Hindmilk as a Rescue Therapy in Very Preterm Infants with Suboptimal Growth Velocity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Enteral Nutrition
2.3. Milk Separation
2.4. Statistical Analyses
3. Results
3.1. Study Population
3.2. Enteral Nutrition and Milk Content
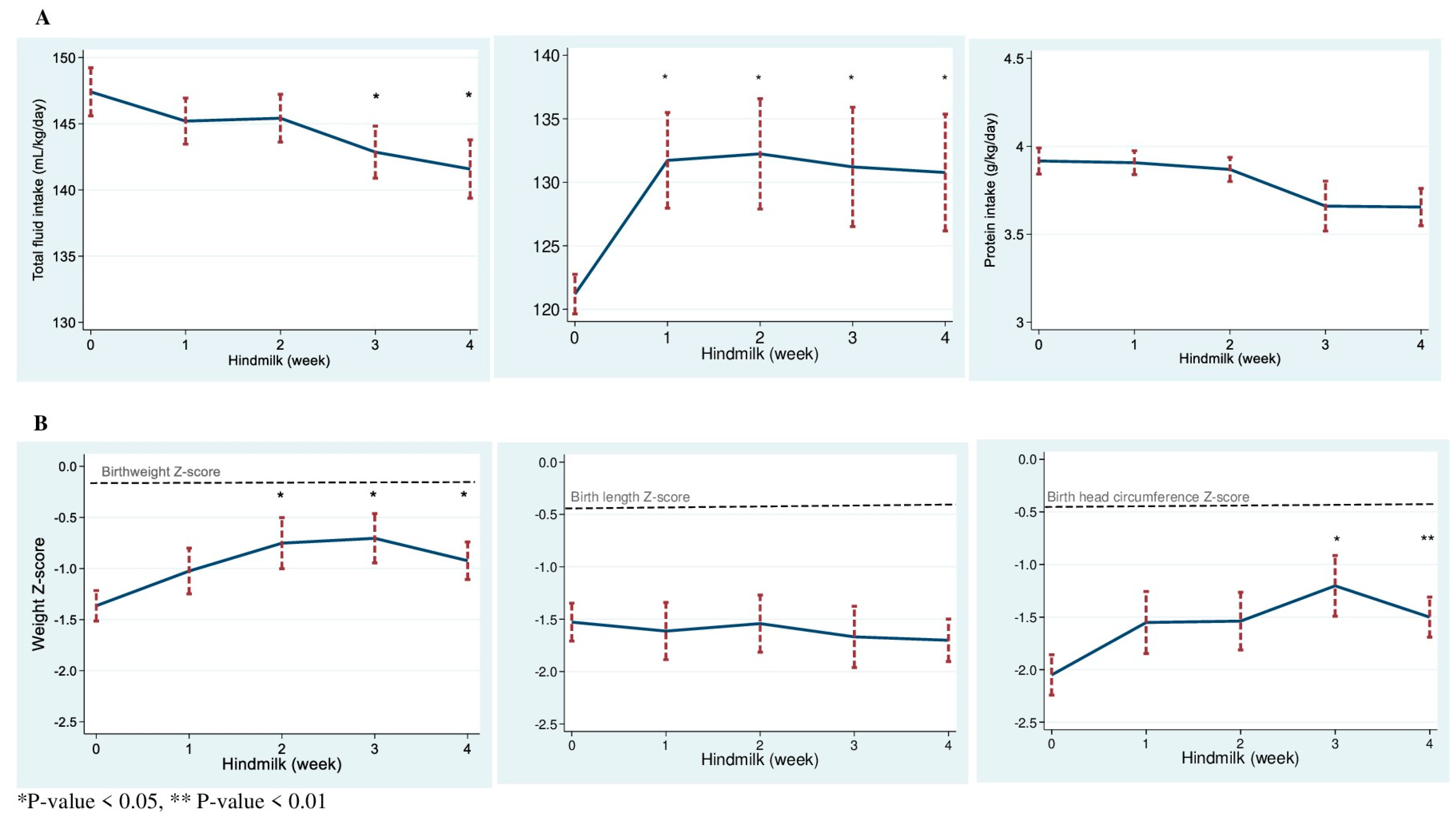
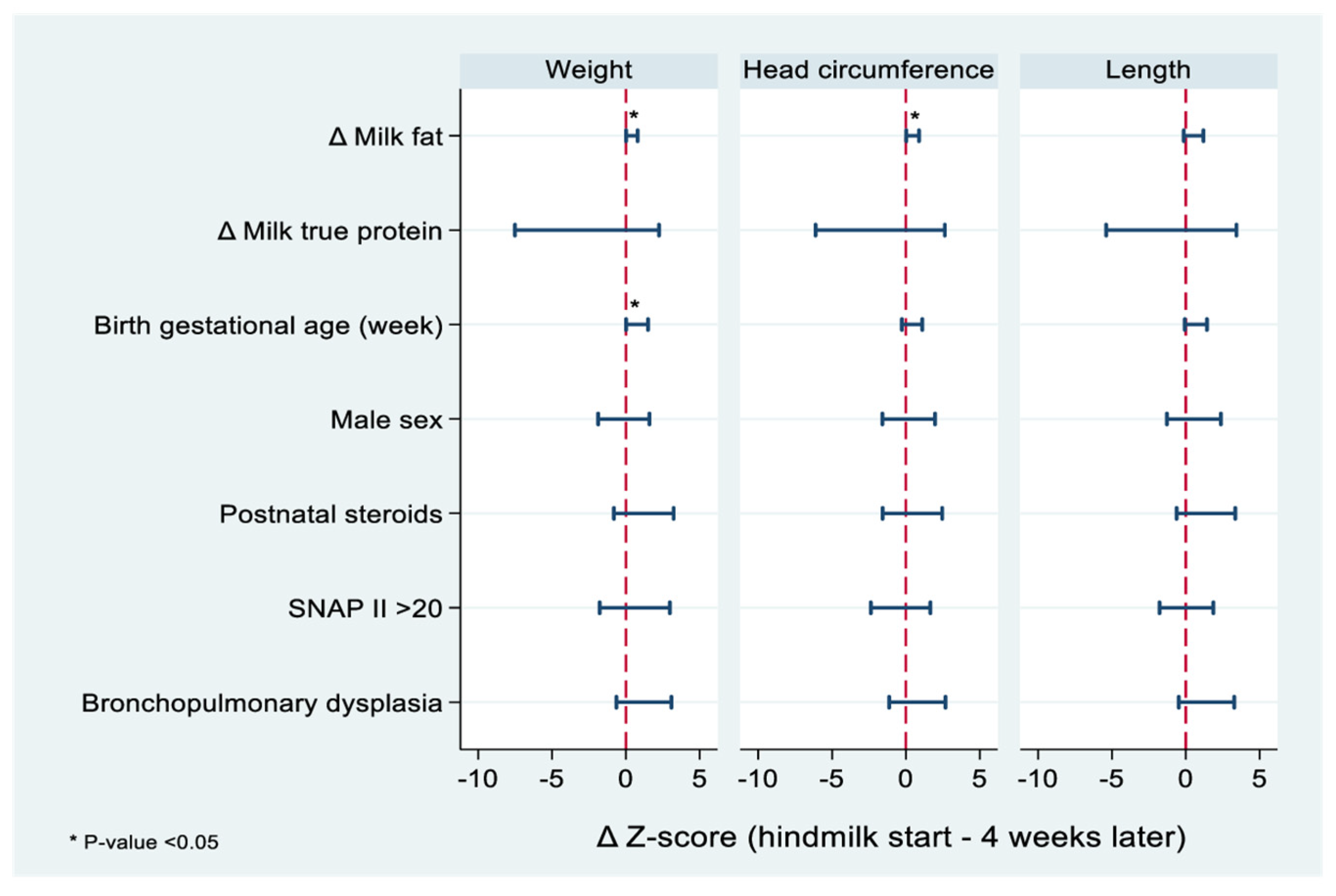
3.3. Growth and Anthropometric Measurements
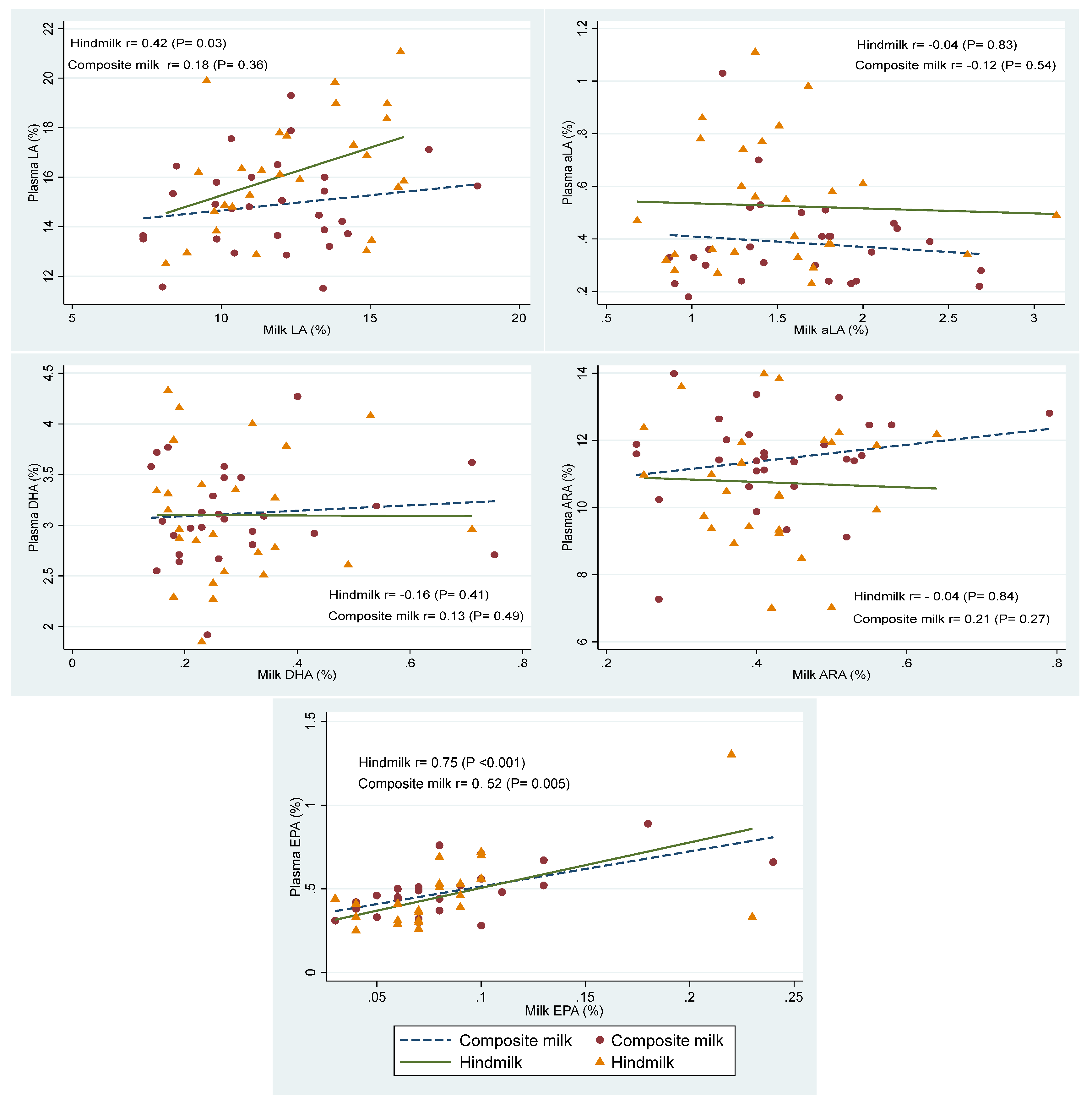
3.4. Biochemical Outcomes
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makker, K.; Ji, Y.; Hong, X.; Wang, X. Antenatal and neonatal factors contributing to extra uterine growth failure (EUGR) among preterm infants in Boston Birth Cohort (BBC). J. Perinatol. 2021, 41, 1025–1032. [Google Scholar] [CrossRef]
- Zozaya, C.; Avila-Alvarez, A.; Couce, M.L.; Rodrigo, F.G.M.; Arruza, L.; Fernandez-Perez, C.; Castro, A.; Cuesta, M.T.; Vacas, B.; Vento, M.; et al. Cohort study showed that growth rate increment has not been enough to prevent growth retardation of preterm infants and raised concerns about unbalanced growth. Acta Paediatr. 2019, 108, 1793–1800. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.K.; Kennedy, K.; Castaneda-Gutierrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Latulippe, M.E.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.H.; et al. Postnatal growth in preterm infants and later health outcomes: A systematic review. Acta Paediatr. 2015, 104, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Agostoni, C.; Bergmann, R.; Ritzenthaler, K.; Shamir, R. Physiological aspects of human milk lipids and implications for infant feeding: A workshop report. Acta Paediatr. 2011, 100, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G.; Horner, M.R.; Bezerra, V.L.; Campanate, M.L. Variation in major constituents of fore- and hindmilk of Brazilian women. J. Trop. Pediatr. 1982, 28, 303–305. [Google Scholar] [CrossRef]
- Valentine, C.J.; Hurst, N.M.; Schanler, R.J. Hindmilk improves weight gain in low-birth-weight infants fed human milk. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 474–477. [Google Scholar] [CrossRef]
- Bishara, R.; Dunn, M.S.; Merko, S.E.; Darling, P. Nutrient composition of hindmilk produced by mothers of very low birth weight infants born at less than 28 weeks’ gestation. J. Hum. Lact. 2008, 24, 159–167. [Google Scholar] [CrossRef]
- Uauy, R.; Hoffman, D.R.; Peirano, P.; Birch, D.G.; Birch, E.E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Fenton, T.R.; Griffin, I.J.; Hoyos, A.; Groh-Wargo, S.; Anderson, D.; Ehrenkranz, R.A.; Senterre, T. Accuracy of preterm infant weight gain velocity calculations vary depending on method used and infant age at time of measurement. Pediatr. Res. 2019, 85, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Hussey, E.K.; Portelli, S.; Fossler, M.J.; Gao, F.; Harris, W.S.; Blum, R.A.; Lates, C.D.; Gould, E.; Abu-Baker, O.; Johnson, S.; et al. Relative Bioavailability of an Emulsion Formulation for Omega-3-Acid Ethyl Esters Compared to the Commercially Available Formulation: A Randomized, Parallel-Group, Single-Dose Study Followed by Repeat Dosing in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2012, 1, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.Y.; Mangani, C.; Ashorn, P.; Harris, W.S.; Maleta, K.; Dewey, K.G. Breast milk from women living near Lake Malawi is high in docosahexaenoic acid and arachidonic acid. Prostaglandins Leukot. Essent. Fat. Acids 2015, 95, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Saadin, K.; Bliden, K.P.; Harris, W.S.; Dinh, B.; Sharma, T.; Tantry, U.S.; Gurbel, P.A. Risk factors associated with plasma omega-3 fatty acid levels in patients with suspected coronary artery disease. Prostaglandins Leukot. Essent. Fat. Acids 2016, 113, 40–45. [Google Scholar] [CrossRef]
- Koba, S.; Takao, T.; Shimizu, F.; Ogawa, M.; Ishii, Y.; Yokota, Y.; Furuyama, F.; Tsunoda, F.; Shoji, M.; Harris, W.S.; et al. Comparison of plasma levels of different species of trans fatty acids in Japanese male patients with acute coronary syndrome versus healthy men. Atherosclerosis 2019, 284, 173–180. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir Crit Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Fenton, T.R. Fenton Preterm Growth Chart: Calculators and Apps. 2022. Available online: https://live-ucalgary.ucalgary.ca/resource/preterm-growth-chart/calculators-apps (accessed on 2 September 2022).
