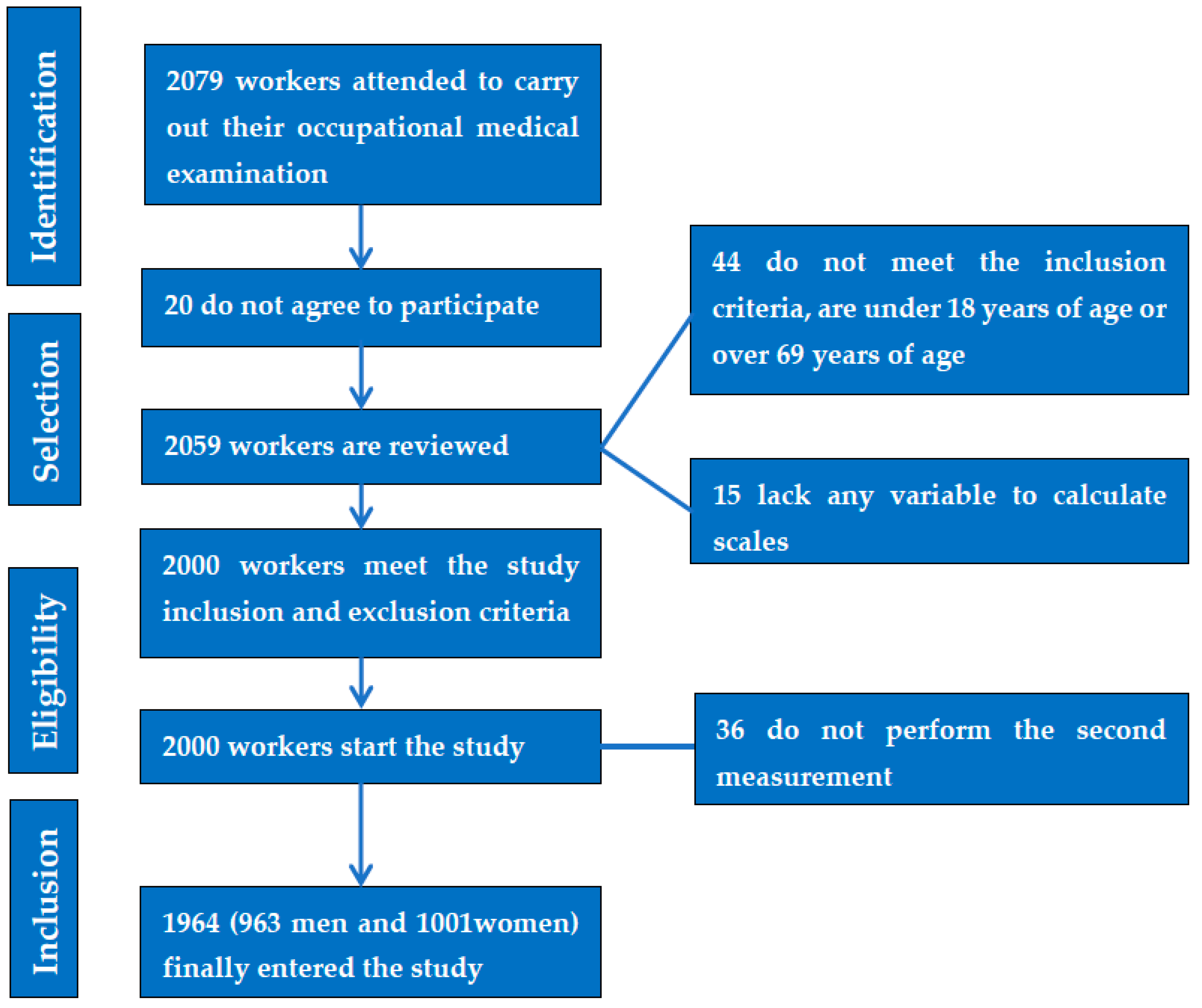
Dietary Intervention on Overweight and Obesity after Confinement by COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
- (a)
- NCEP ATP III (National Cholesterol Education Program Adult Treatment Panel III) [48]. Metabolic syndrome is defined when at least three of the following factors are present: waist circumference greater than 88 cm in women and 102 in men; triglycerides with values higher than 150 mg/dL or if the person is receiving lipid-lowering treatment for this condition; blood pressure in figures greater than 130/85 mm Hg, HDL less than 50 mg/dL in women, or less than 40 in men or specific treatment; and fasting blood glucose greater than 100 mg/dL or antidiabetic treatment.
- (b)
- The International Diabetes Federation (IDF) [49] requires the presence of central obesity assessed as a waist circumference greater than 80 cm in women and 94 cm in men, in addition to two of the other factors mentioned above in the ATP III requirements (triglycerides, HDL-cholesterol, blood pressure, and blood glucose).
- (c)
- The JIS [48] model uses the same criteria as NCEP ATPIII, but with waist circumference cut-off points of 80 cm in women and 94 cm in men.
2.2. Statistical Analysis
2.3. Considerations and/or Ethical Aspects
3. Results
4. Discussion
4.1. Limitations
4.2. Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Controlling the Global Obesity Epidemic. Available online: https://www.who.int/activities/controlling-the-global-obesity-epidemi (accessed on 8 February 2023).
- Centers for Disease Control (CDC) BRFSS. Behavioral Risk Factor Surveillance System Survey Data; US Department of Health and Human Services: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/brfss/index.html (accessed on 8 February 2023).
- Available online: https://ourworldindata.org/causes-of-death (accessed on 8 February 2023).
- Bennasar-Veny, M.; Sergio, F.; López-González, A.; Busquets-Cortés, C.; Yáñez, M.A. Lifestyle and progression to type 2 diabetes in a cohort of workers with prediabetes. Nutrients 2020, 12, 1538. [Google Scholar] [CrossRef]
- Kendel Jovanović, G.; Mrakovcic-Sutic, I.; Pavičić Žeželj, S.; Šuša, B.; Rahelić, D.; Klobučar Majanović, S. The Efficacy of an Energy-Restricted Anti-Inflammatory Diet for the Management of Obesity in Younger Adults. Nutrients 2020, 12, 3583. [Google Scholar] [CrossRef]
- Anekwe, C.V.; Jarrell, A.R.; Townsend, M.J.; Gaudier, G.I.; Hiserodt, J.M.; Stanford, F.C. Socioeconomics of Obesity. Curr. Obes. Rep. 2020, 9, 272–279. [Google Scholar] [CrossRef]
- Ayalon, I.; Bodilly, L.; Kaplan, J. The Impact of Obesity on Critical Illnesses. Shock 2021, 56, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kahn, H.S.; Jackson, S.L.; Steele, E.M.; Gillespie, C.; Yang, Q. Associations between ultra- or minimally processed food intake and three adiposity indicators among US adults: NHANES 2011 to 2016. Obesity 2022, 4, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; de Koning, B.; Lapillonne, A.; Moltu, S.J.; Norsa, L.; et al. Role of Dietary Factors, Food Habits, and Lifestyle in Childhood Obesity Development: A Position Paper From the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Wadolowska, L.; Hamulka, J.; Kowalkowska, J.; Kostecka, M.; Wadolowska, K.; Biezanowska-Kopec, R.; Czarniecka-Skubina, E.; Kozirok, W.; Piotrowska, A. Prudent-Active and Fast-Food-Sedentary Dietary-Lifestyle Patterns: The Association with Adiposity, Nutrition Knowledge and Sociodemographic Factors in Polish Teenagers-The ABC of Healthy Eating Project. Nutrients 2018, 10, 1988. [Google Scholar] [CrossRef] [PubMed]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-Čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wuhan Seafood Market Pneumonia Virus Isolate Wuhan-Hu-1, Complete Genome. 23 de enero de 2020 [citado 7 de 453 febrero de 2020]. Available online: http://www.ncbi.nlm.nih.gov/nuccore/MN908947.3 (accessed on 8 February 2023).
