Post Hoc Analysis of a Randomized Controlled Trial on Fasting and Plant-Based Diet in Rheumatoid Arthritis (NutriFast): Nutritional Supply and Impact on Dietary Behavior
Abstract
:1. Introduction
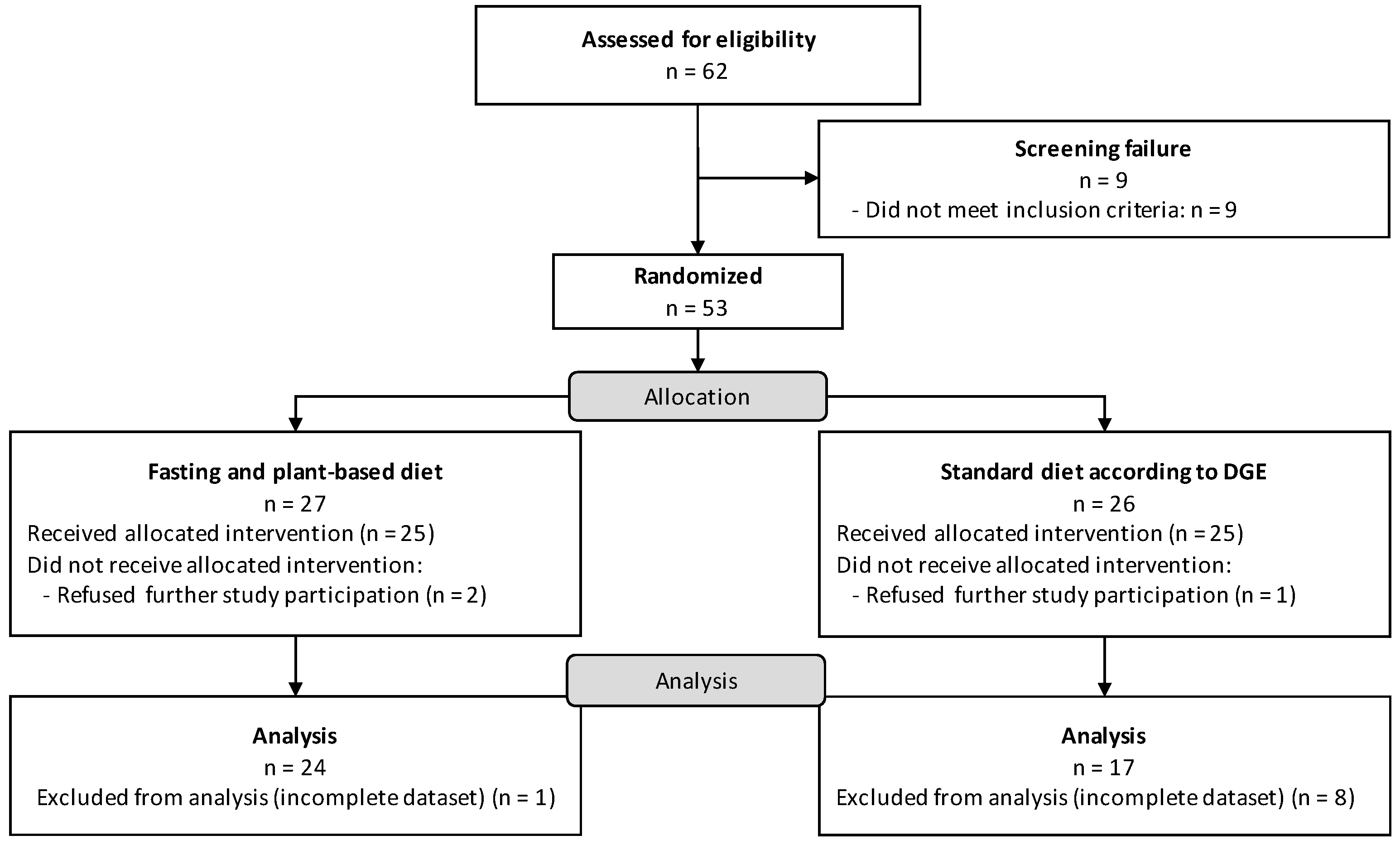
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Intervention
2.3. Study Variables and Instruments
2.4. Statistical Analysis
2.5. Cluster Analysis
3. Results
3.1. Nutrient Supply
| Nutrients | Overall Mean ± SD (n = 41) | Fasting + PBD Mean ± SD (n = 24) | DGE Mean ± SD (n = 17) | Reference Values | p-Value |
|---|---|---|---|---|---|
| Energy intake, kcal/day | 1909.2 ± 744.3 | 1762.7 ± 690.8 | 2115.9 ± 788.4 | 2100.0 | 0.17 |
| Carbohydrates, g/day | 222.2 ± 132.4 | 197.8 ± 93.9 | 256.8 ± 170.3 | 256.1 | 0.30 |
| Sugar, g/day | 72.6 ± 54.8 | 75.1 ± 64.4 | 68.1 ± 32.3 | 61.0 | 0.89 |
| Total fat, g/day | 77.1 ± 29.3 | 76.6 ± 33.8 | 77.8 ± 22.4 | 69.2 | 0.43 |
| Saturated | 20.4 ± 9.5 | 17.3 ± 8.5 | 24.7 ± 9.5 | 16.2–23.1 | 0.02 |
| Monounsaturated | 20.0 ± 13.0 | 21.5 ± 15.5 | 17.5 ± 5.8 | 34.6–46.2 | 0.94 |
| Polyunsaturated | 17.6 ± 9.3 | 19.0 ± 9.4 | 15.6 ± 9.1 | 18.5–27.7 | 0.23 |
| Protein, g/day | 59.4 ± 24.6 | 50.9 ± 22.4 | 71.5 ± 23.0 | 48.0 | 0.003 |
| Dietary fiber, g/day | 31.5 ± 13.6 | 31.3 ± 13.9 | 31.7 ± 13.4 | 35.1 | 0.77 |
| Vitamin E, mg/day | 13.1 ± 7.0 | 14.3 ± 7.8 | 11.4 ± 5.4 | 14.0 | 0.27 |
| Vitamin C, mg/day | 129.8 ± 75.8 | 130.5 ± 88.6 | 128.8 ± 55.6 | 95.0 | 0.58 |
| Vitamin D, µg/day | 3.2 ± 4.4 | 1.7 ± 1.4 | 5.9 ± 6.6 | 2.0–4.0 | 0.04 |
| Thiamine, mg/day | 0.8 ± 0.4 | 0.9 ± 0.4 | 0.8 ± 0.4 | 1.0 | 0.69 |
| Riboflavin, mg/day | 0.9 ± 0.5 | 0.8 ± 0.4 | 1.0 ± 0.6 | 1.1 | 0.18 |
| Vitamin B6, mg/day | 1.1 ± 0.5 | 1.0 ± 0.4 | 1.2 ± 0.6 | 1.4 | 0.49 |
| Biotin, µg/day | 23.6 ± 19.6 | 23.6 ± 21.2 | 23.5 ± 17.3 | 40.0 | 0.84 |
| Folate, µg/day | 214.0 ± 104.8 | 213.3 ± 90.7 | 215.4 ± 131.3 | 300.0 | 0.77 |
| Vitamin B12, µg/day | 1.5 ± 2.2 | 0.6 ± 0.8 | 3.0 ± 3.0 | 4.0 | 0.001 |
| Vitamin A, mg/day | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.7 | 0.02 |
| Vitamin K, µg/day | 68.3 ± 52.2 | 62.5 ± 40.3 | 79.0 ± 69.8 | 60.0 | 0.83 |
| Zinc, mg/day | 6.5 ± 4.2 | 6.5 ± 4.8 | 6.4 ± 3.1 | 8.0 | 0.69 |
| Magnesium, mg/day | 336.5 ± 194.0 | 354.3 ± 222.9 | 303.9 ± 127.5 | 300.0 | 0.80 |
| Copper, µg/day | 1630.5 ± 693.3 | 1631.9 ± 721.9 | 1628.0 ± 668.6 | 1000–1500 | 0.89 |
| Calcium, mg/day | 580.1 ± 336.4 | 513.1 ± 319.8 | 702.9 ± 344.6 | 1000.0 | 0.04 |
| Nutrients | Overall Mean ± SD (n = 41) | Fasting + PBD Mean ± SD (n = 24) | DGE Mean ± SD (n = 17) | Reference Values | p-Value |
|---|---|---|---|---|---|
| Energy intake, kcal/day | 1810.2 ± 585.0 | 1764.4 ± 492.0 | 1874.8 ± 707.1 | 2100.0 | 0.96 |
