Beneficial Effect of Increased Tryptophan Intake on Its Metabolism and Mental State of the Elderly
Abstract
:1. Introduction
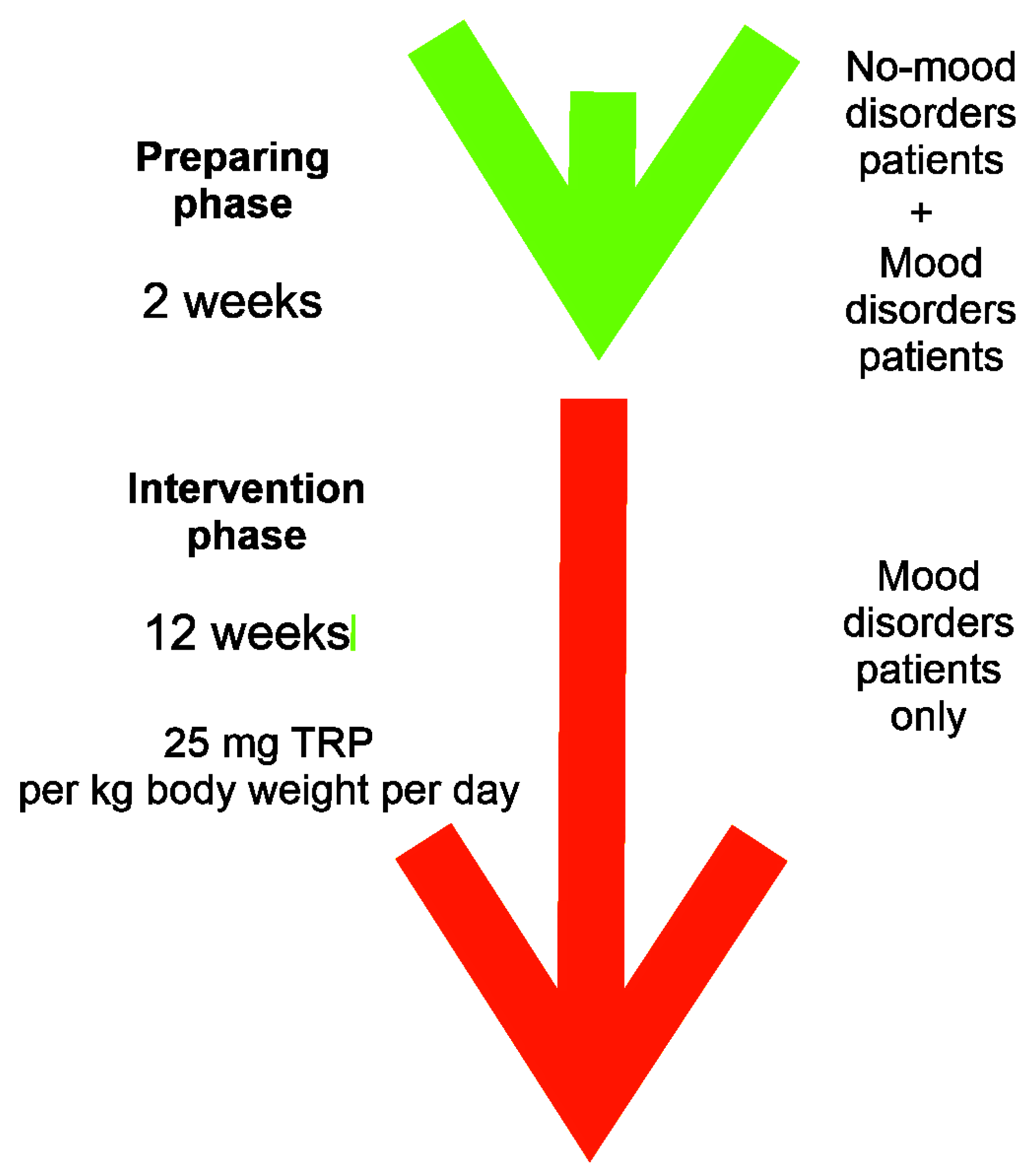
2. Materials and Methods
2.1. Patients
2.2. Laboratory Tests
2.3. Dietary Intervention
2.4. Ethical Issues
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hopkins, F.G.; Cole, S.W. A contribution to the chemistry of proteins: Part I. A preliminary study of the hitherto undescribed products of tryptic digestion. J. Physiol. 1901, 27, 418–428. [Google Scholar] [CrossRef]
- Sainio, E.L.; Pulkki, K.; Young, S.N. Biochemical, nutritional and pharmacological agents. Amino Acids 1996, 10, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. The uniqueness of tryptophan in biology Properties, metabolism, interactions and localization in protein. Int. J. Mol. Sci. 2020, 21, 8776. [Google Scholar] [CrossRef]
- Tanaka, M.; Toth, f.; Polyak, H.; Mandi, Y.; Vecsel, L. Immune influencers in action: Metabolites and enzymes of tryptophan-kynurenine metabolic pathway. Preprints 2021, 2021, 2021080344. [Google Scholar] [CrossRef]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Transmission in Gastrointestinal Tract. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD(+) metabolism and its roles in cellular processes during ageing. Nature reviews. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Jones, S.P.; Guillemin, G.J.; Brew, B.J. The kynurenine pathway in stem cell biology. Int. J. Tryptophan Res. 2013, 6, 57–66. [Google Scholar] [CrossRef]
- Boros, F.A.; Bohár, Z.; Vécsei, L. Genetic alterations affecting the genes encoding the enzymes of the kynurenine pathway and their association with human diseases. Mutat. Res. 2018, 776, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Benson, J.M.; Shepherd, D.M. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol. Sci. 2011, 120, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J. Beneficial actions of microbiota-derived tryptophan metabolites. Neurogastroenterol. Motil. 2018, 30, e13283. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc. Natl. Acad. Sci. USA 1996, 93, 12553–12558. [Google Scholar] [CrossRef]
- Schwarcz, R.; Pellicciari, R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002, 303, 1–10. [Google Scholar] [CrossRef]
- Depression. 2020. Available online: http://who.int/news-room/fact-sheets/detail/depression (accessed on 29 December 2022).
- Alexopoulos, G.S. Depression in the elderly. Lancet 2005, 365, 1961–1970. [Google Scholar] [CrossRef]