- Koh, D. COVID-19 lockdowns throughout the world. Occup. Med. 2020, 70, 322. [Google Scholar] [CrossRef]
- Real Decreto 463/2020, de 14 de Marzo, por el que se Declara el Estado de Alarma Para la Gestión de la Situación de Crisis 461 Sanitaria Ocasionada por el COVID-19. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2020-3692 (accessed on 8 February 2023).
- Ramírez Manent, J.I.; Altisench Jané, B.; Sanchís Cortés, P.; Busquets-Cortés, C.; Arroyo Bote, S.; Masmiquel Comas, L.; López González, Á.A. Impact of COVID-19 Lockdown on Anthropometric Variables, Blood Pressure, and Glucose and Lipid Profile in Healthy Adults: A before and after Pandemic Lockdown Longitudinal Study. Nutrients 2022, 14, 1237. [Google Scholar] [CrossRef] [PubMed]
- Wiechert, M.; Holzapfel, C. Nutrition Concepts for the Treatment of Obesity in Adults. Nutrients 2021, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Available online: https://estilosdevidasaludable.sanidad.gob.es/ (accessed on 8 February 2023).
- Cavero-Redondo, I.; Martinez-Vizcaino, V.; Fernandez-Rodriguez, R.; Saz-Lara, A.; Pascual-Morena, C.; Álvarez-Bueno, C. Effect of Behavioral Weight Management Interventions Using Lifestyle mHealth Self-Monitoring on Weight Loss: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1977. [Google Scholar] [CrossRef]
- Burke, L.E.; Sereika, S.M.; Bizhanova, Z.; Parmanto, B.; Kariuki, J.; Cheng, J.; Beatrice, B.; Cedillo, M.; Loar, I.; Pulantara, I.W.; et al. The Effect of Tailored, Daily, Smartphone Feedback to Lifestyle Self-Monitoring on Weight Loss at 12 Months: The SMARTER Randomized Clinical Trial. J. Med. Internet Res. 2022, 24, e38243. [Google Scholar] [CrossRef]
- Rumbo-Rodríguez, L.; Sánchez-SanSegundo, M.; Ruiz-Robledillo, N.; Albaladejo-Blázquez, N.; Ferrer-Cascales, R.; Zaragoza-Martí, A. Use of Technology-Based Interventions in the Treatment of Patients with Overweight and Obesity: A Systematic Review. Nutrients 2020, 12, 3634. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.; Mareschal, J.; Schwab, N.; Manoogian, E.; Borloz, S.; Ostinelli, G.; Gauthier-Jaques, A.; Umwali, S.; Rodriguez, E.G.; Aeberli, D.; et al. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients 2021, 13, 1042. [Google Scholar] [CrossRef]
- Free, C.; Phillips, G.; Galli, L.; Watson, L.; Felix, L.; Edwards, P.; Patel, V.; Haines, A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Med. 2013, 10, e1001362. [Google Scholar] [CrossRef] [Green Version]
- Fischer, H.H.; Fischer, I.P.; Pereira, R.I.; Furniss, A.L.; Rozwadowski, J.M.; Moore, S.L.; Durfee, M.J.; Raghunath, S.G.; Tsai, A.G.; Havranek, E.P. Text Message Support for Weight Loss in Patients With Prediabetes: A Randomized Clinical Trial. Diabetes Care 2016, 39, 1364–1370. [Google Scholar] [CrossRef]
- Bhardwaj, N.N.; Wodajo, B.; Gochipathala, K.; Paul, D.P., 3rd; Coustasse, A. Can mHealth Revolutionize the Way We Manage Adult Obesity? Perspect. Health Inf. Manag. 2017, 14, 1a. [Google Scholar]
- Wang, Y.; Min, J.; Khuri, J.; Xue, H.; Xie, B.; AKaminsky, L.; JCheskin, L. Effectiveness of Mobile Health Interventions on Diabetes and Obesity Treatment and Management: Systematic Review of Systematic Reviews. JMIR Mhealth Uhealth 2020, 8, e15400. [Google Scholar] [CrossRef]
- Arambepola, C.; Ricci-Cabello, I.; Manikavasagam, P.; Roberts, N.; French, D.P.; Farmer, A. The Impact of Automated Brief Messages Promoting Lifestyle Changes Delivered Via Mobile Devices to People with Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Controlled Trials. J. Med. Internet Res. 2016, 18, e86. [Google Scholar] [CrossRef] [PubMed]
- Schoeppe, S.; Alley, S.; Van Lippevelde, W.; Bray, N.A.; Williams, S.L.; Duncan, M.J.; Vandelanotte, C. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 127. [Google Scholar] [CrossRef]
- Spring, B.; Pellegrini, C.; McFadden, H.G.; Pfammatter, A.F.; Stump, T.K.; Siddique, J.; King, A.C.; Hedeker, D. Multicomponent mHealth Intervention for Large, Sustained Change in Multiple Diet and Activity Risk Behaviors: The Make Better Choices 2 Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e10528. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.R.; Piatt, G.A.; Sen, A.; Plegue, M.A.; De Michele, M.L.; Hafez, D.; Czuhajewski, C.M.; Buis, L.R.; Kaufman, N.; Richardson, C.; et al. The Effect of Technology-Mediated Diabetes Prevention Interventions on Weight: A Meta-Analysis. J. Med. Internet Res. 2017, 19, e76. [Google Scholar] [CrossRef] [PubMed]
- Tsaban, G.; Meir, A.Y.; Rinott, E.; Zelicha, H.; Kaplan, A.; Shalev, A.; Katz, A.; Rudich, A.; Tirosh, A.; Shelef, I.; et al. The effect of green Mediterranean diet on cardiometabolic risk; a randomised controlled trial. Heart 2020, 107, 1054–1061. [Google Scholar] [CrossRef]
- Schuppelius, B.; Peters, B.; Ottawa, A.; Pivovarova-Ramich, O. Time Restricted Eating: A Dietary Strategy to Prevent and Treat Metabolic Disturbances. Front. Endocrinol. 2021, 12, 683140. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Salvany, A.; Bacigalupe, A.; Carrasco, J.M.; Espelt, A.; Ferrando, J.; Borrell, C. del Grupo de Determinantes Sociales de Sociedad Española de Epidemiología. Propuestas de clase social neoweberiana y neomarxista a partir de la Clasificación Nacional de Ocupaciones 2011 [Proposals for social class classification based on the Spanish National Classification of Occupations 2011 using neo-Weberian and neo-Marxist approaches]. Gac. Sanit. 2013, 27, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Miró, O.; Martín-Sánchez, F.J.; Jacob, J.; Andueza, J.A.; Herrero, P.; Llorens, P. Valoración del grado de adherencia a la dieta mediterránea en pacientes con insuficiencia cardiaca: Estudio DIME-EAHFE [Evaluation of the degree of adherence to the Mediterranean diet in patients with heart failure: DIME-EAHFE study]. An. Sist. Sanit. Navar. 2016, 39, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; Ridder, H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry—ISAK: Lower Hutt, New Zealand, 2011. [Google Scholar]