| Carbohydrates, g/day | 211.0 ± 99.6 | 202.4 ± 78.0 | 223.2 ± 125.6 | 256.1 | 0.79 |
| Sugar, g/day | 82.6 ± 52.0 | 85.6 ± 61.3 | 76.7 ± 25.9 | 61.0 | 0.71 |
| Total fat, g/day | 72.0 ± 24.9 | 73.3 ± 25.2 | 70.3 ± 25.0 | 69.2 | 0.94 |
| Saturated | 18.9 ± 9.5 | 15.9 ± 7.7 | 23.2 ± 10.3 | 16.2–23.1 | 0.02 |
| Monounsaturated | 19.4 ± 10.2 | 21.4 ± 11.8 | 15.5 ± 4.2 | 34.6–46.2 | 0.38 |
| Polyunsaturated | 17.0 ± 9.5 | 17.6 ± 9.4 | 16.1 ± 9.7 | 18.5–27.7 | 0.61 |
| Protein, g/day | 56.3 ± 19.8 | 52.7 ± 17.9 | 61.3 ± 21.7 | 48.0 | 0.34 |
| Dietary fiber, g/day | 31.5 ± 18.5 | 29.1 ± 10.2 | 35.0 ± 26.2 | 35.1 | 0.96 |
| Vitamin E, mg/day | 12.5 ± 6.8 | 13.8 ± 6.7 | 10.7 ± 6.6 | 14.0 | 0.05 |
| Vitamin C, mg/day | 159.1 ± 124.6 | 145.8 ± 96.8 | 117.8 ± 157.1 | 95.0 | 0.69 |
| Vitamin D, µg/day | 1.7 ± 2.0 | 1.1 ± 0.8 | 2.9 ± 3.1 | 2.0–4.0 | 0.01 |
| Thiamine, mg/day | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.3 | 1.0 | 0.55 |
| Riboflavin, mg/day | 0.9 ± 0.3 | 0.8 ± 0.3 | 1.0 ± 0.4 | 1.1 | 0.02 |
| Vitamin B6, mg/day | 1.1 ± 0.5 | 1.1 ± 0.4 | 1.2 ± 0.6 | 1.4 | 0.81 |
| Biotin, µg/day | 29.8 ± 34.7 | 32.5 ± 41.7 | 24.2 ± 12.2 | 40.0 | 0.69 |
| Folate, µg/day | 226.8 ± 143.9 | 231.2 ± 133.2 | 218.1 ± 169.1 | 300.0 | 0.16 |
| Vitamin B12, µg/day | 1.0 ± 1.3 | 0.4 ± 0.6 | 2.3 ± 1.5 | 4.0 | 0.00 |
| Vitamin A, mg/day | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.7 | 0.01 |
| Vitamin K, µg/day | 98.8 ± 109.1 | 108.9 ± 128.4 | 78.5 ± 52.0 | 60.0 | 0.84 |
| Zinc, mg/day | 5.9 ± 2.2 | 5.6 ± 1.8 | 6.4 ± 2.8 | 8.0 | 0.70 |
| Magnesium, mg/day | 320.6 ± 118.2 | 319.6 ± 95.5 | 322.7 ± 159.4 | 300.0 | 0.46 |
| Copper, µg/day | 1611.1 ± 531.8 | 1646.8 ± 434.6 | 1539.8 ± 704.6 | 1000–1500 | 0.24 |
| Calcium, mg/day | 569.8 ± 278.5 | 479.9 ± 187.0 | 731.6 ± 357.3 | 1000.0 | 0.02 |
3.2. Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D. Rheumatoid arthritis therapy reappraisal: Strategies, opportunities and challenges. Nat. Rev. Rheumatol. 2015, 11, 276–289. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Vadell, A.K.E.; Bärebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA)-a randomized, controlled crossover trial indicating effects on disease activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef]
- Haugen, M.; Kjeldsen-Kragh, J.; Nordvåg, B.Y.; Førre, O. Diet and disease symptoms in rheumatic diseases—Results of a questionnaire based survey. Clin. Rheumatol. 1991, 10, 401–407. [Google Scholar] [CrossRef]