- Arteaga-Henriquez, G.; Burger, B.; Weidinger, E.; Grosse, L.; Moll, N.; Schuetze, G.; Schwarz, M.; Wijkhuijs, A.; Op de Beeck, G.; Berghmans, R.; et al. Activation and deactivation steps in the tryptophan breakdown pathway in major depressive disorder: A link to the monocyte inflammatory state of patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110226. [Google Scholar] [CrossRef]
- Johannsen, D.L.; Ravussin, E. Obesity in the elderly: Is faulty metabolism to blame? Aging Health 2010, 6, 159–167. [Google Scholar] [CrossRef]
- Oxenkrug, G. Serotonin-Kynurenine Hypothesis of Depression: Historical Overview and Recent Developments. Curr. Drug Targets 2013, 14, 514–521. [Google Scholar] [CrossRef]
- Colle, R.; Masson, P.; Verstuyft, C.; Feve, B.; Werner, E.; Boursier-Neyret, C.; Walther, B.; David, D.J.; Boniface, B.; Falissard, B.; et al. Peripheral tryptophan, serotonin, kynurenine, and their metabolites in major depression: A case control study. Psychiatry Clin. Neurosci. 2020, 74, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A Link between Stress and Depression: Shifts in the Balance between the Kynurenine and Serotonin Pathways of Tryptophan Metabolism and the Etiology and Pathophysiology of Depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Young, G.S.N. The effect of raising and lowering tryptophan levels on human mood and social behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110375. [Google Scholar] [CrossRef]
- Hiratsuka, C.; Fukuwatari, T.; Sano, M.; Saito, K.; Sasaki, S.; Shibata, K. Supplementing Healthy Women with up to 5.0 g/d of L-Tryptophan Has No Adverse Effects. J. Nutr. 2013, 143, 859–866. [Google Scholar] [CrossRef]
- Chojnacki, C.; Popławski, T.; Chojnacki, J.; Fila, M.; Konrad, P.; Blasiak, J. Tryptophan Intake and Metabolism in Older Adults with Mood Disorders. Nutrients 2020, 12, 3183. [Google Scholar] [CrossRef]
- Carrozzino, D.; Patierno, C.; Fava, G.; Guidi, J. The Hamilton Rating Scale for Depression: A Critical Review of Clinimetric Properties of Different Versions. Psychother. Psychosom. 2020, 89, 133–150. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivrs, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 608. [Google Scholar] [CrossRef]
- Bell, C. Tryptophan depletion and its implications for psychiatry. Br. J. Psychiatry 2001, 178, 399–405. [Google Scholar] [CrossRef]
- Ogawa, S.; Fujii, T.; Koga, N.; Hori, H.; Teraishi, T.; Hattori, K.; Noda, T.; Higuchi, T.; Nobutaka, M.; Kunugi, H. Plasma L-tryptophan concentration in major depressive disorder: New data and meta-analysis. J. Clin. Psychiatry 2014, 75, e906–e915. [Google Scholar] [CrossRef]