- López-González, A.A.; Ramírez Manent, J.I.; Vicente-Herrero, M.T.; García Ruiz, E.; Albaladejo Blanco, M.; López Safont, N. Prevalence of diabesity in the Spanish working population: Influence of sociodemographic variables and tobacco consumption. An. Sist. Sanit. Navar. 2022, 45, e0977. [Google Scholar] [CrossRef]
- Mohebbi, V.; Aramayo, A.; Morales, J. Determination of scales related to cardiovascular risk and fatty liver in 5.370 spanish farmers. Med. Balear. 2021, 36, 26–33. [Google Scholar]
- Barchetta, I.; Dule, S.; Bertoccini, L.; Cimini, F.A.; Sentinelli, F.; Bailetti, D.; Marini, G.; Barbonetti, A.; Loche, S.; Cossu, E.; et al. The single-point insulin sensitivity estimator (SPISE) index is a strong predictor of abnormal glucose metabolism in overweight/obese children: A long-term follow-up study. J. Endocrinol. Investig. 2022, 45, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Leng, J.; Cao, W.; Tan, Z.; Meng, W.; Wang, S.; Xu, Y. Fatty liver disease index: A simple screening tool to facilitate diagnosis of nonalcoholic fatty liver disease in the Chinese population. Dig. Dis. Sci. 2013, 58, 3326–3334. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Xun, Y.; Lu, Z.; Shi, J.; Yu, C.; Li, Y. ZJU index: A novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci. Rep. 2015, 5, 16494. [Google Scholar] [CrossRef]
- Ratziu, V.; Giral, P.; Charlotte, F.; Bruckert, E.; Thibault, V.; Theodorou, I.; Khalil, L.; Turpin, G.; Opolon, P.; Poynard, T. Liver fibrosis in overweight patients. Gastroenterology 2000, 118, 1117–1123. [Google Scholar] [CrossRef]
- Burton, R.F. The waist-hip ratio: A flawed index. Ann. Hum. Biol. 2020, 47, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rode, E.; Stusser, B.; Cálix, W.; Orlandi, N.; Rodríguez, J.; Cubas-Dueñas, I.; Echevarría, R.; Álvarez, A. Concordancia diagnóstica entre siete definiciones de síndrome metabólico en adultos con sobrepeso y obesidad [Diagnostic concordance between seven definitions of metabolic syndrome in overweight and obese adults]. Rev. Peru. Med. Exp. Salud Publica 2017, 34, 19–27. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, G.; Serrano Ríos, M. Una nueva definición mundial del síndrome metabólico propuesta por la federación Internacional de Diabetes: Fundamento y resultados [A new international diabetes federation worldwide definition of the metabolic syndrome: The rationale and the results]. Rev. Esp. Cardiol. 2005, 58, 1371–1376, Erratum in Rev. Esp. Cardiol. 2006, 59, 185. [Google Scholar] [CrossRef]
- Sam, S.; Haffner, S.; Davidson, M.H.; D’Agostino RBSr Feinstein, S.; Kondos, G.; Perez, A.; Mazzone, T. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes Care 2009, 32, 1916–1920. [Google Scholar] [CrossRef]
- Nita, C.; Hancu, N.; Rusu, A.; Bala, C.; Roman, G. Hypertensive waist: First step of the screening for metabolic syndrome. Metab. Syndr. Relat. Disord. 2009, 7, 105–110. [Google Scholar] [CrossRef]
- Ponte-Negretti, C.I.; Isea-Perez, J.E.; Lorenzatti, A.J.; Lopez-Jaramillo, P.; Wyss, Q.F.S.; Pintó, X. Atherogenic Dyslipidemia in Latin America: Prevalence, causes and treatment: Expert’s position paper made by The Latin American Academy for the Study of Lipids (ALALIP) Endorsed by the Inter-American Society of Cardiology (IASC), the South American Society of Cardiology (SSC), the Pan-American College of Endothelium (PACE), and the International Atherosclerosis Society (IAS). Int. J. Cardiol. 2017, 243, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.R.; Wang, H.Y.; Chen, S.; Guo, X.F.; Li, Z.; Sun, Y.X. Estimate of prevalent diabetes from cardiometabolic index in general Chinese population: A community-based study. Lipids Health Dis. 2018, 17, 236. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Joint FAO/WHO/UNU Consultative Meeting of Experts on Energy and Protein Requirements (1981: Rome, Italy), Food and Agriculture Organization of the United Nations, World Health Organization & United Nations University. (1985). Energy and protein requirements: Report of a Joint FAO/WHO/UNU Consultative Meeting of Experts, [Rome, 5–17 October 1981]. Available online: https://apps.who.int/iris/handle/10665/40157 (accessed on 8 February 2023).
- López-González, Á.A.; Altisench Jané, B.; Masmiquel Comas, L.; Arroyo Bote, S.; González San Miguel, H.M.; Ramírez Manent, J.I. Impact of COVID-19 Lockdown on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in Adults: A before and after Pandemic Lockdown Longitudinal Study. Nutrients 2022, 14, 2795. [Google Scholar] [CrossRef]
- Hincapie Tabares, D.; Perez Carrillo, V.; Donado Gómez, J.H. Causas de Pérdidas de Pacientes Durante los Ensayos Clínicos con Asignación Aleatoria: Estudio Metaepidemiológico; Salud: Barranquilla, Colombia, 2019; Volume 35, pp. 57–71. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-55522019000100057&lng=en&nrm=iso (accessed on 8 February 2023).