- Tedeschi, S.K.; Frits, M.; Cui, J.; Zhang, Z.Z.; Mahmoud, T.; Iannaccone, C.; Lin, T.C.; Yoshida, K.; Weinblatt, M.E.; Shadick, N.A.; et al. Diet and Rheumatoid Arthritis Symptoms: Survey Results from a Rheumatoid Arthritis Registry. Arthritis Care Res. 2017, 69, 1920–1925. [Google Scholar] [CrossRef]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Sköldstam, L.; Larsson, L.; Lindström, F.D. Effect of fasting and lactovegetarian diet on rheumatoid arthritis. Scand. J. Rheumatol. 1979, 8, 249–255. [Google Scholar] [CrossRef]
- Udén, A.M.; Trang, L.; Venizelos, N.; Palmblad, J. Neutrophil functions and clinical performance after total fasting in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1983, 42, 45–51. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J.; Borchgrevink, C.F.; Laerum, E.; Haugen, M.; Eek, M.; Førre, O.; Mowinkel, P.; Hovi, K. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Hartmann, A.M.; Spoo, M.; Fischer, J.M.; Steckhan, N.; Jeitler, M.; Häupl, T.; Kandil, F.I.; Michalsen, A.; Kessler, C.S. To eat or not to eat—An exploratory randomized controlled trial on fasting and plant-based diet in rheumatoid arthritis (NutriFast-Study). Front. Nutr. 2022, 9, 1030380. [Google Scholar] [CrossRef]
- Kiltz, U.; Braun, J.; Becker, A.; Chenot, J.F.; Dreimann, M.; Hammel, L.; Heiligenhaus, A.; Hermann, K.G.; Klett, R.; Krause, D.; et al. Langfassung zur S3-Leitlinie Axiale Spondyloarthritis inklusive Morbus Bechterew und Frühformen, Update 2019. Z. Rheumatol. 2019, 78, 3–64. [Google Scholar] [CrossRef]
- Wilhelmi De Toledo, F.; Buchinger, A.; Burggrabe, H.; Hölz, G.; Kuhn, C.; Lischka, E.; Lischka, N.; Lützner, H.; May, W.; Ritzmann-Widderich, M.; et al. Fasting Therapy—An Expert Panel Update of the 2002 Consensus Guidelines. Forsch. Komplementärmed./Res. Complement. Med. 2013, 20, 434–443. [Google Scholar] [CrossRef]
- Wilhelmi de Toledo, F.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, e0209353. [Google Scholar] [CrossRef]
- Grundler, F.; Mesnage, R.; Michalsen, A.; Wilhelmi de Toledo, F. Blood Pressure Changes in 1610 Subjects with and without Antihypertensive Medication during Long-Term Fasting. J. Am. Heart Assoc. 2020, 9, e018649. [Google Scholar] [CrossRef]
- Laurens, C.; Grundler, F.; Damiot, A.; Chery, I.; Le Maho, A.L.; Zahariev, A.; Le Maho, Y.; Bergouignan, A.; Gauquelin-Koch, G.; Simon, C.; et al. Is muscle and protein loss relevant in long-term fasting in healthy men? A prospective trial on physiological adaptations. J. Cachexia Sarcopenia Muscle 2021, 12, 1690–1703. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W.; Wu, D.; Hu, F. Metabolic Efficacy of Time-Restricted Eating in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2022, 107, 3428–3441. [Google Scholar] [CrossRef]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178, 1102–1114.e1117. [Google Scholar] [CrossRef]