- Bär, K.J.; Köhler, S.; Schumann, A.; Zepf, F.D.; Wagner, G. Functional consequences of acute tryptophan depletion on raphe nuclei connectivity and network organization in healthy. Neuroimage 2019, 207, 116362. [Google Scholar] [CrossRef]
- Young, S.N.; Smith, S.C.; Phil, R.O.; Ervin, F.R. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology 1985, 87, 173–177. [Google Scholar] [CrossRef]
- Young, S.N. Acute tryptophan depletion in humans: A review of theoretical, practical and ethical aspects. J. Psych. Neurosci. 2013, 38, 294–305. [Google Scholar] [CrossRef]
- Van der Does, A.J.W. The effects of tryptophan depletion on mood and psychiatric symptoms. J. Affect. Disord. 2001, 64, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Toker, L.; Amar, S.; Bersudsky, Y.; Benjamin, J.; Klein, E. The biology of tryptophan depletion and mood disorders. Isr. J. Psychiatry 2010, 47, 46–55. [Google Scholar]
- Booji, L.; Van der Does, A.J.W.; Haffmans, P.M.J.; Riedel, W.J.; Fekkes, D.; Blom, M.J.B. The effects of high-dose and low-dose tryptophan depletion on mood and cognitive functions of remitted depressed patients. J. Psychopharmacol. 2005, 19, 267–275. [Google Scholar] [CrossRef]
- Murphy, S.E.; Longhitano, C.; Ayres, R.E.; Cowen, P.J.; Harmer, C.J. Tryptophan supplementation induces a positive bias in the processing of emotional material in healthy female volunteers. Psychopharmacology 2006, 187, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodriguez, A.B.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly. Age 2013, 35, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Soh, N.L.; Walter, G. Tryptophan and depression: Can diet alone be the answer? Acta Neuropsychiatr. 2011, 23, 3–11. [Google Scholar] [CrossRef]
- Reuter, M.; Zamoscik, V.; Plieger, T.; Bravo, T.; Ugertemendia, L.; Rodriguez, A.B.; Kirsch, P. Tryptophan-rich diet is negatively associated with deprwssion and positively linked to social cognition. Nutr. Res. 2021, 85, 14–20. [Google Scholar] [CrossRef]
- Fernstrom, J.D. A Perspective on the Safety of Supplemental Tryptophan Based on Its Metabolic Fates. J. Nutr. 2016, 146, S2601–S2608. [Google Scholar] [CrossRef]
- Carneiro, I.B.C.; Toscano, A.E.; Lacerda, D.C.; da Cunha, M.S.B.; de Castro, R.M.; Deiro, R.M.; Medeiros, J.M.B. L-tryptophan administration and increase in cerebral serotonin levels: Systemic review. Eur. J. Pharmacol. 2018, 836, 129–135. [Google Scholar] [CrossRef]
- Markus, C.R.; Firk, C.; Gerhardt, C.; Kloek, J.; Smolders, G.F. Effect of different tryptophan sources on amino acids availability to the brain and mood in healthy volunteers. Psychopharmacology 2008, 201, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Effects and side effects associated with the non-nutritional use of tryptophan by humans. J. Nutr. 2012, 142, 2236–2244. [Google Scholar] [CrossRef]
