Maternal Pea Protein Intake Provides Sex-Specific Protection against Dyslipidemia in Offspring from Obese Pregnancies
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef]
- Fruhbeck, G.; Yumuk, V. Obesity: A gateway disease with a rising prevalence. Obes. Facts 2014, 7 (Suppl. S2), 33–36. [Google Scholar] [CrossRef] [PubMed]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; Demerath, E.W. Relationship of insulin, glucose, leptin, IL-6 and TNF-alpha in human breast milk with infant growth and body composition. Pediatr. Obes. 2012, 7, 304–312. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.G.; Desai, M. Developmental programming of appetite/satiety. Ann. Nutr. Metab. 2014, 64 (Suppl. S1), 36–44. [Google Scholar] [CrossRef] [PubMed]
- Berggren, E.K.; Groh-Wargo, S.; Presley, L.; Hauguel-de Mouzon, S.; Catalano, P.M. Maternal fat, but not lean, mass is increased among overweight/obese women with excess gestational weight gain. Am. J. Obstet. Gynecol. 2016, 214, e741–e745. [Google Scholar] [CrossRef]
- Lomas-Soria, C.; Reyes-Castro, L.A.; Rodriguez-Gonzalez, G.L.; Ibanez, C.A.; Bautista, C.J.; Cox, L.A.; Nathanielsz, P.W.; Zambrano, E. Maternal obesity has sex-dependent effects on insulin, glucose and lipid metabolism and the liver transcriptome in young adult rat offspring. J. Physiol. 2018, 596, 4611–4628. [Google Scholar] [CrossRef]
- Menting, M.D.; Mintjens, S.; van de Beek, C.; Frick, C.J.; Ozanne, S.E.; Limpens, J.; Roseboom, T.J.; Hooijmans, C.R.; van Deutekom, A.W.; Painter, R.C. Maternal obesity in pregnancy impacts offspring cardiometabolic health: Systematic review and meta-analysis of animal studies. Obes. Rev. 2019, 20, 675–685. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Leung, C.W.; Li, Y.; Ding, E.L.; Chiuve, S.E.; Hu, F.B.; Willett, W.C. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern. Med. 2014, 174, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. Systematic review and meta-analysis of energy and macronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2012, 70, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2013, 71, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Nansel, T.R.; Cummings, J.R.; Burger, K.; Siega-Riz, A.M.; Lipsky, L.M. Greater Ultra-Processed Food Intake during Pregnancy and Postpartum Is Associated with Multiple Aspects of Lower Diet Quality. Nutrients 2022, 14, 3933. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, M.D.; Ingram, K.H.; Appiah, D.; Rudd, J.; Whitaker, K.M.; Bennett, W.L.; Shikany, J.M.; Jacobs, D.R., Jr.; Lewis, C.E.; Gunderson, E.P. Prepregnancy Protein Source and BCAA Intake Are Associated with Gestational Diabetes Mellitus in the CARDIA Study. Int. J. Environ. Res. Public Health 2022, 19, 14142. [Google Scholar] [CrossRef]
