The Human Gut Virome and Its Relationship with Nontransmissible Chronic Diseases
Abstract
1. Introduction
2. Host-Gut Virome Interactions/Relationships
3. Gut Virome Determination
4. Relationship between the Gut Virome and Metabolic Pathologies
4.1. Metabolic Syndrome
| Disease/Model | Subjects | Determination | Main Findings | References |
|---|---|---|---|---|
| Obesity/humans | 128 obese subjects and 101 lean subjects | Gut virome (GV), bacteriome, and viral–bacterial correlations | -Obese subjects, especially those with type 2 diabetes (T2D), had a lower gut viral richness and diversity than lean controls. -GV may play an important role in the development of obesity and T2D. -Eleven viruses, including Escherichia phage, Geobacillus phage, and Lactobacillus phage, were higher in obese subjects than in lean controls. | [28] |
| Inflammatory Bowel disease (IBD)/humans | 12 household controls, 18 Crohn’s disease patients, and 42 ulcerative colitis patients | Stool samples investigated by virus-like particle enrichment and sequencing as well as bacterial 16S rRNA gene analysis | -Patients with IBD showed a significant increase in Caudovirales bacteriophages in their GV. -Changes in the GV may contribute to intestinal inflammation and bacterial dysbiosis. | [33] |
| Cirrhosis and hepatic encephalopathy/humans | 40 controls and 163 cirrhotic patients | Stool metagenomics for bacteria and phages were analyzed in controls versus cirrhosis, within cirrhotic, hospitalized/not, and pre/post rifaximin | -Bacterial α-/β-diversity worsened from controls through cirrhosis patients. Phage α-diversity was similar in both groups. -No changes in α-/β-diversity of phages or bacteria were seen after postrifaximin treatment in cirrhotic patients. | [47] |
| Obesity and metabolic syndrome/humans | 28 school-aged children (10 with normal weight, 10 obese, and 8 obese + metabolic syndrome) | Characterization of the gut DNA virome using metagenomic sequencing | -Phage richness and diversity of individuals with obese and obese + metabolic syndrome tended to increase with respect to controls. -The abundance of some phages correlated with gut bacterial taxa and with anthropometric and biochemical parameters altered in obese and obese + metabolic syndrome. | [50] |
| Bile acid metabolism/mice | 7 germ-free C57BL/6J mice | Phage-induced repression of a tryptophan-rich sensory protein and repression of bile acid deconjugation | -Phages’ presence in the gut can affect the microbial metabolism of bile acids. -Phague BV01 and other phages from the family Salyersviridae are ubiquitous in the human gut, can infect a broad range of Bacteroides hosts, and affect bile acid metabolism. | [24] |
| Cerebral ischemia/mice | 6 adult C57BL/6J mice | Determination of GV composition by shotgun metagenomics in fecal samples | -Following focal ischemia, the abundances of two viral taxa decreased, and those of five viral taxa increased compared with previous cohorts. -Abundances of Clostridia-like phages and Erysipelatoclostridiaceae-like phages were decreased in the stroke compared with previous cohorts | [36] |
| IBD/humans | 40 fecal samples | Stool samples investigated by bioinformatics viral sequencing and bacterial 16S rRNA gene analysis | -Changes in GV and increased numbers of temperate phage sequences were found in individuals with Crohn’s disease. -Incorporating both bacteriome and GV composition offered better discrimination power between health and disease. | [49] |