- Hiratsuka, C.; Sano, M.; Fukuwatari, T.; Shibata, K. Time-Dependent Effects of L-Tryptophan Administration on Urinary Excretion of L-Tryptophan Metabolites. J. Nutr. Sci. Vitaminol. 2014, 60, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Konrad, P.; Błońska, A.; Mędrek-Socha, M.; Przybyłowska-Sygut, K.; Chojnacki, J.; Popławski, T. Altered Tryptophan Metabolism on the Kynurenine Pathway in Depressive Patients with Small Intestinal Bacterial Overgrowth. Nutrients 2022, 14, 3217. [Google Scholar] [CrossRef]
- Kumar, A.M.; Weiss, S.; Fernandez, J.B.; Cruess, D.; Eisdorfer, C. Peripheral serotonin levels in women: Role of aging and ethnicity. Gastroenterology 1998, 44, 211–216. [Google Scholar] [CrossRef]
- Frick, B.; Schroecksnadel, K.; Neurauter, G.; Leblhuber, F.; Fuchs, D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin. Biochem. 2004, 37, 684–687. [Google Scholar] [CrossRef]
- Devanand, D.P. Dysthymic disorder in the elderly population. Int. Psychogeriatr. 1998, 18, 407–430. [Google Scholar] [CrossRef]
- Alexopoulos, G.S. Mechanism and treatment of late-life depression. Transl. Psychiatry 2019, 9, 188. [Google Scholar] [CrossRef]
- Granero, R. Role of Nutrition and Diet on Healthy Mental State. Nutrients 2022, 14, 750. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, E.J.; Kim, A.; Lee, H.J.; Choi, H.J.; Yang, S.J. Nutritional Factors Affecting Mental Health. Clin. Nutr. Res. 2016, 5, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbottle, L. The effect of nutrition on older people’s mental health. Br. J. Community Nurs. 2020, 24, S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Wetterling, T. Feeding disability in elder psychiatric patients. Psychiatr. Prax. 2015, 42, 42–46. [Google Scholar] [CrossRef] [PubMed]
- German, L.; Kahana, C.; Rosenfeld, V.; Zabrowsky, I.; Wiezer, Z.; Fraser, D.; Shahar, D.R. Depressive symptoms are associated with food insufficiency and nutritional deficiencies in poor community—Dewelling elderly people. J. Nutr. Health Aging 2011, 15, 3–8. [Google Scholar] [CrossRef]
- Opie, R.S.; Itsiopoulos, C.; Parletta, N.; Sanchez-Villegas, A.; Akbaraly, T.N.; Ruusune, A.; Jacka, F.N. Dietary recommendations for the prevention of depression. Nutr. Neurosci. 2017, 20, 161–171. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Agarval, S.; Fulgoni, V.L. Tryptophan Intake in the US Adult Population Is Not Related to liver or Kidney Function but Is Associated with Depression and Sleep Outcomes. J. Nutr. 2016, 146, 26095–26155. [Google Scholar] [CrossRef]