- Doyle, L.W.; Davis, P.G.; Morley, C.J.; McPhee, A.; Carlin, J.B.; Investigators, D.S. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: A multicenter, international, randomized, controlled trial. Pediatrics 2006, 117, 75–83. [Google Scholar] [CrossRef]
- Embleton, N.E.; Pang, N.; Cooke, R.J. Postnatal malnutrition and growth retardation: An inevitable consequence of current recommendations in preterm infants? Pediatrics 2001, 107, 270–273. [Google Scholar] [CrossRef]
- Rochow, N.; Fusch, G.; Choi, A.; Chessell, L.; Elliott, L.; McDonald, K.; Kuiper, E.; Purcha, M.; Turner, S.; Chan, E.; et al. Target fortification of breast milk with fat, protein, and carbohydrates for preterm infants. J. Pediatr. 2013, 163, 1001–1007. [Google Scholar] [CrossRef]
- Rigourd, V.; Lopera, I.; Cata, F.; Benoit, G.; Jacquemet, B.; Lapillonne, A. Role of Daily Milk Volume and Period of Lactation in Nutrient Content of Human Milk: Results from a Prospective Study. Nutrients 2020, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [PubMed]
- Slusher, T.; Hampton, R.; Bode-Thomas, F.; Pam, S.; Akor, F.; Meier, P. Promoting the exclusive feeding of own mother’s milk through the use of hindmilk and increased maternal milk volume for hospitalized, low birth weight infants (<1800 grams) in Nigeria: A feasibility study. J. Hum. Lact. 2003, 19, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Ogechi, A.A.; William, O.; Fidelia, B.T. Hindmilk and weight gain in preterm very low-birthweight infants. Pediatr. Int. 2007, 49, 156–160. [Google Scholar] [CrossRef]
- Lindquist, S.; Hernell, O. Lipid digestion and absorption in early life: An update. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 314–320. [Google Scholar] [CrossRef]
- Roberts, S.B.; Young, V.R. Energy costs of fat and protein deposition in the human infant. Am. J. Clin. Nutr. 1988, 48, 951–955. [Google Scholar] [CrossRef]
- Maas, C.; Mathes, M.; Bleeker, C.; Vek, J.; Bernhard, W.; Wiechers, C.; Peter, A.; Poets, C.F.; Franz, A.R. Effect of Increased Enteral Protein Intake on Growth in Human Milk-Fed Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2017, 171, 16–22. [Google Scholar] [CrossRef]
- Tonkin, E.L.; Collins, C.T.; Miller, J. Protein Intake and Growth in Preterm Infants: A Systematic Review. Glob. Pediatr. Health 2014, 1, 2333794X14554698. [Google Scholar] [CrossRef]
- Fenton, T.R.; Groh-Wargo, S.; Gura, K.; Martin, C.R.; Taylor, S.N.; Griffin, I.J.; Rozga, M.; Moloney, L. Effect of Enteral Protein Amount on Growth and Health Outcomes in Very-Low-Birth-Weight Preterm Infants: Phase II of the Pre-B Project and an Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2021, 121, 2287–2300.e12. [Google Scholar] [CrossRef]
- Alburaki, W.; Yusuf, K.; Dobry, J.; Sheinfeld, R.; Alshaikh, B. High Early Parenteral Lipid in Very Preterm Infants: A Randomized-Controlled Trial. J. Pediatr. 2021, 228, 16–23.e11. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, N.J.; Kim, S.Y. Safety and Efficacy of Early High Parenteral Lipid Supplementation in Preterm Infants: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1535. [Google Scholar] [CrossRef] [PubMed]