| Metabolic syndrome/humans | 196 participants with metabolic syndrome preceding cardiometabolic disease | Bulk whole genome and virus-like particle communities | -GV from metabolic syndrome patients exhibited low richness and diversity. -Viral clusters revealed that Candidatus Heliusviridae, a highly widespread gut phage, was found in >90% of metabolic syndrome patients. | [37] |
| Environmental enteric dysfunction and low growth rate/humans | 94 children without diarrhea or human immunodeficiency virus | Gut bacterial and GV sequencing and analysis | - Three differentially abundant phages were identified in GV, depending on child growth velocities. -A positive correlation was found between bacteria and bacteriophage richness in children with subsequent adequate/moderate growth. | [35] |
| IBD/humans | Fecal samples from 24 children, 12 with inflammatory bowel disease and 12 controls | Identification of viral sequences and bacterial microbiota sequencing | -Caudovirales’ relative abundance was greater than that of Microviridae in both inflammatory bowel disease patients and healthy controls. -Caudovirales was more abundant in Chron´s disease patients than in ulcerative colitis patients, but not than in control patients. -Pediatric inflammatory bowel disease patients can be distinguished from healthy controls by bacterial community composition. | [34] |
| Crohn´s disease/mice | 12–23 BALB/CYJ mice | Disruption of normal resident microbiota with streptomycin sulphate administration and phage therapy | -A single day of treatment with a phage cocktail significantly decreased the number of adherent invasive Escherichia coli in feces. -A single dose of the phage cocktail reduced dextran sodium sulphate-induced colitis symptoms in mice. | [60] |
| Colorectal cancer (CRC) and colonic adenoma/humans | 71 colorectal cancer patients, 63 adenoma patients, and 91 healthy controls | Metagenomic sequencing of the gut microbiome and microbial interactions in adenoma and colorectal cancer patients | -Uncultured CrAssphage was higher in healthy controls and positively associated with beneficial butyrate-producing bacteria in gut microbiota (GM). -GV was much more dynamic than the GM as the disease progressed. | [52] |
| CRC/humans | 90 human subjects, (30 healthy controls, 30 of whom had adenomas, and 30 of whom had carcinomas) | Stool samples analyzed by 16S rRNA gene, whole shotgun metagenomics, and purified virus metagenomic sequencing | -The CRC-associated GV consisted primarily of temperate bacteriophages. -Phages influenced cancer by directly modulating the influential bacteria. | [39] |
| Enteric pathogens/mice | 100 C57BL/6J mice | Viruses generated from molecular clones were used to infect cell lines to liberate virions. Subsequently, clones were used to infect mice that were euthanized and investigated for results of viral infections | -Chronic murine astrovirus complements defects in adaptive immunity by elevating cell-intrinsic IFN-λ in the intestinal epithelial barrier in immunodeficient mice. -Elements of the GV can protect against enteric pathogens in an immunodeficient host. | [51] |
| Alcoholic hepatitis/humans | 89 patients with alcoholic hepatitis, 36 with alcohol use disorder, and 17 healthy people as controls | Metagenomic sequencing of virus-like particles from fecal samples, fractionated using differential filtration techniques | -Patients with alcohol use disorder showed increased viral diversity in fecal samples compared to controls and patients with alcoholic hepatitis. -History of antibiotic treatment was associated with higher GV diversity. -Specific viral taxa, such as Staphylococcus phages and Herpesviridae, were associated with increased hepatic disease severity. | [1] |