- Chirico, M.; Custer, J.; Shoyombo, H.; Cooper, C.; Meldeum, S.; Fantzer, R.; Trivedi, M.H.; Rathouz, P.; Toups, M.S. Kynurenine pathway metabolites selectively associate with impaired associative memory function in depression. Brain Behav. Immun.-Health 2020, 8, 100126. [Google Scholar] [CrossRef]
- Savitz, J.; Drevets, W.C.; Wurfel, B.E.; Ford, B.N.; Bellgowan, P.S.F.; Victor, T.A.; Bodurka, J.; Teague, T.J.; Dantzer, R. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav. Immun. 2015, 46, 55–59. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Mai, N.; Wen, Y.; Shang, D.; Hu, L.; Chen, B.; Zhang, B.; Ning, Y. Kynurenine pathway in late-life depression with memory deficit. Psychiatry Res. 2018, 269, 45–49. [Google Scholar] [CrossRef]



| Feature a | No Mood Disorders | Mood Disorders |
|---|---|---|
| Age (years) | 74.9 ± 7.3 | 76.2 ± 8.6 |
| Gender (M/F) | 18/22 | 14/26 |
| BMI (kg/m2) | 23.8 ± 1.9 | 22.4 ± 2.3 |
| CRP (mg/L) | 1.83 ± 0.33 | 3.64 ± 2.83 |
| AST (U/L) | 16.4 ± 3.7 | 19.1 ± 6.2 |
| ALT (U/L) | 16.1 ± 5.2 | 21.2 ± 8.9 |
| TSH (µIU/mL/L) | 2.07 ± 0.92 | 1.68 ± 1.22 |
| HbA1c (mmol/mol) | 36.3 ± 3.4 | 1.68 ± 1.22 |
| Creatinine (mg/dL) | 0.75 ± 0.23 | 0.86 ± 0.21 |
| GFR (mL/min) | 98.6 ± 10.7 | 89.1 ± 10.3 |
| ISI score | 9.6 ± 1.19 | 19.7 ± 6.2 *** |
| HAM-D score | 7.01 ± 1.12 | 20.8 ± 4.26 *** |
| TRP (mg daily) | 1256 ± 193 | 806 ± 174 *** |
| TRP (mg/body weight daily) | 21.2 ± 4.23 | 12.5 ± 3.96 *** |
| Pair of Variables a | Spearman’s ρ | p |
|---|---|---|
| HAM-D and TRP | −0.574738 | <0.001 |
| HAM-D and 5-HIAA | −0.450369 | <0.001 |
| HAM-D and KYN | 0.537382 | <0.001 |
| HAM-D and KYNA | −0.229663 | <0.05 |
| HAM-D and QA | 0.739648 | <0.001 |
| HAM-D and KYNA/KYN | −0.484408 | <0.001 |
| HAM-D and KYNA/QA | −0.540872 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnacki, C.; Gąsiorowska, A.; Popławski, T.; Konrad, P.; Chojnacki, M.; Fila, M.; Blasiak, J. Beneficial Effect of Increased Tryptophan Intake on Its Metabolism and Mental State of the Elderly. Nutrients 2023, 15, 847. https://doi.org/10.3390/nu15040847
Chojnacki C, Gąsiorowska A, Popławski T, Konrad P, Chojnacki M, Fila M, Blasiak J. Beneficial Effect of Increased Tryptophan Intake on Its Metabolism and Mental State of the Elderly. Nutrients. 2023; 15(4):847. https://doi.org/10.3390/nu15040847
Chicago/Turabian StyleChojnacki, Cezary, Anita Gąsiorowska, Tomasz Popławski, Paulina Konrad, Marcin Chojnacki, Michal Fila, and Janusz Blasiak. 2023. "Beneficial Effect of Increased Tryptophan Intake on Its Metabolism and Mental State of the Elderly" Nutrients 15, no. 4: 847. https://doi.org/10.3390/nu15040847