- Lou, M.F.; Shen, W.; Fu, R.S.; Zhang, X.Y.; Wang, D.H. Maternal dietary protein supplement confers long-term sex-specific beneficial consequences of obesity resistance and glucose tolerance to the offspring in Brandt’s voles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 182, 38–44. [Google Scholar] [CrossRef]
- McCrory, M.A.; Hamaker, B.R.; Lovejoy, J.C.; Eichelsdoerfer, P.E. Pulse consumption, satiety, and weight management. Adv. Nutr. 2010, 1, 17–30. [Google Scholar] [CrossRef]
- Adam, C.L.; Gratz, S.W.; Peinado, D.I.; Thomson, L.M.; Garden, K.E.; Williams, P.A.; Richardson, A.J.; Ross, A.W. Effects of Dietary Fibre (Pectin) and/or Increased Protein (Casein or Pea) on Satiety, Body Weight, Adiposity and Caecal Fermentation in High Fat Diet-Induced Obese Rats. PLoS ONE 2016, 11, e0155871. [Google Scholar] [CrossRef]
- Li, H.; Prairie, N.; Udenigwe, C.C.; Adebiyi, A.P.; Tappia, P.S.; Aukema, H.M.; Jones, P.J.; Aluko, R.E. Blood pressure lowering effect of a pea protein hydrolysate in hypertensive rats and humans. J. Agric. Food Chem. 2011, 59, 9854–9860. [Google Scholar] [CrossRef]
- Lasekan, J.B.; Gueth, L.; Khan, S. Influence of Dietary Golden Pea Proteins Versus Casein on Plasma and Hepatic Lipids in Rats. Nutr. Res. 1995, 15, 71–84. [Google Scholar] [CrossRef]
- Spielmann, J.; Shukla, A.; Brandsch, C.; Hirche, F.; Stangl, G.I.; Eder, K. Dietary lupin protein lowers triglyceride concentrations in liver and plasma in rats by reducing hepatic gene expression of sterol regulatory element-binding protein-1c. Ann. Nutr. Metab. 2007, 51, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Heyne, G.W.; Plisch, E.H.; Melberg, C.G.; Sandgren, E.P.; Peter, J.A.; Lipinski, R.J. A Simple and Reliable Method for Early Pregnancy Detection in Inbred Mice. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 368–371. [Google Scholar] [PubMed]
- DePeters, E.J.; Hovey, R.C. Methods for collecting milk from mice. J. Mammary Gland Biol. Neoplasia 2009, 14, 397–400. [Google Scholar] [CrossRef]
- Paul, H.A.; Hallam, M.C.; Reimer, R.A. Milk Collection in the Rat Using Capillary Tubes and Estimation of Milk Fat Content by Creamatocrit. J. Vis. Exp. 2015, 106, e53476. [Google Scholar] [CrossRef]