- Hartmann, A.M.; Dell’Oro, M.; Kessler, C.S.; Schumann, D.; Steckhan, N.; Jeitler, M.; Fischer, J.M.; Spoo, M.; Kriegel, M.A.; Schneider, J.G.; et al. Efficacy of therapeutic fasting and plant-based diet in patients with rheumatoid arthritis (NutriFast): Study protocol for a randomised controlled clinical trial. BMJ Open 2021, 11, e047758. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Curcumin and Curcuma longa Extract in the Treatment of 10 Types of Autoimmune Diseases: A Systematic Review and Meta-Analysis of 31 Randomized Controlled Trials. Front. Immunol. 2022, 13, 896476. [Google Scholar] [CrossRef]
- Pagliari, S.; Forcella, M.; Lonati, E.; Sacco, G.; Romaniello, F.; Rovellini, P.; Fusi, P.; Palestini, P.; Campone, L.; Labra, M.; et al. Antioxidant and Anti-Inflammatory Effect of Cinnamon (Cinnamomum verum J. Presl) Bark Extract after In Vitro Digestion Simulation. Foods 2023, 12, 452. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Ayman Salkini, M.; Ibnouf Ahmed, E.O.; Yusufoglu, H.S. Evaluation of the composition and in vitro antimicrobial, antioxidant, and anti-inflammatory activities of Cilantro (Coriandrum sativum L. leaves) cultivated in Saudi Arabia (Al-Kharj). Saudi J. Biol. Sci. 2021, 28, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Gesellschaft für Ernährung (DGE) ÖgfEÖ; Schweizerische Gesellschaft für Ernährung (SGE). Referenzwerte für Die Nährstoffzufuhr, 2nd ed.; Ausgabe Ed: Bonn, Germany, 2021. [Google Scholar]
- Devlin, U.M.; McNulty, B.A.; Nugent, A.P.; Gibney, M.J. The use of cluster analysis to derive dietary patterns: Methodological considerations, reproducibility, validity and the effect of energy mis-reporting. Proc. Nutr. Soc. 2012, 71, 599–609. [Google Scholar] [CrossRef]
- Hasan, B.; Thompson, W.G.; Almasri, J.; Wang, Z.; Lakis, S.; Prokop, L.J.; Hensrud, D.D.; Frie, K.S.; Wirtz, M.J.; Murad, A.L.; et al. The effect of culinary interventions (cooking classes) on dietary intake and behavioral change: A systematic review and evidence map. BMC Nutr. 2019, 5, 29. [Google Scholar] [CrossRef]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. Adv. Exp. Med. Biol. 2021, 1291, 15–39. [Google Scholar] [CrossRef]
- Luongo, D.; Treppiccione, L.; Sorrentino, A.; Ferrocino, I.; Turroni, S.; Gatti, M.; Di Cagno, R.; Sanz, Y.; Rossi, M. Immune-modulating effects in mouse dendritic cells of lactobacilli and bifidobacteria isolated from individuals following omnivorous, vegetarian and vegan diets. Cytokine 2017, 97, 141–148. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef]