| Viral entities/humans | 662 samples from 1-year-old children | Processing of metagenomics and metaviromics datasets | -Viral enrichment during sample processing showed a loss of a significant part of the GV and did not represent integrated bacteria containing dormant phages (prophagues). -Approximately 65–83% of the viral populations in the metavirome were not aligned with the metagenome data. | [61] |
| Nonalcoholic fatty liver diseases (NAFLD)/humans | 73 patients with NAFLD | RNA and DNA virus-like particles from fecal samples | -Patients with NAFLD and cirrhosis showed a significant decrease in intestinal viral diversity compared with controls. -Advanced NAFLD was associated with a reduction in the proportion of phages compared with other intestinal viruses. | [62] |
| IBD/humans | 54 Patients with IBD and 23 healthy controls | Virus-like particles were purified from stool samples and characterized by DNA and RNA sequencing and VLP particle counts | -Viral populations associated with IBD showed perturbations with respect to healthy controls. -Anelloviridae showed a higher prevalence in IBD compared to healthy controls, and Analloviridae DNA levels were biomarkers of the effectiveness of immunosuppression. -IBD subjects had a higher ratio of Caudovirales to Microviridae phages compared to healthy controls. | [54] |
| CRC/humans | 80 colorectal primary tumors tissues and corresponding normal colorectal tissues | GV and bacteriome analysis for CRC tissues | -The number of viral species increased whereas bacterial species decreased in CRC tissues compared with healthy ones. -Phages were the most preponderant viral species in CRC tissues, and the main families were Myoviridae, Siphoviridae, and Podoviridae. -Primary CRC tissues were enriched for Enterobacteria, Bacillus, Proteus, and Streptococcus phages, together with their pathogenic hosts in contrast to normal tissues. | [45] |
| Type 2 diabetes (T2D)/humans | 71 T2D patients and 74 healthy controls | Whole-community metagenomic sequencing data of fecal samples | -Significant increase in the number of gut phages in fecal samples was found in the T2D group. -Significant alterations of the gut phageome cannot be explained simply by covariation with the altered bacterial hosts. | [46] |
| Cognitive maintenance/humans | 120 subjects, 60 with obesity and 60 without obesity | Neuropsychological assessment in humans, extraction of fecal genomic DNA and whole-genome shotgun sequencing | -GV was dominated by Caudovirales and Microviridae phages. -Subjects with increased Caudovirales and Siphoviridae levels in the gut microbiome performed better cognitive status. -Phages should be considered novel actors in the microbiome–brain axis. | [46] |
| Cognitive maintenance/mice | 11 mice were orally gavaged with saline and fecal material from humans | Behavioral testing in mice and study of gene expression in mouse prefrontal cortex | -Microbiota transplantation from human donors with increased specific Caudovirales levels led to increased scores in novel object recognition. -Phages should be considered novel actors in the microbiome–brain axis. | [53] |
| CRC/humans | 74 patients with CRC and 92 healthy controls | Shotgun metagenomic analyses of viromes of fecal samples | -Gut phage community diversity was significantly increased in patients with CRC compared with controls. -GV dysbiosis was associated with early- and late-stage CRC. | [63] |
| Fructose intake/mice | 25 C57BL/6J mice per group were used for phage production, and 36 mice were used for the in vivo dietary crossover study | Lactobacillus reuteri survival and phage production during gastrointestinal transit in mice | -Fructose intake activated the Ack pathway, involved in generating acetic acid, which promotes phage production. | [64] |