- Rideout, T.C.; Harding, S.V.; Jones, P.J. Consumption of plant sterols reduces plasma and hepatic triglycerides and modulates the expression of lipid regulatory genes and de novo lipogenesis in C57BL/6J. mice. Mol. Nutr. Food Res. 2010, 54 (Suppl. S1), S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.V.; Rideout, T.C.; Jones, P.J. Hepatic nuclear sterol regulatory binding element protein 2 abundance is decreased and that of ABCG5 increased in male hamsters fed plant sterols. J. Nutr. 2010, 140, 1249–1254. [Google Scholar] [CrossRef]
- Ma, X.; Wu, L.; Wang, Y.; Han, S.; El-Dalatony, M.M.; Feng, F.; Tao, Z.; Yu, L.; Wang, Y. Diet and human reproductive system: Insight of omics approaches. Food Sci. Nutr. 2022, 10, 1368–1384. [Google Scholar] [CrossRef]
- Wu, S.; Divall, S.; Nwaopara, A.; Radovick, S.; Wondisford, F.; Ko, C.; Wolfe, A. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes 2014, 63, 1270–1282. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur. J. Clin. Nutr. 2009, 63, 78–86. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, G.; Zhang, T.; Zhang, H.; Gao, X.; Xing, X.; Zhao, J.; Yang, F. Effects of dietary protein level on nutrients digestibility and reproductive performance of female mink (Neovison vison) during gestation. Anim. Nutr. 2015, 1, 65–69. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, W.; Huang, S.; Le Maho, Y.; Habold, C.; Zhang, Z. Impacts of Dietary Protein and Niacin Deficiency on Reproduction Performance, Body Growth, and Gut Microbiota of Female Hamsters (Tscherskia triton) and Their Offspring. MicroBiol. Spectr. 2022, 10, e0015722. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Li, D.; Li, N.; Dindot, S.V.; Satterfield, M.C.; Bazer, F.W.; Wu, G. Nutrition, epigenetics, and metabolic syndrome. Antioxid. Redox Signal. 2012, 17, 282–301. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Protein intake and ovulatory infertility. Am. J. Obstet. Gynecol. 2008, 198, e211–e217. [Google Scholar] [CrossRef] [PubMed]
- Bertino, M. Effects of high fat, protein supplemented diets on maternal behavior in rats. Physiol. Behav. 1982, 29, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.A.; Rasmussen, K.M.; Myers, T.R. Consumption of a high fat diet impairs reproductive performance in Sprague-Dawley rats. J. Nutr. 1997, 127, 64–69. [Google Scholar] [CrossRef]