- Xu, X.; Wang, M.; Wang, Z.; Chen, Q.; Chen, X.; Xu, Y.; Dai, M.; Wu, B.; Li, Y. The bridge of the gut–joint axis: Gut microbial metabolites in rheumatoid arthritis. Front. Immunol. 2022, 13, 1007610. [Google Scholar] [CrossRef]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef]
- Scher, J.U.; Littman, D.R.; Abramson, S.B. Review: Microbiome in Inflammatory Arthritis and Human Rheumatic Diseases. Arthritis Rheumatol. 2016, 68, 35–45. [Google Scholar] [CrossRef]
- Smiljanovic, B.; Grützkau, A.; Sörensen, T.; Grün, J.R.; Vogl, T.; Bonin, M.; Schendel, P.; Stuhlmüller, B.; Claussnitzer, A.; Hermann, S.; et al. Synovial tissue transcriptomes of long-standing rheumatoid arthritis are dominated by activated macrophages that reflect microbial stimulation. Sci. Rep. 2020, 10, 7907. [Google Scholar] [CrossRef]
- Cheng, M.; Zhao, Y.; Cui, Y.; Zhong, C.; Zha, Y.; Li, S.; Cao, G.; Li, M.; Zhang, L.; Ning, K.; et al. Stage-specific roles of microbial dysbiosis and metabolic disorders in rheumatoid arthritis. Ann. Rheum. Dis. 2022, 81, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.M.; Joyce Wu, H.J.; Mauro, D.; Schett, G.; Ciccia, F. The gut-joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, C.A.; van de Put, M.; Bisschops, M.; Walrabenstein, W.; de Jonge, C.S.; Herrema, H.; van Schaardenburg, D. The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review. Nutrients 2021, 13, 3208. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.I.; Efimova, D.A.; Nikogosov, D.A.; Osipenko, D.A.; et al. Microbiome Responses to an Uncontrolled Short-Term Diet Intervention in the Frame of the Citizen Science Project. Nutrients 2018, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Valter, D.L.; Mark, P.M. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Schmidt, N.S.; Lorentz, A. Dietary restrictions modulate the gut microbiota: Implications for health and disease. Nutr. Res. 2021, 89, 10–22. [Google Scholar] [CrossRef]
- Häupl, T.; Sörensen, T.; Boyer, M.; Scheder-Bieschin, J.; Smiljanovic, B.; Steckhan, N.; Burmester, G.-R.; Stuhlmüller, B.; Kessler, C.; Bonin, M.; et al. SAT0249 Reduction of monocyte activation by bowel cleanse and one week fasting suggests permanent pathogenetic triggering from the gut in rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 986–987. [Google Scholar] [CrossRef]
- Kang, J.; Shi, X.; Fu, J.; Li, H.; Ma, E.; Chen, W. Effects of an Intermittent Fasting 5:2 Plus Program on Body Weight in Chinese Adults with Overweight or Obesity: A Pilot Study. Nutrients 2022, 14, 4734. [Google Scholar] [CrossRef]