| Malnutrition/humans | 8 monozygotic and 12 dizygotic twin pairs | Shotgun pyrosequencing of VLP-derived DNA | -Phage plus members of the Anelloviridae and Circoviridae families of eukaryotic viruses discriminate discordant from concordant healthy pairs. | [43] |
| Type 1 diabetes (T1D)/humans | 103 T1D children and their mothers | Determination of virus antibodies, enterovirus RNA, and enzyme immunoassay analysis | -Autoantibody-positive children had more enterovirus infections than autoantibody-negative children before the appearance of autoantibodies. -Enterovirus infections seem to be associated with the induction of β-cell autoimmunity in young children with increased genetic susceptibility to T1D. | [65] |
| High-fat diet/mice | 12 C57BL/6J pregnant female mice | Mice were administered with subtherapeutic antibiotic dosages or no antibiotic and subsequently analyzed for GV composition and 16S rRNA metagenomics | -High-fat diet significant shift away from the relatively abundant Siphoviridae, accompanied by increases in phages from the Microviridae family. -Phage structural genes significantly decreased after the transition to a high-fat diet. | [41] |
| IBD/humans and mice | Fecal samples collected from 3 ulcerative colitis patients in remission and 3 unrelated healthy controls were transferred to C57BL/6 mice | Fecal virus-like particles (VLPs) isolated from ulcerative colitis patients and healthy controls were transferred to mice | -VLPs isolated from ulcerative colitis patients specifically altered the relative abundances of several bacterial taxa involved in IBD progression in mice. -Phages are dynamic regulators of GM and implicate the GV in modulating intestinal inflammation and disease. | [31] |
| T1D/humans | Fecal samples from 11 children who had developed serum autoantibodies associated with T1D and healthy controls | Detection of phage and eukaryotic viral sequences | -GV of T1D subjects was less diverse than those of controls. Lower phage diversity in cases than in controls. -Specific components of the GV were both directly and inversely associated with the development of human autoimmune disease. -Among eukaryotic viruses, there was a significant enrichment of Circoviridae-related sequences in controls in comparison with T1D patients. | [44] |
| Hypertension/humans | 196 samples | Viral and bacterial metagenomic investigation of fecal samples | -Virus could have higher discrimination power than bacteria to differentiate healthy prehypertension samples from hypertension patients | [48] |
4.2. Obesity, Diabetes and Malnutrition
4.3. Liver Diseases
4.4. Cancer
5. Intestinal Diseases Mediated by Bacteria
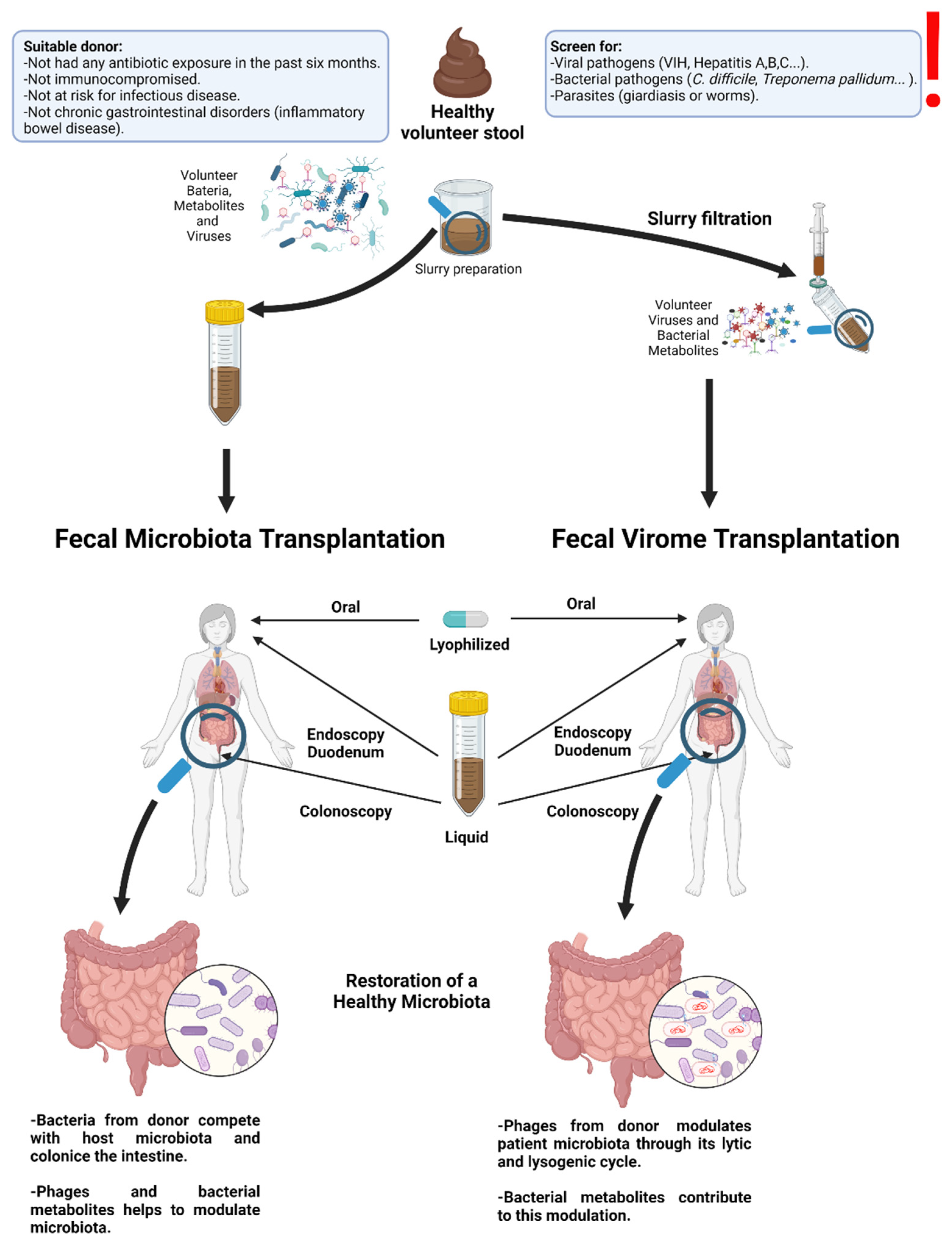
6. Therapies including Transfer of Gut Viruses
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansen, J.; Plichta, D.R.; Nissen, J.N.; Jespersen, M.L.; Shah, S.A.; Deng, L.; Stokholm, J.; Bisgaard, H.; Nielsen, D.S.; Sørensen, S.J.; et al. Genome binning of viral entities from bulk metagenomics data. Nat. Commun. 2022, 13, 965. [Google Scholar] [CrossRef] [PubMed]
- Roca-Saavedra, P.; Mendez-Vilabrille, V.; Miranda, J.M.; Nebot, C.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Food additives, contaminants and other minor components: Effects on human gut microbiota—A review. J. Physiol. Biochem. 2018, 74, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-origin prebiotics based on chitin: An alternative for the future? a critical review. Foods 2020, 9, 782. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. eBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Kennedy, E.A.; Holtz, L.R. Gut virome in early life: Origins and implications. Curr. Opin. Virol. 2022, 55, 101233. [Google Scholar] [CrossRef]