- Shi, X.; Huang, Z.; Zhou, G.; Li, C. Dietary Protein From Different Sources Exerted a Great Impact on Lipid Metabolism and Mitochondrial Oxidative Phosphorylation in Rat Liver. Front. Nutr. 2021, 8, 719144. [Google Scholar] [CrossRef]
- Maurer, A.D.; Chen, Q.; McPherson, C.; Reimer, R.A. Changes in satiety hormones and expression of genes involved in glucose and lipid metabolism in rats weaned onto diets high in fibre or protein reflect susceptibility to increased fat mass in adulthood. J. Physiol. 2009, 587, 679–691. [Google Scholar] [CrossRef]
- Brandsch, C.; Shukla, A.; Hirche, F.; Stangl, G.I.; Eder, K. Effect of proteins from beef, pork, and turkey meat on plasma and liver lipids of rats compared with casein and soy protein. Nutrition 2006, 22, 1162–1170. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Zhou, D.; Pan, Y.X. A low-protein diet during gestation in rats activates the placental mammalian amino acid response pathway and programs the growth capacity of offspring. J. Nutr. 2010, 140, 2116–2120. [Google Scholar] [CrossRef]
- Chamson-Reig, A.; Thyssen, S.M.; Arany, E.; Hill, D.J. Altered pancreatic morphology in the offspring of pregnant rats given reduced dietary protein is time and gender specific. J. Endocrinol. 2006, 191, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Bautista, C.J.; Bautista, R.J.; Montano, S.; Reyes-Castro, L.A.; Rodriguez-Pena, O.N.; Ibanez, C.A.; Nathanielsz, P.W.; Zambrano, E. Effects of maternal protein restriction during pregnancy and lactation on milk composition and offspring development. Br. J. Nutr. 2019, 122, 141–151. [Google Scholar] [CrossRef]
- Savitikadi, P.; Pullakhandam, R.; Kulkarni, B.; Kumar, B.N.; Reddy, G.B.; Reddy, V.S. Chronic Effects of Maternal Low-Protein and Low-Quality Protein Diets on Body Composition, Glucose-Homeostasis and Metabolic Factors, Followed by Reversible Changes upon Rehabilitation in Adult Rat Offspring. Nutrients 2021, 13, 4129. [Google Scholar] [CrossRef] [PubMed]
- Hallam, M.C.; Reimer, R.A. A maternal high-protein diet predisposes female offspring to increased fat mass in adulthood whereas a prebiotic fibre diet decreases fat mass in rats. Br. J. Nutr. 2013, 110, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Bautista, C.J.; Reyes-Castro, L.A.; Bautista, R.J.; Ramirez, V.; Elias-Lopez, A.L.; Hernandez-Pando, R.; Zambrano, E. Different Protein Sources in the Maternal Diet of the Rat during Gestation and Lactation Affect Milk Composition and Male Offspring Development during Adulthood. Reprod. Sci. 2021, 28, 2481–2494. [Google Scholar] [CrossRef] [PubMed]