- Afshin, A.; Babalola, D.; Mclean, M.; Yu, Z.; Ma, W.; Chen, C.-Y.; Arabi, M.; Mozaffarian, D. Information Technology and Lifestyle: A Systematic Evaluation of Internet and Mobile Interventions for Improving Diet, Physical Activity, Obesity, Tobacco, and Alcohol Use. J. Am. Heart Assoc. 2016, 5, e003058. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Stewart, R.A.H.; Benatar, J.R. Intensity and duration of lifestyle interventions for long-term weight loss and association with mortality: A meta-analysis of randomised trials. BMJ Open 2019, 9, e029966. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sánchez, L.; Gómez-Sánchez, M.; Lugones-Sánchez, C.; Rodríguez-Sánchez, E.; Tamayo-Morales, O.; Gonzalez-Sánchez, S.; Magallón-Botaya, R.; Ramirez-Manent, J.I.; Recio-Rodriguez, J.I.; Agudo-Conde, C.; et al. Long-Term Effectiveness of a Smartphone App and a Smart Band on Arterial Stiffness and Central Hemodynamic Parameters in a Population with Overweight and Obesity (Evident 3 Study): Randomised Controlled Trial. Nutrients 2022, 14, 4758. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.S.; Ali, E.; Harris, L.; Messow, C.M.; Brosnahan, N.T.; Thom, G.; McCombie, E.L.; Barnes, A.C.; Sattar, N.; Taylor, R.; et al. Antihypertensive medication needs and blood pressure control with weight loss in the Diabetes Remission Clinical Trial (DiRECT). Diabetologia 2021, 64, 1927–1938. [Google Scholar] [CrossRef]
- Lugones-Sanchez, C.; Recio-Rodriguez, J.I.; Agudo-Conde, C.; Repiso-Gento, I.; Adalia, E.G.; Ramirez-Manent, J.I.; Sanchez-Calavera, M.A.; Rodriguez-Sanchez, E.; Gomez-Marcos, M.A.; Garcia-Ortiz, L.; et al. EVIDENT 3 Investigators. Long-term Effectiveness of a Smartphone App Combined With a Smart Band on Weight Loss, Physical Activity, and Caloric Intake in a Population With Overweight and Obesity (Evident 3 Study): Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e30416. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Zolghadri, S.; Cholewka, A.; Myśliński, W. The Role of Intermittent Energy Restriction Diet on Metabolic Profile and Weight Loss among Obese Adults. Nutrients 2022, 14, 1509. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Larsen, T.M.; Westerterp-Plantenga, M.; Macdonald, I.; Martinez, J.A.; Handjiev, S.; Poppitt, S.; Hansen, S.; Ritz, C.; Astrup, A.; et al. Men and women respond differently to rapid weight loss: Metabolic outcomes of a multi-centre intervention study after a low-energy diet in 2500 overweight, individuals with pre-diabetes (PREVIEW). Diabetes Obes. Metab. 2018, 20, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Gupta, S.R.; Moustafa, A.F.; Chao, A.M. Sex/Gender Differences in Obesity Prevalence, Comorbidities, and Treatment. Curr. Obes. Rep. 2021, 10, 458–466. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef]
- Tricò, D.; Moriconi, D.; Berta, R.; Baldi, S.; Quinones-Galvan, A.; Guiducci, L.; Taddei, S.; Mari, A.; Nannipieri, M. Effects of Low-Carbohydrate versus Mediterranean Diets on Weight Loss, Glucose Metabolism, Insulin Kinetics and β-Cell Function in Morbidly Obese Individuals. Nutrients 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Dellis, D.; Tsilingiris, D.; Eleftheriadou, I.; Tentolouris, A.; Sfikakis, P.P.; Dellis, G.; Karanasiou, M.; Meimari, A.; Dimosthenopoulos, C.; Lazarou, S.; et al. Carbohydrate restriction in the morning increases weight loss effect of a hypocaloric Mediterranean type diet: A randomized, parallel group dietary intervention in overweight and obese subjects. Nutrition 2020, 71, 110578. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Kawada, N.; Japan Study Group Of Nafld Jsg-Nafld. The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 29, 3863. [Google Scholar] [CrossRef]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N. EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 3, 875. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Manent, J.I.; Martínez-Almoyna, E.; López, C.; Busquets-Cortés, C.; González San Miguel, H.; López-González, Á.A. Relationship between Insulin Resistance Risk Scales and Non-Alcoholic Fatty Liver Disease and Liver Fibrosis Scales in 219,477 Spanish Workers. Metabolites 2022, 12, 1093. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Zeng, L.Q.; Bai, H.; Li, J.; Zhou, J.; Zhou, G.Y.; Fang, C.W.; Wang, F.; Qin, X.J. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020, 36, 101635. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Llompart, I.; Ugarriza, L.; Martínez, J.A.; Tur, J.A.; et al. Increased Adherence to the Mediterranean Diet after Lifestyle Intervention Improves Oxidative and Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants 2022, 11, 1440. [Google Scholar] [CrossRef]