- Lim, E.S.; Zhou, Y.; Zhao, G.; Bauer, I.K.; Droit, L.; Ndao, I.M.; Warner, B.B.; Tarr, P.I.; Wang, D.; Holtz, L.R. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 2015, 21, 1228–1234. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the human gut: The “known unknown” of the microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef]
- Yutin, N.; Makarova, K.S.; Gussow, A.B.; Krupovic, M.; Segall, A.; Edwards, R.A.; Koonin, E.V. Discovery of an expansive bacteriophage family that includes the most abundant viruses from the human gut. Nat. Microbiol. 2018, 3, 38–46. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Clooney, A.G.; Sutton, T.D.S.; Ryan, F.J.; Daly, K.M.; Nolan, J.A.; McDonnell, S.A.; Khokhlova, E.V.; Draper, L.A.; Forde, A.; et al. The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe 2019, 26, 527–541.e5. [Google Scholar] [CrossRef]
- Dutilh, B.E.; Cassman, N.; McNair, K.; Sanchez, S.E.; Silva, G.G.Z.; Boling, L.; Barr, J.J.; Speth, D.R.; Seguritan, V.; Aziz, R.K.; et al. A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nat. Commun. 2014, 5, 4498. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Sun, Y.; Wan, Y.; Yeoh, Y.K.; Zhang, F.; Cheung, C.P.; Chen, N.; Luo, J.; Wang, W.; Sung, J.J.Y.; et al. Human-gut-DNA virome variations across geography, ethnicity, and urbanization. Cell Host Microbe 2020, 28, 741–751.e4. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.C.; Zablocki, O.; Zayed, A.A.; Howell, A.; Bolduc, B.; Sullivan, M.B. The gut virome database reveals age-dependent patterns of virome diversity in the human gut. Cell Host Microbe 2020, 28, 724–740.e8. [Google Scholar] [CrossRef] [PubMed]
- Khan Mirzaei, M.; Khan, M.A.A.; Ghosh, P.; Taranu, Z.E.; Taguer, M.; Ru, J.; Chowdhury, R.; Kabir, M.M.; Deng, L.; Mondal, D.; et al. Bacteriophages isolated from stunted children can regulate gut bacterial communities in an age-specific manner. Cell Host Microbe 2020, 27, 199–212.e5. [Google Scholar] [CrossRef]
- Minot, S.; Sinha, R.; Chen, J.; Li, H.; Keilbaugh, S.A.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. The human gut virome: Inter-individual variation and dynamic response to diet. Genome Res. 2011, 21, 1616–1625. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 2019, 25, 803–814.e5. [Google Scholar] [CrossRef]
- Edwards, R.A.; McNair, K.; Faust, K.; Raes, J.; Dutilh, B.E. Computational approaches to predict bacteriophage-host relationships. FEMS Microbiol. Rev. 2016, 40, 258–272. [Google Scholar] [CrossRef]
- Lam, S.; Bai, X.; Shkoporov, A.N.; Park, H.; Wu, X.; Lan, P.; Zuo, T. Roles of the gut virome and mycobiome in faecal microbiota transplantation. Lancet Gastroenterol. Hepatol. 2022, 7, 472–484. [Google Scholar] [CrossRef]
- Łoś, M.; Węgrzyn, G. Pseudolysogeny. Adv. Virus Res. 2012, 82, 339–349. [Google Scholar] [CrossRef]
- Mäntynen, S.; Laanto, E.; Oksanen, H.M.; Poranen, M.M.; Díaz-Muñoz, S.L. Black box of phage–bacterium interactions: Exploring alternative phage infection strategies. Open Biol. 2021, 11, 210188. [Google Scholar] [CrossRef]
- Rascovan, N.; Duraisamy, R.; Desnues, C. Metagenomics and the human virome in asymptomatic individuals. Annu. Rev. Microbiol. 2016, 70, 17. [Google Scholar] [CrossRef]