- Torres, L.; Lee, J.L.; Park, S.; Di Lorenzo, R.C.; Branam, J.P.; Fraser, S.A.; Salisbury, B.A. Retention, Fasting Patterns, and Weight Loss with an Intermittent Fasting App: Large-Scale, 52-Week Observational Study. JMIR Mhealth Uhealth 2022, 10, e35896. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Li, Y.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Willett, W.C.; Hu, F.B.; Sun, Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: Two prospective longitudinal cohort studies. BMJ 2016, 355, i5796. [Google Scholar] [CrossRef] [PubMed]
- Sebe, M.; Tsutsumi, R.; Senoura, S.; Kishi, J.; Iuchi, M.; Mishima, Y.; Tsutsumi, Y.M.; Kuroda, M.; Harada, N.; Nakaya, Y.; et al. Saturated fatty acids intake is associated with muscle atrophy in rheumatoid arthritis. JCSM Rapid Commun. 2022, 5, 86–101. [Google Scholar] [CrossRef]
- Edefonti, V.; Parpinel, M.; Ferraroni, M.; Boracchi, P.; Schioppo, T.; Scotti, I.; Ubiali, T.; Currenti, W.; De Lucia, O.; Cutolo, M.; et al. A Posteriori Dietary Patterns and Rheumatoid Arthritis Disease Activity: A Beneficial Role of Vegetable and Animal Unsaturated Fatty Acids. Nutrients 2020, 12, 3856. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The impact of dietary protein intake on longevity and metabolic health. eBioMedicine 2019, 43, 632–640. [Google Scholar] [CrossRef]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Petersen, K.; Barger, K.; Hansen, K.E.; Anderson, C.A.M.; Baer, D.J.; Lampe, J.W.; Rasmussen, H.; Matthan, N.R. Perspective: Design and Conduct of Human Nutrition Randomized Controlled Trials. Adv. Nutr. 2020, 12, 4–20. [Google Scholar] [CrossRef]

| Nutrients | Overall Mean ± SD (n = 41) | Fasting + PBD (n = 24) | DGE (n = 17) | Reference Values | p-Value |
|---|---|---|---|---|---|
| Energy intake, kcal/day | 1932.1 ± 879.7 | 1880.5 ± 466.4 | 2005.0 ± 1269.8 | 2100.0 | 0.49 |
| Carbohydrates, g/day | 215.7 ± 112.8 | 214.8 ± 60.7 | 216.8 ± 162.8 | 256.1 | 0.21 |
| Sugar, g/day | 80.0 ± 42.0 | 81.9 ± 43.1 | 77.4 ± 42.0 | 61.0 | 0.78 |
| Total fat, g/day | 78.9 ± 37.1 | 76.7 ± 25.7 | 82.2 ± 49.7 | 69.2 | 0.73 |
| Saturated | 26.4 ± 11.4 | 24.8 ± 9.6 | 28.7 ± 13.6 | 16.2–23.1 | 0.46 |
| Monounsaturated | 19.11 ± 9.5 | 19.5 ± 8.9 | 18.6 ± 10.8 | 34.6–46.2 | 0.58 |
| Polyunsaturated | 13.6 ± 8.5 | 14.6 ± 9.2 | 12.2 ± 7.4 | 18.5–27.7 | 0.60 |
| Protein, g/day | 69.0 ± 35.8 | 63.0 ± 18.4 | 77.5 ± 50.9 | 48.0 | 0.51 |
| Dietary fiber, g/day | 22.6 ± 11.0 | 21.9 ± 10.8 | 23.7 ± 11.6 | 35.1 | 0.54 |
| Vitamin E, mg/day | 11.5 ± 9.3 | 11.3 ± 11.2 | 11.7 ± 6.0 | 14.0 | 0.24 |
| Vitamin C, mg/day | 118.1 ± 86.2 | 113.6 ± 67.0 | 124.5 ± 109.8 | 95.0 | 1.00 |
| Vitamin D, µg/day | 3.3 ± 4.2 | 2.2 ± 2.0 | 4.8 ± 5.8 | 2.0–4.0 | 0.25 |
| Thiamine, mg/day | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.9 ± 0.6 | 1.0 | 0.65 |