- Cai, H.; Qin, Y.-L.; Shi, Z.-Y.; Chen, J.-H.; Zeng, M.-J.; Zhou, W.; Chen, R.-Q.; Chen, Z.-Y. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef]
- Meir, A.Y.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Cantero, I.; Abete, I.; del Bas, J.M.; Caimari, A.; Arola, L.; Zulet, M.A.; Martinez, J.A. Changes in lysophospholipids and liver status after weight loss: The RESMENA study. Nutr. Metab. 2018, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, e128308. [Google Scholar] [CrossRef]
- Willems, A.E.M.; Sura-de Jong, M.; van Beek, A.P.; Nederhof, E.; van Dijk, G. Effects of macronutrient intake in obesity: A meta-analysis of low-carbohydrate and low-fat diets on markers of the metabolic syndrome. Nutr. Rev. 2021, 79, 429–444. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Dursun, M.; Besiroglu, H.; Otunctemur, A.; Ozbek, E. Association between cardiometabolic index and erectile dysfunction: A new index for predicting cardiovascular disease. Kaohsiung J. Med. Sci. 2016, 32, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Sun, G.; Jia, P.; Qian, H.; Sun, Y. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad Med. 2018, 130, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Xiong, H.; Zhang, H.; Hu, C.; Lu, S.; Zou, Y. Association between the cardiometabolic index and non-alcoholic fatty liver disease: Insights from a general population. BMC Gastroenterol. 2022, 22, 20. [Google Scholar] [CrossRef]
- Higashiyama, A.; Wakabayashi, I.; Okamura, T.; Kokubo, Y.; Watanabe, M.; Takegami, M.; Honda-Kohmo, K.; Okayama, A.; Miyamoto, Y. The Risk of Fasting Triglycerides and its Related Indices for Ischemic Cardiovascular Diseases in Japanese Community Dwellers: The Suita Study. J. Atheroscler. Thromb. 2021, 28, 1275–1288. [Google Scholar] [CrossRef]
- Ascaso, J.F.; Millán, J.; Hernández-Mijares, A.; Blasco, M.; Brea, Á.; Díaz, Á.; Pedro-Botet, J.; Pintó, X. Atherogenic Dyslipidaemia 2019. Consensus document of the Atherogenic Dyslipidaemia Group of the Spanish Arteriosclerosis Society. Clin. Investig. Arterioscler. 2020, 32, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Cortés, C.; López, C.; Paublini, H.; Arroyo Bote, S.; López-González, Á.A.; Ramírez-Manent, J.I. Relationship between Atherogenic Dyslipidaemia and Lipid Triad with Different Scales of Overweight and Obesity in 418,343 Spanish Workers. J. Nutr. Metab. 2022, 2022, 9946255. [Google Scholar] [CrossRef]
- Cheatham, S.W.; Stull, K.R.; Fantigrassi, M.; Motel, I. The efficacy of wearable activity tracking technology as part of a weight loss program: A systematic review. J. Sports Med. Phys. Fit. 2018, 58, 534–548. [Google Scholar] [CrossRef]
- Thivel, D.; Doucet, E.; Julian, V.; Cardenoux, C.; Boirie, Y.; Duclos, M. Nutritional compensation to exercise- vs. diet-induced acute energy deficit in adolescents with obesity. Physiol. Behav. 2017, 176, 159–164. [Google Scholar] [CrossRef]
- Danielsen, K.K.; Svendsen, M.; Mæhlum, S.; Sundgot-Borgen, J. Changes in body composition, cardiovascular disease risk factors, and eating behavior after an intensive lifestyle intervention with high volume of physical activity in severely obese subjects: A prospective clinical controlled trial. J. Obes. 2013, 2013, 325464. [Google Scholar] [CrossRef]
- Gast, J.; Campbell Nielson, A.; Hunt, A.; Leiker, J.J. Intuitive eating: Associations with physical activity motivation and BMI. Am. J. Health Promot. 2015, 29, e91–e99. [Google Scholar] [CrossRef]
- Castro, E.A.; Carraça, E.V.; Cupeiro, R.; López-Plaza, B.; Teixeira, P.J.; González-Lamuño, D.; Peinado, A.B. The Effects of the Type of Exercise and Physical Activity on Eating Behavior and Body Composition in Overweight and Obese Subjects. Nutrients 2020, 12, 557. [Google Scholar] [CrossRef] [Green Version]
| Metabolic Rate at Rest Based on Weight and Age | |||
|---|---|---|---|
| Age (Years) | Men | Women | |
| 0–2 | (60.9 × P) − 54 | (61.0 × P) − 51 | |
| 3–9 | (22.7 × P) + 495 | (22.5 × P) + 499 | |
| 10–17 | (17.5 × P) + 651 | (12.2 × P) + 746 | |
| 18–29 | (15.3 × P) + 679 | (14.7 × P) + 496 | |
| 30–59 | (11.6 × P) + 879 | (8.7 × P) + 829 | |
| ≥60 | (13.5 × P) + 487 | (10.5 × P) + 596 | |
| Total energy expenditure according to resting metabolic rate (RMR) | |||
| Physical activity intensity | Light | Moderate | High |
| Men | 1.55 | 1.78 | 2.10 |
| Women | 1.56 | 1.64 | 1.82 |
| Classification of activities by intensity | |||
| Light | People who spend several hours a day in sedentary activities, who do not regularly do sport, use the car to get around, spend most of their leisure time watching TV, reading, using the computer or video games. E.g., sitting or standing most of the time, walking on flat ground, doing light housework, board games, sewing, cooking, studying, driving, writing on a computer, office workers, etc. Those who performed light or moderate activity 2 or 3 times a week were classified in this section. | ||