- Zhang, T.; Breitbart, M.; Lee, W.H.; Run, J.-Q.; Wei, C.L.; Soh, S.W.L.; Hibberd, M.L.; Liu, E.T.; Rohwer, F.; Ruan, Y. RNA viral community in human feces: Prevalence of plant pathogenic viruses. PLoS Biol. 2006, 4, 108. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kim, M.S.; Kim, E.; Cheon, J.H.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Seo, S.U.; Shin, S.H.; Choi, S.S.; et al. Enteric viruses ameliorate gut inflammation via toll-like receptor 3 and toll-like receptor 7-mediated interferon-β production. Immunity 2016, 44, 889–900. [Google Scholar] [CrossRef]
- Chelluboina, B.; Kieft, K.; Breister, A.; Anantharaman, K.; Vemuganti, R. Gut virome dysbiosis following focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 2022, 42, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.; Ferrer-Orta, C.; Verdaguer, N. Viral RNA-dependent RNA polymerases: A structural overview. Subdell Biochem. 2018, 88, 39–71. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Xiao, M.; Li, A. Advances and challenges in cataloging the human gut virome. Cell Host Microbe 2022, 30, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.C.; Gerhardt, K.; Zhong, Z.-P.; Bolduc, B.; Temperton, B.; Konstantinidis, K.T.; Sullivan, M.B. MetaPop: A pipeline for macro- and microdiversity analyses and visualization of microbial and viral metagenome-derived populations. Microbiome 2022, 10, 49. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef]
- Park, A.; Zhao, G. Mining the virome for insights into type 1 diabetes. DNA Cell Biol. 2018, 37, 422–425. [Google Scholar] [CrossRef]
- Monaco, C.L.; Gootenberg, D.B.; Zhao, G.; Handley, S.A.; Ghebremichael, M.S.; Lim, E.S.; Lankowski, A.; Baldridge, M.T.; Wilen, C.B.; Flagg, M.; et al. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe 2016, 19, 311–322. [Google Scholar] [CrossRef]
- Yang, K.; Niu, J.; Zuo, T.; Sun, Y.; Xu, Z.; Tang, W.; Liu, Q.; Zhang, J.; Ng, E.K.W.; Wong, S.K.H.; et al. Alterations in the gut virome in obesity and type 2 diabetes mellitus. Gastroenterology 2021, 161, 1257–1269.e13. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Citro, V.; Cataldi, M. Findings from studies are congruent with obesity having a viral origin, but what about obesity-related nafld? Viruses 2021, 13, 1285. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Sikaroodi, M.; Shamsaddini, A.; Henseler, Z.; Santiago-Rodriguez, T.; Acharya, C.; Fagan, A.; Hylemon, P.B.; Fuchs, M.; Gavis, E.; et al. Interaction of bacterial metagenome and virome in patients with cirrhosis and hepatic encephalopathy. Gut 2021, 70, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Galtier, M.; De Sordi, L.; Sivignon, A.; de Vallée, A.; Maura, D.; Neut, C.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages targeting adherent invasive Escherichia coli strains as a promising new treatment for Crohn’s disease. J. Crohn’s Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef]
- Fernandes, M.A.; Verstraete, S.G.; Phan, T.; Deng, X.; Stekol, E.; Lamere, B.; Lynch, S.V.; Heyman, M.B.; Delwart, E. Enteric virome and bacterial microbiota in children with ulcerative colitis and crohn disease. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 30–36. [Google Scholar] [CrossRef]
- Clooney, A.G.; Sutton, T.D.S.; Shkoporov, A.N.; Holohan, R.K.; Daly, K.M.; O’Regan, O.; Ryan, F.J.; Draper, L.A.; Plevy, S.E.; Ross, R.P.; et al. Whole-virome analysis sheds light on viral dark matter in inflammatory bowel disease. Cell Host Microbe 2019, 26, 764–778.e5. [Google Scholar] [CrossRef]
- Desai, C.; Handley, S.A.; Rodgers, R.; Rodriguez, C.; Ordiz, M.I.; Manary, M.J.; Holtz, L.R. Growth velocity in children with environmental enteric dysfunction is associated with specific bacterial and viral taxa of the gastrointestinal tract in Malawian children. PLoS Negl. Trop. Dis. 2020, 14, e0008387. [Google Scholar] [CrossRef]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 2019, 25, 285–299.e8. [Google Scholar] [CrossRef]