| Riboflavin, mg/day | 0.9 ± 0.5 | 0.8 ± 0.4 | 1.1 ± 0.5 | 1.1 | 0.02 |
| Vitamin B6, mg/day | 1.1 ± 0.6 | 1.0 ± 0.4 | 1.3 ± 0.7 | 1.4 | 0.09 |
| Biotin, µg/day | 25.2 ± 15.6 | 25.6 ± 17.9 | 24.7 ± 12.5 | 40.0 | 0.52 |
| Folate, µg/day | 190.8 ± 97.1 | 176.7 ± 81.2 | 210.2 ± 116.2 | 300.0 | 0.40 |
| Vitamin B12, µg/day | 3.1 ± 3.9 | 1.8 ± 1.4 | 5.0 ± 5.4 | 4.0 | 0.05 |
| Vitamin A, mg/day | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.7 | 0.87 |
| Vitamin K, µg/day | 57.4 ± 46.0 | 59.5 ± 53.9 | 54.5 ±33.9 | 60.0 | 0.90 |
| Zinc, mg/day | 21.6 ± 85.8 | 31.8 ± 112.7 | 7.4 ± 6.2 | 8.0 | 0.60 |
| Magnesium, mg/day | 696.5 ± 2306.9 | 982.6 ± 3028.3 | 300.3 ± 141.4 | 300.0 | 0.46 |
| Copper, µg/day | 3422.0 ± 11557.9 | 4823.3 ± 15178.5 | 1481.8 ± 747.8 | 1000–1500 | 0.40 |
| Calcium, mg/day | 1625.9 ± 5734.2 | 2306.1 ± 7536.5 | 684.1 ± 291.1 | 1000.0 | 0.16 |
| Cluster 1 | Cluster 2 | Total | |
|---|---|---|---|
| DGE | 17 | 6 | 23 |
| Fasting + PBD | 15 | 9 | 24 |
| Total | 32 | 15 | 47 |
| Cluster 1 | Cluster 2 | Total | |
|---|---|---|---|
| DGE | 22 | 1 | 23 |
| Fasting + PBD | 19 | 5 | 24 |
| Total | 41 | 6 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartmann, A.M.; D’Urso, M.; Dell’Oro, M.; Koppold, D.A.; Steckhan, N.; Michalsen, A.; Kandil, F.I.; Kessler, C.S. Post Hoc Analysis of a Randomized Controlled Trial on Fasting and Plant-Based Diet in Rheumatoid Arthritis (NutriFast): Nutritional Supply and Impact on Dietary Behavior. Nutrients 2023, 15, 851. https://doi.org/10.3390/nu15040851
Hartmann AM, D’Urso M, Dell’Oro M, Koppold DA, Steckhan N, Michalsen A, Kandil FI, Kessler CS. Post Hoc Analysis of a Randomized Controlled Trial on Fasting and Plant-Based Diet in Rheumatoid Arthritis (NutriFast): Nutritional Supply and Impact on Dietary Behavior. Nutrients. 2023; 15(4):851. https://doi.org/10.3390/nu15040851
Chicago/Turabian StyleHartmann, Anika M., Marina D’Urso, Melanie Dell’Oro, Daniela A. Koppold, Nico Steckhan, Andreas Michalsen, Farid I. Kandil, and Christian S. Kessler. 2023. "Post Hoc Analysis of a Randomized Controlled Trial on Fasting and Plant-Based Diet in Rheumatoid Arthritis (NutriFast): Nutritional Supply and Impact on Dietary Behavior" Nutrients 15, no. 4: 851. https://doi.org/10.3390/nu15040851
APA StyleHartmann, A. M., D’Urso, M., Dell’Oro, M., Koppold, D. A., Steckhan, N., Michalsen, A., Kandil, F. I., & Kessler, C. S. (2023). Post Hoc Analysis of a Randomized Controlled Trial on Fasting and Plant-Based Diet in Rheumatoid Arthritis (NutriFast): Nutritional Supply and Impact on Dietary Behavior. Nutrients, 15(4), 851. https://doi.org/10.3390/nu15040851