| Moderate | Walking at 5 km/h, carrying out heavy housework (cleaning windows, sweeping, etc.), carpenters, construction workers (except hard jobs), chemical and electrical industries, mechanized agricultural tasks, golf, childcare, etc. Activities in which objects are moved or handled in a moderate way. They were classified in this section if more than 30 min/day of moderate activity and up to 20 min/week of vigorous activity were carried out. | ||
| High | People who walk long distances on a daily basis, use a bicycle to get around, carry out activities of great physical effort, or do sports that require a high level of effort for several hours. E.g., non-mechanized agricultural tasks, mining, forestry, digging, cutting firewood, mowing by hand, climbing, mountaineering, playing soccer, tennis, jogging, dancing, skiing, etc. They were classified in this section if they engaged in moderate or vigorous activity every day. | ||
| Men n = 963 | Women n = 1001 | ||
|---|---|---|---|
| % | % | p-Value | |
| 18–29 years | 15.6 | 17.9 | 0.185 |
| 30–39 years | 28.0 | 26.4 | |
| 40–49 years | 33.0 | 33.0 | |
| 50–59 years | 18.2 | 18.5 | |
| 60–69 years | 5.2 | 4.4 | |
| Social class I | 64.2 | 57.6 | <0.001 |
| Social class II | 11.0 | 13.5 | |
| Social class III | 24.8 | 28.9 | 0.490 |
| Non-smokers | 84.7 | 84.8 | |
| Smokers | 15.3 | 15.2 | |
| Low physical exercise | 25.5 | 33.2 | <0.001 |
| Moderate physical exercise | 27.7 | 27.7 | |
| High physical exercise | 46.7 | 39.1 | |
| Low adherence Mediterranean diet | 61.2 | 58.2 | 0.113 |
| High adherence Mediterranean diet | 38.8 | 41.8 |
| Men | n = 963 | Women | n = 1001 | |||
|---|---|---|---|---|---|---|
| Dietary Intervention Program | Basal | After | Basal | After | ||
| Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value | |
| Systolic blood pressure | 129.4 (13.8) | 127.7 (12.4) | <0.001 | 116.7 (15.0) | 116.0 (12.7) | <0.001 |
| Diastolic blood pressure | 81.4 (10.9) | 78.9 (9.6) | <0.001 | 76.6 (10.1) | 73.4 (9.6) | <0.001 |
| Glycaemia | 95.0 (17.8) | 92.0 (17.9) | <0.001 | 89.5 (11.7) | 88.3 (13.3) | <0.001 |
| Total cholesterol | 194.0 (35.2) | 190.3 (36.7) | <0.001 | 190.1 (34.7) | 187.0 (34.5) | <0.001 |
| HDL-c | 47.2 (11.9) | 50.0 (10.4) | <0.001 | 59.1 (12.9) | 59.1 (12.6) | 0.224 |
| LDL-c | 126.0 (30.9) | 123.6 (73.6) | <0.001 | 115.3 (31.0) | 111.5 (30.1) | <0.001 |
| Triglycerides | 117.8 (81.8) | 102.7 (56.7) | <0.001 | 81.9 (47.7) | 80.5 48.3) | <0.001 |
| Weight | 82.7 (14.8) | 80.4 (14.0) | <0.001 | 63.9 (13.3) | 63.7 (13.4) | <0.001 |
| Waist circumference | 91.8 (12.3) | 88.1 (12.3) | <0.001 | 77.7 (12.0) | 76.1 (11.7) | <0.001 |
| Hip circumference | 104.1 (8.6) | 99.8 (8.2) | <0.001 | 101.2 (10.2) | 97.6 (10.9) | <0.001 |
| BMI | 26.7 (4.3) | 26.0 (4.2) | <0.001 | 24.3 (4.9) | 24.1 (4.8) | <0.001 |
| WtHR | 0.52 (0.07) | 0.50 (0.07) | <0.001 | 0.48 (0.08) | 0.47 (0.07) | <0.001 |
| WthipR | 0.88 (0.07) | 0.88 (0.07) | 0.337 | 0.77 (0.07) | 0.77 (0.08) | 0.297 |
| % Fat mass | 20.1 (7.9) | 19.6 (7.4) | 0.01 | 28.9 (7.9) | 28.8 (7.8) | 0.084 |
| % Visceral fat | 8.2 (4.5) | 7.5 (4.9) | <0.001 | 4.7 (3.3) | 4.4 (3.1) | <0.001 |
| TyG index | 8.5 (0.6) | 8.3 (0.5) | 0.01 | 8.1 (0.5) | 8.1 (0.5) | 0.339 |
| TyG-BMI index | 226.6 (45.8) | 217.3 (42.3) | <0.001 | 197.8 (47.2) | 195.5 (46.4) | <0.001 |
| TyG-waist index | 779.0 (141.8) | 736.7 (131.2) | <0.001 | 631.8 (122.8) | 618.0 (119.1) | <0.001 |
| TyG-WtHR index | 4.4 (0.8) | 4.2 (0.8) | <0.001 | 3.9 (0.8) | 3.8 (0.8) | 0.02 |
| METS-IR | 40.3 (9.0) | 38.0 (8.2) | <0.001 | 33.6 (8.6) | 33.1 (8.2) | <0.001 |
| SPISE-IR | 1.7 (0.5) | 1.6 (0.5) | <0.001 | 1.4 (0.5) | 1.3 (0.5) | <0.001 |
| LAP | 40.8 (44.0) | 30.0 (29.4) | <0.001 | 20.9 (24.3) | 19.4 (23.2) | <0.001 |
| FLI | 41.1 (29.4) | 33.9 (29.0) | <0.001 | 17.6 (23.3) | 15.5 (20.9) | <0.001 |
| HSI | 36.4 (6.4) | 33.7 (6.5) | <0.001 | 36.8 (7.2) | 33.8 (6.2) | <0.001 |
| FLD | 31.7 (5.4) | 30.8 (5.5) | <0.001 | 28.1 (5.6) | 27.7 (5.3) | <0.001 |
| BAAT | 1.1 (1.1) | 0.8 (0.9) | <0.001 | 0.8 (1.0) | 0.6 (0.8) | <0.001 |
| nº factors MS ATP III | 1.5 (1.4) | 1.1 (1.2) | <0.001 | 1.0 (1.2) | 0.8 (1.1) | <0.001 |
| nº factors MS JIS | 1.6 (1.4) | 1.2 (1.3) | <0.001 | 1.2 (1.3) | 1.0 (1.2) | <0.001 |
| CMI | 1.6 (1.7) | 1.2 (1.0) | <0.001 | 0.8 (0.9) | 0.7 (0.8) | <0.001 |
| Men | n = 963 | Women | n = 1001 | |||||
|---|---|---|---|---|---|---|---|---|
| Dietary Intervention Program | Basal | After | Basal | After | ||||
| % | % | p-Value | Difference % | % | % | p-Value | Difference % | |
| Hypertension | 25.5 | 21.8 | <0.001 | −14.5 | 12.1 | 6.7 | <0.001 | −44.6 |
| Glycaemia: >100 mg/dL | 29.6 | 18.4 | <0.001 | −37.8 | 11.7 | 9.5 | <0.001 | −18.8 |