- Beauport, L.; Schneider, J.; Faouzi, M.; Hagmann, P.; Huppi, P.S.; Tolsa, J.F.; Truttmann, A.C.; Fischer Fumeaux, C.J. Impact of Early Nutritional Intake on Preterm Brain: A Magnetic Resonance Imaging Study. J. Pediatr. 2017, 181, 29–36.e21. [Google Scholar] [CrossRef] [PubMed]
- Lepping, R.J.; Honea, R.A.; Martin, L.E.; Liao, K.; Choi, I.Y.; Lee, P.; Papa, V.B.; Brooks, W.M.; Shaddy, D.J.; Carlson, S.E.; et al. Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev. Psychobiol. 2019, 61, 5–16. [Google Scholar] [CrossRef]
- Böckmann, K.A.; von Stumpff, A.; Bernhard, W.; Shunova, A.; Minarski, M.; Frische, B.; Warmann, S.; Schleicher, E.; Poets, C.F.; Franz, A.R. Fatty acid composition of adipose tissue at term indicates deficiency of arachidonic and docosahexaenoic acid and excessive linoleic acid supply in preterm infants. Eur. J. Nutr. 2021, 60, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Udell, T.; Gibson, R.A.; Makrides, M.; Grp, P.S. The effect of alpha-linolenic acid and linoleic acid on the growth and development of formula-fed infants: A systematic review and meta-analysis of randomized controlled trials. Lipids 2005, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C.; International Society for the Study of Fatty Acids and Lipids. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Carlson, S.E.; Schipper, L.; Brenna, J.T.; Agostoni, C.; Calder, P.C.; Forsyth, S.; Legrand, P.; Abrahamse-Berkeveld, M.; van de Heijning, B.J.M.; van der Beek, E.M.; et al. Perspective: Moving Toward Desirable Linoleic Acid Content in Infant Formula. Adv. Nutr. 2021, 12, 2085–2098. [Google Scholar] [CrossRef]

| Characteristics | n = 34 |
|---|---|
| Gestational age, week, mean (SD) | 26.5 (1.6) |
| Birthweight, grams, mean (SD) | 855 (208) |
| Maternal age, year, mean (SD) | 31 (4.8) |
| Prim gravida mother, n (%) | 17 (50.0) |
| Parity, median (IQR) | 0 (0, 1) |
| Gestation hypertension, n (%) | 3 (8.8) |
| Cesarean section, n (%) | 23 (67.6) |
| Twin pregnancy, n (%) | 12 (54.5) |
| Chorioamnionitis, n (%) | 3 (8.8) |
| Antenatal steroid, n (%) | 31 (91.1) |
| SNAP II, median (IQR) | 9 (0, 20) |
| Duration of mechanical ventilation, median (IQR) | 10 (3, 23) |
Respiratory support at start, n (%)
| 2 (5.9) 31 (91.2) 1 (2.9) |
| Postnatal steroid, n (%) | 7 (25.9) |
| Bronchopulmonary dysplasia, n (%) | 13 (38.2) |
| Severe intraventricular hemorrhage, n (%) | 2 (5.9) |
| Patent ductus arteriosus required treatment, n (%) | 20 (58.8) |
| Severe retinopathy of prematurity, n (%) | 1 (2.9) |
| Culture-proven sepsis, n (%) | 2 (6.0) |
| Necrotizing enterocolitis, n (%) | 1 (2.9) |
| Gestational age at discharge, mean (SD) | 40.1 (2.9) |
| Age at starting hindmilk, day, median (IQR) | 33.5 (26, 38.5) |
| Duration of parenteral nutrition during total hospital stay, day, median (IQR) | 17 (11, 25) |
| Duration of first course parenteral nutrition, day, median (IQR) | 14 (9, 23) |
| Duration of central line, day, median (IQR) | 10 (6, 17) |
| Birthweight Z-score, mean (SD) | −0.21 (0.93) |
| Birth length Z-score, mean (SD) | −0.43 (1.26) |
| Birth head circumference z-score, mean (SD) | −0.44 (0.93) |
| Discharge weight Z-score, mean (SD) | −1.34 (1.0) |