- Tajaddini, A.; Kendig, M.D.; Prates, K.V.; Westbrook, R.F.; Morris, M.J. Male Rat Offspring Are More Impacted by Maternal Obesity Induced by Cafeteria Diet than Females-Additive Effect of Postweaning Diet. Int. J. Mol. Sci. 2022, 23, 1442. [Google Scholar] [CrossRef] [PubMed]
- Kulhanek, D.; Abrahante Llorens, J.E.; Buckley, L.; Tkac, I.; Rao, R.; Paulsen, M.E. Female and male C57BL/6J. offspring exposed to maternal obesogenic diet develop altered hypothalamic energy metabolism in adulthood. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E448–E466. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.L.; Samuelsson, A.M.; Argenton, M.; Dhonye, H.; Kalamatianos, T.; Poston, L.; Taylor, P.D.; Coen, C.W. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS ONE 2009, 4, e5870. [Google Scholar] [CrossRef]
- Samuelsson, A.M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.; Piersma, A.H.; Ozanne, S.E.; Twinn, D.F.; Remacle, C.; et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef]
- Chang, E.; Hafner, H.; Varghese, M.; Griffin, C.; Clemente, J.; Islam, M.; Carlson, Z.; Zhu, A.; Hak, L.; Abrishami, S.; et al. Programming effects of maternal and gestational obesity on offspring metabolism and metabolic inflammation. Sci. Rep. 2019, 9, 16027. [Google Scholar] [CrossRef]
- Nivoit, P.; Morens, C.; Van Assche, F.A.; Jansen, E.; Poston, L.; Remacle, C.; Reusens, B. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia 2009, 52, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Andres, A.; Hull, H.R.; Shankar, K.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Longitudinal body composition of children born to mothers with normal weight, overweight, and obesity. Obesity 2015, 23, 1252–1258. [Google Scholar] [CrossRef]
- Dakin, R.S.; Walker, B.R.; Seckl, J.R.; Hadoke, P.W.; Drake, A.J. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int. J. Obes. 2015, 39, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Carlin, G.; Chaumontet, C.; Blachier, F.; Barbillon, P.; Darcel, N.; Delteil, C.; van der Beek, E.M.; Kodde, A.; van de Heijning, B.J.M.; Tome, D.; et al. Perinatal exposure of rats to a maternal diet with varying protein quantity and quality affects the risk of overweight in female adult offspring. J. Nutr. Biochem. 2020, 79, 108333. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Duranti, M.; Magni, C.; Morandi, S.; D’Agostina, A.; Arnoldi, A. Proteins of white lupin seed, a naturally isoflavone-poor legume, reduce cholesterolemia in rats and increase LDL receptor activity in HepG2 cells. J. Nutr. 2004, 134, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.F.; Hallowell, H.A.; Higgins, K.V.; Liles, M.R.; Hood, W.R. Maternal Dietary Protein Intake Influences Milk and Offspring Gut Microbial Diversity in a Rat (Rattus norvegicus) Model. Nutrients 2019, 11, 2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]

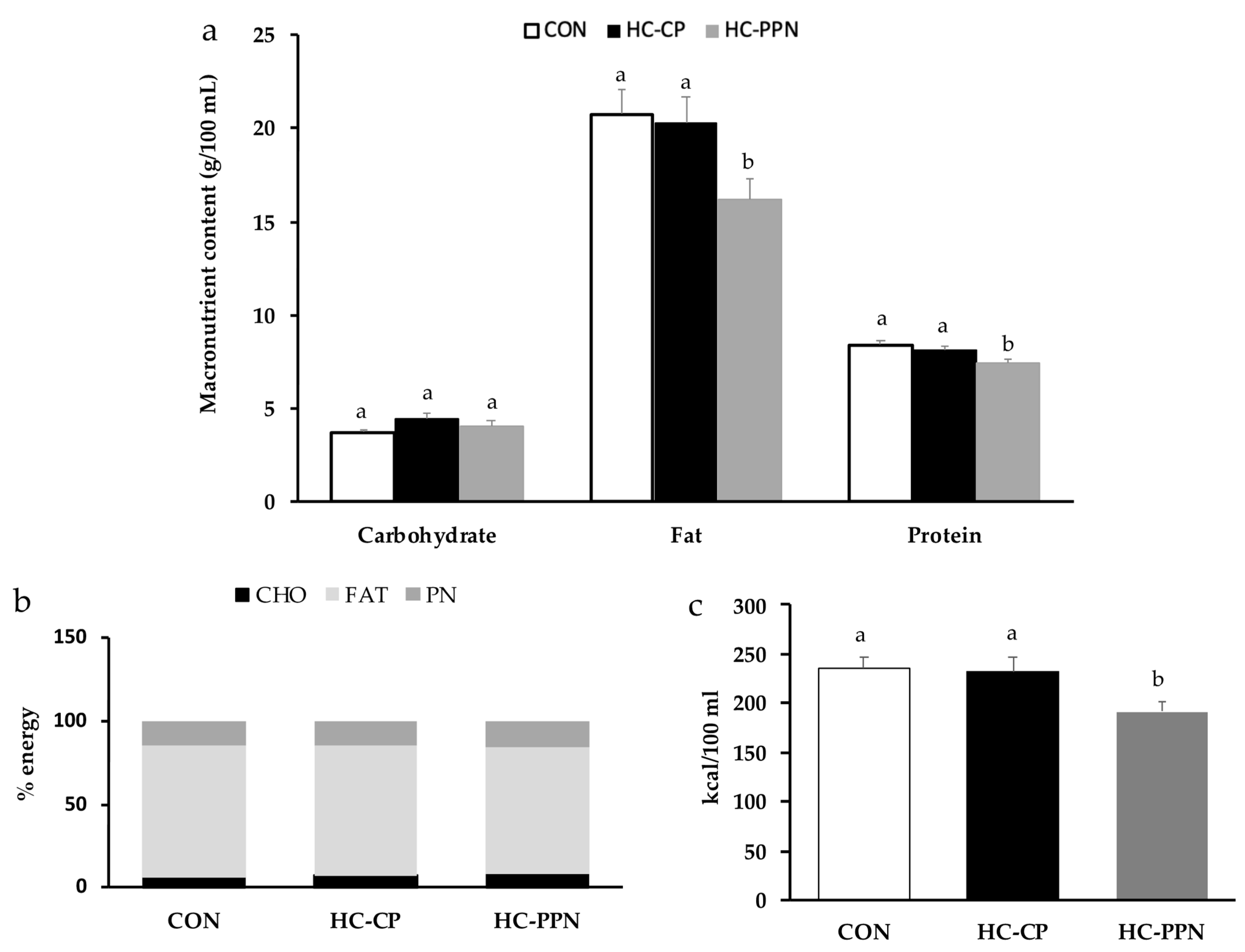

| Experimental Diets 1 | |||
|---|---|---|---|
| Ingredient | CON | HC-CP | HC-PPN |
| Casein | 19.0 | 23.3 | 5.3 |
| L-Cystine | 0.3 | 0.3 | 0.4 |
| Corn starch | 52.1 | 8.5 | 9.3 |
| Maltodextrin | 14.2 | 11.7 | 10.0 |
| Sucrose | 0.4 | 20.6 | 19.3 |
| Cellulose, BW200 | 4.7 | 5.8 | 3.0 |
| Yellow pea protein isolate | - | - | 25.0 |
| Soybean oil | 2.4 | 2.9 | 3.0 |
| Lard | 1.9 | 20.7 | 18.5 |
| Mineral mix | 4.7 | 5.8 | 5.8 |
| Vitamin mix | 0.1 | 0.1 | 0.1 |
| Choline bitartrate | 0.2 | 0.2 | 0.2 |
| Energy contribution | |||
| Total energy (kcal/g) | 3.8 | 4.8 | 4.6 |
| % energy from fat | 10.0 | 45.2 | 45.9 |
| % energy from protein | 20.1 | 20.1 | 20.7 |
| % energy from carbohydrate | 69.8 | 34.7 | 35.7 |
| Outcome | CON | HC-CP | HC-PPN |
|---|---|---|---|
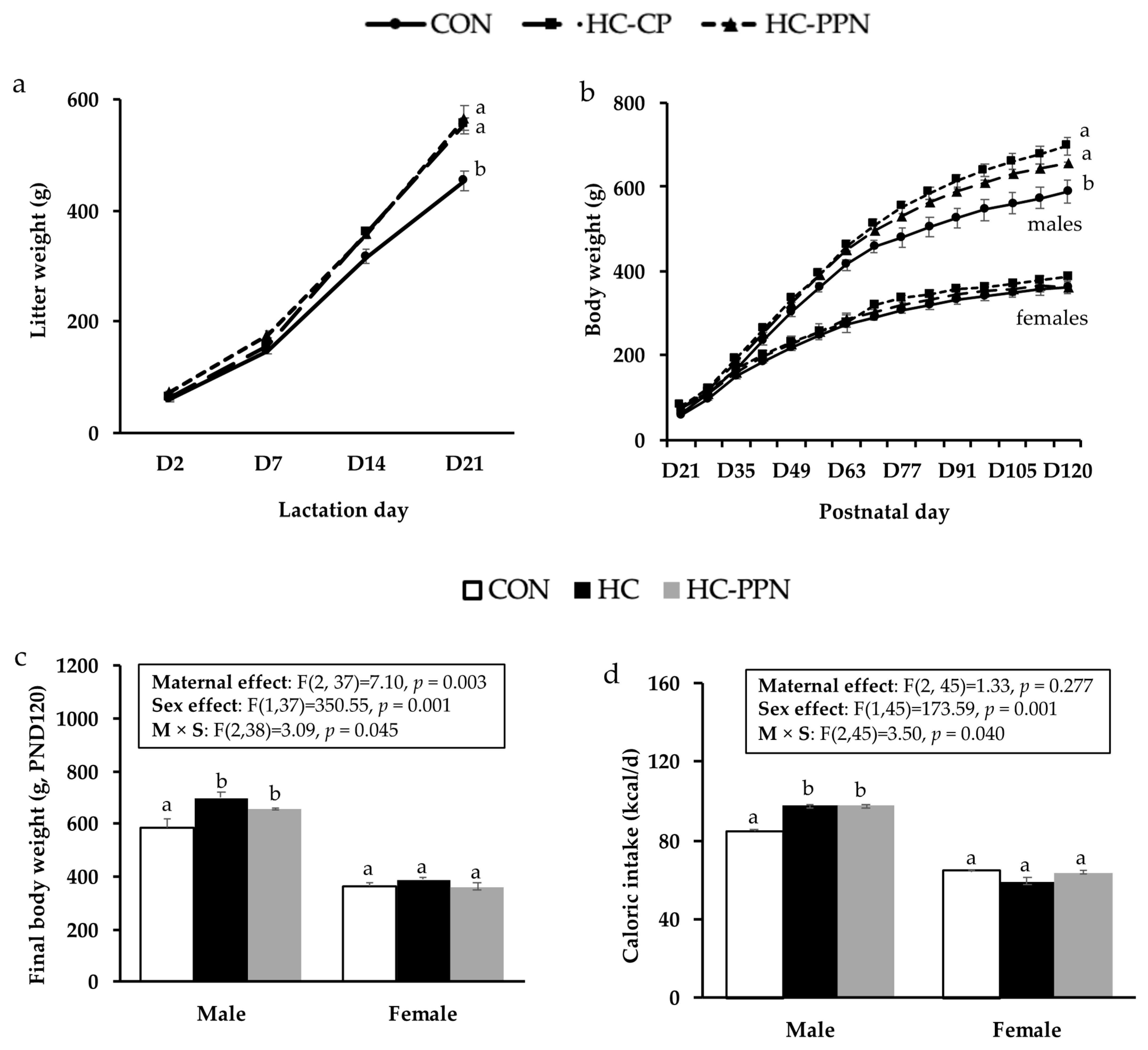
| Body weight (g) | |||
| Initial | 90.7 ± 6.7 a | 94.3 ± 2.1 a | 94.61 ± 1.3 a |
| End of pre-pregnancy | 291.6 ± 8.4 a | 372.5 ± 15.1 b | 386.7 ± 12.4 b |
| End of gestation | 446.9 ± 11.3 a | 488.3 ± 17.4 b | 519.6 ± 14.7 b |
| End of lactation | 336.1 ± 11.2 a | 345.9 ± 9.6 a | 355.4 ± 13.5 a |
| Feed intake (kcal/d) | |||
| Pre-pregnancy | 63.3 ± 1.7 a | 80.2 ± 3.7 b | 77.3 ± 3.1 b |
| Gestation | 85.1 ± 3.1 a | 98.2 ± 4.5 b | 104.6 ± 7.3 b |
| Lactation | 124.3 ± 4.4 a | 130.8 ± 3.3 b | 132.4 ± 4.8 b |
| Breeding outcomes | |||
| Reproductive success (%) * | 90.00 | 43.75 | 72.73 |
| Time to pregnancy (days) | 2.5 ± 0.5 a | 2.8 ± 0.4 a | 3.0 ± 0.3 a |
| Litter size at birth (# pups) | 14.3 ± 0.5 a | 14.0 ± 0.6 a | 13.6 ± 0.9 a |
| Litter weight at birth (g) | 88.9 ± 3.1 a | 88.4 ± 5.2 a | 93.7 ± 7.7 a |
| Average pup weight at birth (g) | 6.2 ± 0.3 a | 6.3 ± 0.2 a | 6.82 ± 0.1 a |
| Metabolic parameters | |||
| Pre-pregnancy | |||
| Glucose (mg/dL) | 76.1 ± 6.7 | 72.9 ± 7.4 | 84.3 ± 12.4 |
| Insulin (µIU/mL) | 65.7 ± 7.5 | 57.86 ± 14.5 | 47.5 ± 9.9 |