| Total cholesterol: high | 43.3 | 37.7 | <0.001 | −12.9 | 38.8 | 32.4 | <0.001 | −16.5 |
| LDL-c high | 46.4 | 35.8 | <0.001 | −22.8 | 28.9 | 25.0 | <0.001 | −13.5 |
| Triglycerides: high | 18.7 | 16.2 | <0.001 | −13.4 | 6.7 | 5.9 | <0.001 | −11.9 |
| BMI: overweight/obesity | 63.2 | 52.0 | <0.001 | −17.7 | 35.4 | 32.2 | <0.001 | −9.0 |
| WtHR: high | 58.9 | 42.0 | <0.001 | −28.7 | 30.6 | 26.9 | <0.001 | −12.1 |
| WtHipR | 18.1 | 16.2 | <0.001 | −10.5 | 16.0 | 13.3 | <0.001 | −16.9 |
| % Fat mass: very high | 15.0 | 12.5 | <0.001 | −16.7 | 9.9 | 8.0 | <0.001 | −19.2 |
| Visceral fat: high | 23.7 | 19.0 | <0.001 | −19.8 | 3.7 | 2.6 | <0.001 | −29.7 |
| TyG index: high | 23.4 | 15.6 | <0.001 | −33.3 | 7.4 | 7.1 | <0.001 | −4.1 |
| Triglycerides/HDL-c: high | 38.3 | 32.1 | <0.001 | −16.2 | 12.9 | 11.7 | <0.001 | −9.3 |
| METS-IR: high | 14.0 | 9.7 | <0.001 | −30.7 | 5.7 | 4.7 | <0.001 | −17.5 |
| SPISE-IR: high | 16.8 | 11.5 | <0.001 | −31.5 | 6.3 | 5.7 | <0.001 | −9.5 |
| LAP: high | 28.7 | 20.2 | <0.001 | −29.6 | 11.3 | 10.2 | <0.001 | −9.7 |
| FLI: high | 28.4 | 22.5 | <0.001 | −20.8 | 8.4 | 6.6 | <0.001 | −21.4 |
| HSI: high | 44.9 | 39.5 | <0.001 | −12.0 | 27.8 | 25.6 | <0.001 | −7.9 |
| ZJU: high | 35.8 | 29.6 | <0.001 | −17.3 | 24.2 | 21.7 | <0.001 | −10.3 |
| FLD: high | 15.0 | 13.9 | <0.001 | −7.3 | 7.3 | 5.7 | <0.001 | −21.9 |
| BAAT: high | 31.8 | 21.5 | <0.001 | −32.4 | 12.8 | 9.4 | <0.001 | −26.6 |
| MS NCEP ATPIII | 21.8 | 12.8 | <0.001 | −41.3 | 8.7 | 7.7 | <0.001 | −11.5 |
| MS IDF | 23.4 | 13.4 | <0.001 | −42.7 | 10.8 | 8.6 | <0.001 | −20.4 |
| MS JIS | 26.2 | 15.9 | <0.001 | −39.3 | 11.1 | 9.3 | <0.001 | −16.2 |
| Hypertriglyceridemic waist | 12.8 | 9.0 | <0.001 | −29.7 | 5.0 | 4.5 | <0.001 | −10 |
| Hypertensive waist | 24.9 | 18.1 | <0.001 | −27.3 | 13.5 | 9.6 | <0.001 | −28.9 |
| Atherogenic dyslipidemia | 12.8 | 8.4 | <0.001 | −34.4 | 4.1 | 3.8 | <0.001 | −7.3 |
| Lipid triad | 7.8 | 3.4 | <0.001 | −56.4 | 2.1 | 1.5 | <0.001 | −28.6 |
| Men | 30–39 Years | 40–49 Years | 50–59 Years | 60–69 Years | Social Class II | Social Class III | Smokers | Moderate PHE | Low PHE | Low MD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Hypertension | 1.8 (1.7–1.9) | 1.2 (1.1–1.3) | 1.5 (1.4–1.6) | 2.7 (2.5–2.9) | 3.9 (3.7–4.2) | ns | 1.3 (1.1–1.4) | 1.1 (1.0–1.2) | 1.2 (1.0–1.4) | 1.9 (1.7–2.1) | 1.3 (1.1–1.5) |
| Obesity (BMI) | 1.4 (1.3–1.4) | 1.4 (1.3–1.6) | 1.9 (1.7–2.1) | 2.8 (2.6–3.1) | 3.7 (3.5–3.9) | 1.2 (1.1–1.4) | 1.5 (1.3–1.7) | 0.9 (0.9–1.0) | 1.3 (1.2–1.4) | 2.5 (2.1–2.9) | 1.6 (1.4–1.9) |
| Fat mass: very high | 1.7 (1.6–1.8) | 1.5 (1.4–1.7) | 1.8 (1.6–1.9) | 2.2 (2.0–2.4) | 3.1 (2.8–3.4) | 1.1 (1.0–1.2) | 1.4 (1.3–1.6) | 1.1 (1.0–1.1) | 1.5 (1.4–1.6) | 3.8 (3.4–4.3) | 1.7 (1.6–1.8) |
| Visceral fat: high | 1.2 (1.2–1.3) | 1.4 (1.3–1.6) | 1.7 (1.6–1.9) | 2.1 (2.0–2.2) | 3.3 (32.1–3.6) | ns | 1.5 (1.4–1.6) | 1.2 (1.0–1.4) | 1.7 (1.5–1.9) | 3.0 (2.7–3.3) | 1.9 (1.7–2.2) |
| TyG: high | 1.9 (1.8–1.9) | 1.1 (1.0–1.2) | 1.4 (1.2–1.6) | 1.8 (1.5–2.0) | 2.2 (2.0–2.5) | 1.2 (1.0–1.4) | 1.6 (1.4–1.9) | ns | 1.6 (1.4–1.8) | 2.0 (1.7–2.2) | 1.8 (1.7–1.9) |
| TG/HDL: high | 1.8 (1.7–1.9) | 1.3 (1.2–1.5) | 1.6 (1.4–1.8) | 2.0 (1.9–2.2) | 2.5 (2.2 -2.7) | ns | 1.5 (1.3–1.6) | ns | 1.5 (1.4–1.7) | 2.6 (2.4–2.8) | 1.6 (1.4–1.7) |
| METS-IR: high | 2.2 (2.0–2.3) | 1.2 (1.0–1.3) | 1.4 (1.3–1.5) | 1.7 (1.6–1.9) | 2.1 (1.8–2.4) | ns | 1.7 (1.6–1.8) | ns | 1.6 (1.4–1.8) | 2.1 (2.0–2.2) | 1.8 (1.7–1.8) |
| SPISE: high | 1.7 (1.6–1.8) | ns | 1.2 (1.1–1.4) | 1.6 (1.5–1.8) | 2.0 (1.8–2.2) | ns | 1.4 (1.3–1.6) | 1.1 (1.0–1.2) | 1.8 (1.7–1.9) | 2.4 (2.1–2.6) | 1.5 (1.4–1.6) |
| LAP: high | 1.6 (1.5–1.7) | 1.2 (1.1–1.3) | 1.8 (1.6–2.1) | 2.0 (1.8–2.2) | 2.4 (2.1–2.7) | ns | 1.5 (1.3–1.7) | ns | 1.9 (1.6–2.1) | 2.9 (2.6–3.1) | 2.0 (1.8–2.2) |
| FLI: high | 1.4 (1.3–1.5) | ns | 1.3 (1.1–1.5) | 1.8 (1.5–2.1) | 2.9 (2.6–3.3) | 1.1 (1.0–1.2) | 1.4 (1.3–1.5) | ns | 1.5 (1.4–1.6) | 2.8 (2.7–3.0) | 1.4 (1.3–1.6) |
| HSI: high | 1.5 (1.4–1.5) | ns | 1.4 (1.3–1.6) | 1.8 (1.7–1.9) | 2.5 (2.3–2.7) | ns | 1.7 (1.5–1.9) | ns | 1.6 (1.4–1.7) | 2.5 (2.3–2.7) | 1.6 (1.5–1.8) |
| ZJU: high | 1.3 (1.2–1.4) | ns | 1.5 (1.4–1.7) | 2.1 (1.9–2.4) | 2.9 (2.6–3.1) | ns | 1.4 (1.2–1.5) | ns | 1.9 (1.7–2.2) | 2.9 (2.8–3.0) | 1.4 (1.3–1.6) |
| FLD: high | 1.6 (1.5–1.7) | 1.1 (1.0–1.2) | 1.4 (1.3–1.6) | 1.9 (1.7–2.2) | 2.5 (2.3–2.7) | ns | 1.3 (1.2–1.3) | ns | 1.4 (1.2–1.6) | 2.2 (2.0–2.4) | 1,7 (1.5–1.9) |
| MS ATPIII | 2.3 (2.2–2.4) | 1.8 (1.6–1.9) | 2.4 (2.2–2.5) | 2.6 (2.5–2.8) | 3.3 (3.1–3.5) | 1.4 (1.2–1.6) | 1.9 (1.7–2.1) | 1.3 (1.1–1.4) | 1.6 (1.3–1.8) | 2.7 (2.4–2.9) | 1.9 (1.6–2.1) |
| MS IDF | 2.2 (2.1–2.3) | 1.6 (1.4–1.8) | 1.9 (1.8–2.1) | 2.3 (2.1–2.6) | 2.7 (2.5–3.0) | 1.3 (1.1–1.5) | 2.0 (1.9–2.2) | 1.2 (1.0–1.3) | 1.4 (1.3–1.6) | 2.8 (2.7–3.0) | 1.7 (1.6–1.9) |