| Discharge length Z-score, mean (SD) | −2.49 (1.42) |
| Content | Composite Milk | Hindmilk | Mean Difference (95%CI) | p-Value |
|---|---|---|---|---|
| Crude protein, g/dL, mean (SD) | 1.5 (0.3) | 1.5 (0.3) | 0.03 (−0.05, 0.12) | 0.44 |
| True protein, g/dL, mean (SD) | 1.1 (0.2) | 1.1 (0.2) | 0.01 (−0.05, 0.08) | 0.71 |
| Fat, g/dL, mean (SD) | 3.4 (1.0) | 4.7 (1.8) | 1.3 (0.7, 2.0) | <0.001 |
| Carbohydrate, g/dL, mean (SD) | 7.9 (0.3) | 7.9 (0.3) | 0.09 (−0.03, 0.22) | 0.13 |
| Calorie, kcal/dL, mean (SD) | 68 (10) | 79 (17) | 11.9 (5.5, 18.3) | <0.001 |
| Composite Milk (7 Days before Hindmilk) | Hindmilk (14 Days from Start) | |||||
|---|---|---|---|---|---|---|
| Estimated Values Based on Composite Milk Reference | Values Based on Milk Analysis | Additional Fortification | Estimated Values Based on Composite Milk Reference before Adjustment * | Values Based on Hindmilk Analysis | Additional Fortification | |
| Energy intake, mean (SD), Kcal/kg/day | 100.3 (6.4) | 100.8 (15.2) | 19.3 (6.8) | 97.1 (4.8) ** | 114.9 (20.5) | 22.3 (10.9) |
| Protein intake, mean (SD), g/kg/day | 1.77 (0.24) | 1.58 (0.29) | 2.15 (0.40) | 1.60 (0.08) | 1.57 (0.27) | 2.28 (0.39) |
| Common Name | Fatty Acid | Composite Milk Content (µg/mL) | Hindmilk Content (µg/mL) | Mean Difference (µg/mL) | p Value |
|---|---|---|---|---|---|
| Capric acid | C10:0 | 8.5 (4.2) | 9.7 (3.6) | 1.2 (−0.5, 2.9) | 0.18 |
| Lauric acid | C12:0 | 37.8 (19.2) | 40.7 (16.3) | 2.9 (−3.9, 9.8) | 0.39 |
| Myristic acid | C14:0 | 38.3 (18.3) | 43.9 (21.5) | 5.6 (−3.2, 14.4) | 0.20 |
| Palmitic acid | C16:0 | 122.0 (63.6) | 149.4 (73.3) | 27.4 (−3.9, 58.7) | 0.06 |
| Palmitelaisic acid | C16:1 n7t | 0.6 (0.3) | 0.7 (0.4) | 0.1 (−0.1, 0.3) | 0.20 |
| Palmitoleic acid | C16:1 n7 | 12.1 (7.6) | 13.7 (6.6) | 1.6 (−1.4, 4.7) | 0.28 |
| Stearic acid | C18:0 | 31.8 (17.0) | 38.1 (19.5) | 6.2 (−2.0, 14.5) | 0.13 |
| Elaidic acid | C18:1t | 3.1 (1.8) | 3.7 (2.5) | 0.6 (−0.4, 1.6) | 0.21 |
| Oleic acid | C18:1 n9 | 217.5 (85.6) | 252.6 (101) | 35.0 (−10.9, 81.0) | 0.13 |
| Linoelaidic acid | C18:2 n6t | 1.4 (0.6) | 1.8 (1.0) | 0.4 (−0.0, 0.7) | 0.07 |
| Linoleic acid | C18:2 n6 | 66.3 (27.6) | 82.5 (37.8) | 16.2 (3.7, 28.7) | 0.01 |
| Arachidic acid | C20:0 | 0.9 (0.4) | 1.1 (0.6) | 0.1 (−0.1, 0.4) | 0.18 |
| γ-Linolenic acid | C18:3 n6 | 0.9 (0.3) | 1.0 (0.5) | 0.1 (−0.0, 0.3) | 0.13 |
| Eicosenoic acid | C20:1 n9 | 2.7 (1.6) | 3.0 (1.3) | 0.3 (−0.3. 0.9) | 0.32 |
| α-Linolenic acid | C18:3 n3 | 8.6 (3.1) | 10.0 (5.4) | 1.4 (−0.4, 3.2) | 0.13 |
| Eicosadienoic acid | C20:2 n6 | 1.6 (0.8) | 1.9 (0.9) | 0.3 (−0.1, 0.6) | 0.09 |
| Behenic acid | C22:0 | 0.5 (0.2) | 0.6 (0.3) | 0.1 (−0.1, 0.2) | 0.30 |
| Dihomo-g-linolenic | C20:3 n6 | 2.3 (0.9) | 2.6 (1.1) | 0.3 (−0.2, 0.8) | 0.21 |
| Arachidonic acid | C20:4 n6 | 2.3 (2.6) | 2.6 (1.0) | 1.2 (−0.1, 0.8) | 0.11 |
| Lignoceric acid | C24:0 | 0.3 (0.2) | 0.4 (0.2) | 0.0 (−0.1, 0.1) | 0.58 |
| Eicosapentaenoic acid | C20:5 n3 | 0.5 (0.4) | 0.7 (0.7) | 0.2 (−0.1, 0.4) | 0.25 |
| Nervonic acid | C24:1 n9 | 0.4 (0.2) | 0.4 (0.1) | 0.0 (−0.1, 0.1) | 0.78 |
| Docosatetraenoic acid | C22:4 n6 | 0.5 (0.2) | 0.6 (0.2) | 0.1 (−0.0, 0.2) | 0.23 |
