronic lung disease | 15 (8.2%) | 8 (6.5%) | 7 (11.7%) |
| Severe heart disease | 14 (7.7%) | 7 (5.7%) | 7 (11.7%) |
| Hypertension | 30 (16.4%) | 12 (9.8%) | 18 (30%) |
| Hypercholesterolemia | 29 (15.8%) | 14 (11.4%) | 15 (25.0%) |
| Diabetes | 20 (10.9%) | 18 (14.6%) | 2 (3.3%) |
| Liver/gut-disease | 7 (3.8%) | 4 (3.3%) | 3 (5.0%) |
| Cancer or cancer related | 6 (3.3%) | 5 (4.1%) | 1 (1.7%) |
| Other | 26 (14.2%) | 9 (7.3% | 11 (18.3%) |
| None | 29 (15.8%) | 17 (13.8%) | 12 (20%) |
| ‘I don’t know’ | 18 (9.8%) | 17 (13.8%) | 1 (1.7%) |
| #1 | “Importantly, there are still times when I need to get into second wind, even on the ketogenic diet during exercise. But getting into second wind feels faster and smoother while following a ketogenic diet and I usually don’t have to stop and rest prior to getting into second wind (but I may slow down, for example, prior to getting into second wind). But in terms of activities of daily living, the ketogenic diet makes all my symptoms of McArdle’s go away—things like walking across a parking lot, drying my hair with a blow dryer, lifting things, etc. All of those things become easy and normal. But when actually doing exercise, such as quick walking, biking or walking uphill, I still need to get into second wind. But being on the ketogenic diet makes this easier and less painful.” |
| #2 | “I have been and continue to be a strong advocate of keto/low carb for McArdle disease—it allows us to do things we thought not possible. I never dreamed I would be able to jog for 50 min non-stop, yet in the past when in ketosis I have done so…” |
| #3 | “Some of my improvement in symptoms was due to weight loss, 14.5 Kg. Being diagnosed at age 76 my normal diet was deeply ingrained and although I felt better in ketosis, I preferred to keep eating what I always had.” |
| #4 | “A LCKD is very helpful in reducing pre-second wind symptoms thereby making ADLs much easier. I am also able to do more aerobically—push the anaerobic threshold further. It is not always easy to maintain a LCKD, but the effects are well worth it.” |
| #5 | “I was on a 3-month period of Keto diet. I went on a family vacation and was amazed at my ability to walk as much as 15 K steps without pain or cramping.” |
| #6 | “Hard to maintain compliance without putting a lot of effort into shopping and meal preparation.” |
| #7 | “It would be so much easier to stay on a ketogenic diet if the resources were cheaper and more readily available. You really have to enjoy cooking from scratch on a keto diet—and sometimes it would just be nice to have crunchy food.” |
| #8 | “Not sure if the small improvement effects I felt when I was on ketonic diet were real or due to placebo effect.” |
| #9 | “Keto diet is expensive to maintain but worth the effort to me. Reduced daily pain, cramping, and quicker recovery from episodes of cramping and fatigue.” |
| #10 | “I found for me, I couldn’t do it permanently as it was too restricted and I was getting sick of the same foods.” |
| #11 | “The efforts exceeded the effects by a small margin. I definitely saw a solid reduction in McArdle symptoms. The most apparent being that I did not feel as if I needed to stop and rest before the second wind came on. I would say that it smoothed out the curve. I would still get a little tired before the second wind came on but the transition was more subtle, and I could last longer doing an activity.” |
| #12 | “Diet helped very much, I benefited very much. Only maintaining it without the support of a dietician is very difficult. It requires a lot of discipline. Also, a lot of foods contain carbohydrates. The same goes for vegetables. It’s really hard to find what you can and cannot eat.” (Translated from Dutch) |
| #13 | “I notice a very big difference in daily things and with training. However, activities that take a lot of strength are still too heavy. So it helps me a lot with aerobic efforts but anaerobic efforts remain heavier” (Translated from Dutch) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Løkken, N.; Voermans, N.C.; Andersen, L.K.; Karazi, W.; Reason, S.L.; Zweers, H.; Wilms, G.; Santalla, A.; Susanibar, E.; Lucia, A.; et al. Patient-Reported Experiences with a Low-Carbohydrate Ketogenic Diet: An International Survey in Patients with McArdle Disease. Nutrients 2023, 15, 843. https://doi.org/10.3390/nu15040843
Løkken N, Voermans NC, Andersen LK, Karazi W, Reason SL, Zweers H, Wilms G, Santalla A, Susanibar E, Lucia A, et al. Patient-Reported Experiences with a Low-Carbohydrate Ketogenic Diet: An International Survey in Patients with McArdle Disease. Nutrients. 2023; 15(4):843. https://doi.org/10.3390/nu15040843
Chicago/Turabian StyleLøkken, Nicoline, Nicol C. Voermans, Linda K. Andersen, Walaa Karazi, Stacey L. Reason, Heidi Zweers, Gustav Wilms, Alfredo Santalla, Edward Susanibar, Alejandro Lucia, and et al. 2023. "Patient-Reported Experiences with a Low-Carbohydrate Ketogenic Diet: An International Survey in Patients with McArdle Disease" Nutrients 15, no. 4: 843. https://doi.org/10.3390/nu15040843
APA StyleLøkken, N., Voermans, N. C., Andersen, L. K., Karazi, W., Reason, S. L., Zweers, H., Wilms, G., Santalla, A., Susanibar, E., Lucia, A., & Vissing, J. (2023). Patient-Reported Experiences with a Low-Carbohydrate Ketogenic Diet: An International Survey in Patients with McArdle Disease. Nutrients, 15(4), 843. https://doi.org/10.3390/nu15040843