| Glucose:insulin | 1.2 ± 0.1 | 1.8 ± 0.4 | 2.3 ± 0.4 |
| Male Offspring | Female Offspring | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | CON | HC-CP | HC-PPN | CON | HC-CP | HC-PPN | Maternal Effect | Sex Effect | M × S |
| Newly weaned | |||||||||
| Total-C | 132.79 ± 10.38 a | 107.4 ± 15.15 ab | 83.08 ± 12.30 b | 130.82 ± 7.23 a | 124.21 ± 22.14 ab | 107.36 ± 9.17 b | 0.03 | 0.23 | 0.57 |
| HDL-C | 78.93 ± 8.78 a | 70.23 ± 12.34 a | 53.54 ± 0.90 b | 73.94 ± 3.13 a | 67.87 ± 11.25 a | 47.73 ± 7.91 b | 0.02 | 0.40 | 0.99 |
| LDL/VLDL-C | 53.86 ± 9.93 | 50.56 ± 8.37 | 53.38 ± 9.21 | 56.88 ± 7.14 | 56.34 ± 11.70 | 53.92 ± 1.77 | 0.97 | 0.70 | 0.97 |
| Adult | |||||||||
| Total-C | 210.92 ± 2.85 a | 356.82 ± 13.30 b | 280.11 ± 15.24 c | 322.90 ± 62.49 a | 286.30 ± 38.86 a | 271.42 ± 15.88 a | 0.23 | 0.69 | 0.03 |
| HDL-C | 96.27 ± 15.83 | 120.67 ± 16.12 | 120.00 ± 11.05 | 135.71 ± 16.01 | 106.63 ± 8.36 | 108.97 ± 13.71 | 0.99 | 0.67 | 0.12 |
| LDL/VLDL-C | 133.50 ± 6.72 a | 236.16 ± 5.73 b | 160.11 ± 7.47 c | 187.19 ± 49.44 a | 150.00 ± 16.67 a | 162.45 ± 11.04 a | 0.32 | 0.61 | 0.03 |
| Triglyceride | 109.71 ± 5.89 a | 68.67 ± 23.17 a | 30.80 ± 8.97 b | 19.85 ± 5.08 | 32.57 ± 12.64 | 27.88 ± 6.05 | 0.048 | 0.001 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rideout, T.C.; Andreani, G.A.; Pembroke, J.; Choudhary, D.; Browne, R.W.; Mahmood, S.; Patel, M.S. Maternal Pea Protein Intake Provides Sex-Specific Protection against Dyslipidemia in Offspring from Obese Pregnancies. Nutrients 2023, 15, 867. https://doi.org/10.3390/nu15040867
Rideout TC, Andreani GA, Pembroke J, Choudhary D, Browne RW, Mahmood S, Patel MS. Maternal Pea Protein Intake Provides Sex-Specific Protection against Dyslipidemia in Offspring from Obese Pregnancies. Nutrients. 2023; 15(4):867. https://doi.org/10.3390/nu15040867
Chicago/Turabian StyleRideout, Todd C., Gabriella A. Andreani, Jillian Pembroke, Divya Choudhary, Richard W. Browne, Saleh Mahmood, and Mulchand S. Patel. 2023. "Maternal Pea Protein Intake Provides Sex-Specific Protection against Dyslipidemia in Offspring from Obese Pregnancies" Nutrients 15, no. 4: 867. https://doi.org/10.3390/nu15040867
APA StyleRideout, T. C., Andreani, G. A., Pembroke, J., Choudhary, D., Browne, R. W., Mahmood, S., & Patel, M. S. (2023). Maternal Pea Protein Intake Provides Sex-Specific Protection against Dyslipidemia in Offspring from Obese Pregnancies. Nutrients, 15(4), 867. https://doi.org/10.3390/nu15040867