| Docosapentaenoic acid | C22:5 n6 | 0.2 (0.2) | 0.2 (0.1) | 0.0 (−0.0, 0.1) | 0.28 |
| Docosapentaenoic acid | C22:5 n3 | 1.0 (0.6) | 1..2 (0.8) | 0.2 (−0.1, 0.6) | 0.15 |
| Docosahexaenoic acid | C22:6 n3 | 1.6 (1.5) | 2.0 (1.4) | 0.3 (−0.3, 0.9) | 0.27 |
| Total fatty acids | - | 564 (226) | 665 (266) | 101 (−16, 218) | 0.07 |
| Common Name | Plasma Fatty Acids | Before the Start of Hindmilk (%) (n = 30) | After Hindmilk (%) (n = 27) | p Value |
|---|---|---|---|---|
| Myristic acid | C14:0 | 1.16 (0.44) | 1.20 (0.66) | 0.33 |
| Palmitic acid | C16:0 | 23.56 (1.14) | 23.0 (1.15) | 0.04 |
| Palmitelaisic acid | C16:1 n7t | 0.12 (0.05) | 0.13 (0.04) | 0.91 |
| Palmitoleic acid | C16:1 n7 | 1.62 (0.61) | 1.07 (0.43) | <0.001 |
| Stearic acid | C18:0 | 12.24 (0.86) | 12.65 (1.46) | 0.14 |
| Elaidic acid | C18:1t | 0.37 (0.09) | 0.39 (0.12) | 0.24 |
| Oleic acid | C18:1 n9 | 22.63 (2.74) | 22.67 (3.16) | 0.97 |
| Linoelaidic acid | C18:2 n6t | 0.19 (0.04) | 0.18 (0.05) | 0.38 |
| Linoleic acid | C18:2 n6 | 14.75 (0.89) | 16.26 (3.37) | 0.002 |
| Arachidic acid | C20:0 | 0.22 (0.05) | 0.22 (0.05) | 0.85 |
| γ-Linolenic acid | C18:3 n6 | 0.24 (0.07) | 0.18 (0.07) | <0.001 |
| Eicosenoic acid | C20:1 n9 | 0.32 (0.05) | 0.33 (0.06) | 0.17 |
| α-Linolenic acid | C18:3 n3 | 0.38 (0.17) | 0.51 (0.23) | 0.007 |
| Eicosadienoic acid | C20:2 n6 | 0.31 (0.06) | 0.31 (0.06) | 0.97 |
| Behenic acid | C22:0 | 0.65 (0.15) | 0.57 (0.19) | 0.03 |
| Dihomo-g-linolenic | C20:3 n6 | 2.22 (0.39) | 2.01 (0.51) | 0.07 |
| Arachidonic acid | C20:4 n6 | 11.53 (1.36) | 10.84 (1.81) | 0.10 |
| Lignoceric acid | C24:0 | 0.54 (0.22) | 0.50 (0.20) | 0.60 |
| Eicosapentaenoic acid | C20:5 n3 | 0.49 (0.17) | 0.48 (0.22) | 0.46 |
| Nervonic acid | C24:1 n9 | 0.86 (0.38) | 0.88 (0.39) | 0.36 |
| Docosatetraenoic acid | C22:4 n6 | 1.22 (0.20) | 1.26 (0.27) | 0.47 |
| Docosapentaenoic acid | C22:5 n6 | 0.49 (0.11) | 0.45 (0.12) | 0.28 |
| Docosapentaenoic acid | C22:5 n3 | 0.76 (0.19) | 0.83 (0.26) | 0.29 |
| Docosahexaenoic acid | C22:6 n3 | 3.09 (0.49) | 3.11 (0.64) | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaikh, B.N.; Festival, J.; Reyes Loredo, A.; Yusuf, K.; Towage, Z.; Fenton, T.R.; Wood, C. Hindmilk as a Rescue Therapy in Very Preterm Infants with Suboptimal Growth Velocity. Nutrients 2023, 15, 929. https://doi.org/10.3390/nu15040929
Alshaikh BN, Festival J, Reyes Loredo A, Yusuf K, Towage Z, Fenton TR, Wood C. Hindmilk as a Rescue Therapy in Very Preterm Infants with Suboptimal Growth Velocity. Nutrients. 2023; 15(4):929. https://doi.org/10.3390/nu15040929
Chicago/Turabian StyleAlshaikh, Belal N., Jannette Festival, Adriana Reyes Loredo, Kamran Yusuf, Zainab Towage, Tanis R. Fenton, and Christel Wood. 2023. "Hindmilk as a Rescue Therapy in Very Preterm Infants with Suboptimal Growth Velocity" Nutrients 15, no. 4: 929. https://doi.org/10.3390/nu15040929
APA StyleAlshaikh, B. N., Festival, J., Reyes Loredo, A., Yusuf, K., Towage, Z., Fenton, T. R., & Wood, C. (2023). Hindmilk as a Rescue Therapy in Very Preterm Infants with Suboptimal Growth Velocity. Nutrients, 15(4), 929. https://doi.org/10.3390/nu15040929