| MS JIS | 2.4 (2.3–2.5) | 1.4 (1.3–1.6) | 1.8 (1.7–2.0) | 2.2 (2.0–2.5) | 2.8 (2.6–3.1) | 1.2 (1.1–1.4) | 1.6 (1.4–1.8) | 1.3 (1.2–1.3) | 1.5 (1.3–1.7) | 3.0 (2.8–3.3) | 1.3 (1.2–1.4) |
| AD | 2.8 (2.7–2.9) | 1.3 (1.1–1.6) | 1.7 (1.5–1.9) | 2.2 (1.9–2.6) | 2.9 (2.6–3.3) | 1.3 (1.1–1.5) | 1.6 (1.5–1.8) | 1.4 (1.3–1.5) | 1.9 (1.7–2.1) | 2.9 (2.7–3.2) | 1.8 (1.7–1.9) |
| Men | 30–39 Years | 40–49 Years | 50–59 Years | 60–69 Years | Social Class II | Social Class III | Smokers | Moderate PHE | Low PHE | Low MD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Hypertension | 1.7 (1.5–1.9) | 1.1 (1.0–1.2) | 1.6 (1.5–1.7) | 2.4 (2.2–2.6) | 3.6 (3.4–3.8) | ns | 1.3 (1.1–1.5) | 1.1 (1.0–1.3) | 1.3 (1.2–1.4) | 2.2 (2.0–2.4) | 1.6 (1.5–1.7) |
| Obesity (BMI) | 1.5 (1.4–1.7) | 1.3 (1.2–1.4) | 1.8 (1.6–2.0) | 2.6 (2.4–2.8) | 3.5 (3.3–3.8) | 1.3 (1.1–1.4) | 1.6 (1.5–1.7) | 0.8 (0.8–0.9) | 1.4 (1.3–1.5) | 3.0 (2.8–3.2) | 1.9 (1.8–2.0) |
| Fat mass: very high | 1.4 (1.3–1.4) | 1.4 (1.3–1.6) | 1.7 (1.6–1.8) | 2.3 (2.1–2.5) | 3.3 (3.0–3.5) | 1.2 (1.1–1.3) | 1.4 (1.3–1.5) | 1.3 (1.2–1.3) | 1.6 (1.5–1.7) | 2.9 (2.7–3.1) | 1.6 (1.5–1.7) |
| Visceral fat: high | 1.3 (1.1–1.4) | 1.3 (1.2–1.5) | 1.7 (1.6–1.9) | 2.4 (2.2–2.6) | 3.0 (2.8–3.2) | 1.1 (1.0–1.3) | 1.3 (1.1–1.4) | 1.2 (1.1–1.3) | 1.7 (1.5–1.9) | 3.4 (3.3–3.6) | 1.9 (1.8–2.1) |
| TyG: high | 1.8 (1.6–2.0) | ns | 1.5 (1.4–1.6) | 2.2 (2.1–2.4) | 2.9 (2.8–3.0) | ns | 1.2 (1.1–1.3) | ns | 1.7 (1.6–1.7) | 3.3 (3.1–3.5) | 1.8 (1.7–1.9) |
| TG/HDL: high | 1.7 (1.6–1.8) | 1.3 (1.2–1.5) | 1.5 (1.4–1.6) | 2.1 (2.0–2.2) | 2.7 (2.5–2.8) | 1.2 (1.1–1.3) | 1.7 (1.6–1.8) | ns | 1.7 (1.6–1.8) | 3.1 (3.0–3.2) | 2.0 (1.9–2.1) |
| METS-IR: high | 2.1 (2.0–2.3) | 1.1 (1.0–1.3) | 1.4 (1.3–1.5) | 1.9 (1.8–2.0) | 2.3 (2.2–2.5) | ns | 1.4 (1.3–1.5) | 1.1 (1.0–1.2) | 1.5 (1.4–1.6) | 2.9 (2.6–3.2) | 1.7 (1.5–1.8) |
| SPISE: high | 1.6 (1.4–1.7) | ns | 1.2 (1.1–1.3) | 2.2 (2.1–2.3) | 2.4 (2.2–2.5) | ns | 1.5 (1.4–1.6) | 1.2 (1.1–1.3) | 1.8 (1.6–1.9) | 2.7 (2.5–2.8) | 1.6 (1.4–1.8) |
| LAP: high | 1.5 (1.4–1.6) | 1.3 (1.2–1.4) | 1.6 (1.5–1.7) | 2.2 (2.0–2.3) | 2.6 (2.5–2.8) | ns | 1.3 (1.2–1.4) | ns | 1.5 (1.4–1.6) | 3.3 (3.2–3.4) | 1.9 (1.7–2.1) |
| FLI: high | 1.3 (1.2–1.4) | ns | 1.3 (1.2–1.4) | 1.9 (1.8–2.0) | 2.4 (2.2–2.6) | ns | 1.5 (1.4–1.7) | ns | 1.6 (1.5–1.7) | 3.0 (2.8–3.2) | 1.8 (1.6–2.0) |
| HSI: high | 1.5 (1.3–1.7) | ns | 1.2 (1.1–1.3) | 1.8 (1.7–1.9) | 2.5 (2.4–2.5) | ns | 1.6 (1.4–1.7) | ns | 1.5 (1.4–1.6) | 2.7 (2.5–2.9) | 1.6 (1.5–1.7) |
| ZJU: high | 1.4 (1.3–1.5) | ns | 1.4 (1.2–1.5) | 2.0 (1.8–2.1) | 2.9 (2.7–3.0) | ns | 1.4 (1.3–1.5) | ns | 1.3 (1.2–1.3) | 2.5 (2.4–2.6) | 1.4 (1.3–1.5) |
| FLD: high | 1.5 (1.4–1.6) | 1.2 (1.1–1.3) | 1.7 (1.6–1.9) | 2.0 (1.8–2.1) | 2.6 (2.4–2.8) | ns | 1.3 (1.2–1.5) | ns | 1.5 (1.4–1.6) | 2.6 (2.4–2.8) | 1.7 (1.5–1.9) |
| MS ATPIII | 2.2 (2.1–2.4) | 1.9 (1.8–2.1) | 2.3 (2.1–2.5) | 2.6 (2.5–2.8) | 3.1 (3.0–3.2) | 1.5 (1.4–1.6) | 2.0 (1.8–2.1) | 1.4 (1.3–1.5) | 1.6 (1.5–1.8) | 3.1 (3.0–3.2) | 1.9 (1.8–2.1) |
| MS IDF | 2.0 (1.9–2.1) | 1.5 (1.4–1.5) | 2.0 (1.8–2.1) | 2.5 (2.4–2.6) | 2.9 (2.8–3.0) | 1.4 (1.4–1.5) | 1.8 (1.7–1.9) | 1.5 (1.4–1.6) | 1.7 (1.6–1.8) | 3.2 (3.0–3.4) | 2.0 (1.9–2.1) |
| MS JIS | 2.3 (2.2–2.5) | 1.5 (1.4–1.6) | 1.9 (1.7–2.0) | 2.4 (2.3–2.5) | 3.1 (3.0–3.3) | 1.3(1.1–1.4) | 1.8 (1.7–1.9) | 1.4 (1.3–1.5) | 1.9 (1.7–2.1) | 2.9 (2.7–3.2) | 1.6 (1.5–1.7) |
| AD | 2.7 (2.6–2.8) | 1.3 (1.2–1.5) | 1.8 (1.6–1.9) | 2.4 (2.3–2.6) | 2.9 (2.7–3.0) | 1.4 (1.4–1.5) | 2.0 (1.8–2.1) | 1.5 (1.4–1.6) | 1.8 (1.5–1.7) | 3.4 (3.3–3.6) | 2.1 (2.0–2.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Manent, J.I.; Tomás-Gil, P.; Martí-Lliteras, P.; Coll Villalonga, J.L.; Martínez-Almoyna Rifá, E.; López-González, Á.A. Dietary Intervention on Overweight and Obesity after Confinement by COVID-19. Nutrients 2023, 15, 912. https://doi.org/10.3390/nu15040912
Ramírez-Manent JI, Tomás-Gil P, Martí-Lliteras P, Coll Villalonga JL, Martínez-Almoyna Rifá E, López-González ÁA. Dietary Intervention on Overweight and Obesity after Confinement by COVID-19. Nutrients. 2023; 15(4):912. https://doi.org/10.3390/nu15040912
Chicago/Turabian StyleRamírez-Manent, José Ignacio, Pilar Tomás-Gil, Pau Martí-Lliteras, Josep Lluis Coll Villalonga, Emilio Martínez-Almoyna Rifá, and Ángel Arturo López-González. 2023. "Dietary Intervention on Overweight and Obesity after Confinement by COVID-19" Nutrients 15, no. 4: 912. https://doi.org/10.3390/nu15040912
APA StyleRamírez-Manent, J. I., Tomás-Gil, P., Martí-Lliteras, P., Coll Villalonga, J. L., Martínez-Almoyna Rifá, E., & López-González, Á. A. (2023). Dietary Intervention on Overweight and Obesity after Confinement by COVID-19. Nutrients, 15(4), 912. https://doi.org/10.3390/nu15040912