- Ingle, H.; Lee, S.; Ai, T.; Orvedahl, A.; Rodgers, R.; Zhao, G.; Sullender, M.; Peterson, S.T.; Locke, M.; Liu, C.-T.; et al. Viral complementation of immunodeficiency confers protection against enteric pathogens via interferon-λ. Nat. Microbiol. 2019, 4, 1120–1128. [Google Scholar] [CrossRef]
- Liang, W.; Feng, Z.; Rao, S.; Xiao, C.; Xue, X.; Lin, Z.; Zhang, Q.; Qi, W. Diarrhea may be underestimated: A missing link in 2019 novel coronavirus. Gut 2020, 69, 1141–1143. [Google Scholar] [CrossRef]
- Sinha, A.; Li, Y.; Mirzaei, M.K.; Shamash, M.; Samadfam, R.; King, I.L.; Maurice, C.F. Transplantation of bacteriophages from ulcerative colitis patients shifts the gut bacteriome and exacerbates the severity of DSS colitis. Microbiome 2022, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Lu, X.-J.; Zhang, Y.; Cheung, C.P.; Lam, S.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Zhao, R.; Chan, P.K.S.; et al. Gut mucosal virome alterations in ulcerative colitis. Gut 2019, 68, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Sadeharju, K.; Hämäläinen, A.-M.; Knip, M.; Lönnrot, M.; Koskela, P.; Virtanen, S.M.; Ilonen, J.; Åkerblom, H.K.; Hyöty, H. Enterovirus infections as a risk factor for type I diabetes: Virus analyses in a dietary intervention trial. Clin. Exp. Immunol. 2003, 132, 271–277. [Google Scholar] [CrossRef]
- Zhao, G.; Vatanen, T.; Droit, L.; Park, A.; Kostic, A.D.; Poon, T.W.; Vlamakis, H.; Siljander, H.; Härkönen, T.; Hämäläinen, A.-M.; et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc. Natl. Acad. Sci. USA 2017, 114, E6166–E6175. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; You, X.; Mai, G.; Tokuyasu, T.; Liu, C. A human gut phage catalog correlates the gut phageome with type 2 diabetes. Microbiome 2018, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Castells-Nobau, A.; Arnoriaga-Rodríguez, M.; Garre-Olmo, J.; Puig, J.; Ramos, R.; Martínez-Hernández, F.; Burokas, A.; Coll, C.; Moreno-Navarrete, J.M.; et al. Caudovirales bacteriophages are associated with improved executive function and memory in flies, mice, and humans. Cell Host Microbe 2022, 30, 340–356.e8. [Google Scholar] [CrossRef]
- Bikel, S.; López-Leal, G.; Cornejo-Granados, F.; Gallardo-Becerra, L.; García-López, R.; Sánchez, F.; Equihua-Medina, E.; Ochoa-Romo, J.P.; López-Contreras, B.E.; Canizales-Quinteros, S.; et al. Gut dsDNA virome shows diversity and richness alterations associated with childhood obesity and metabolic syndrome. iScience 2021, 24, 102900. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yang, P.; Zhong, C.; Ning, K. The Human Gut Virome in Hypertension. Front. Microbiol. 2018, 9, 3150. [Google Scholar] [CrossRef]
- de Jonge, P.A.; Wortelboer, K.; Scheithauer, T.P.M.; van den Born, B.J.H.; Zwinderman, A.H.; Nobrega, F.L.; Dutilh, B.E.; Nieuwdorp, M.; Herrema, H. Gut virome profiling identifies a widespread bacteriophage family associated with metabolic syndrome. Nat. Commun. 2022, 13, 3594. [Google Scholar] [CrossRef]
- Campbell, D.E.; Ly, L.K.; Ridlon, J.M.; Hsiao, A.; Whitaker, R.J.; Degnan, P.H. Infection with bacteroides phage bv01 alters the host transcriptome and bile acid metabolism in a common human gut microbe. Cell Rep. 2020, 32, 108142. [Google Scholar] [CrossRef]
- Jiang, L.; Lang, S.; Duan, Y.; Zhang, X.; Gao, B.; Chopyk, J.; Schwanemann, L.K.; Ventura-Cots, M.; Bataller, R.; Bosques-Padilla, F.; et al. Intestinal virome in patients with alcoholic hepatitis. Hepatology 2020, 72, 2182–2196. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, G.D.; Duhaime, M.B.; Ruffin, M.T., IV; Koumpouras, C.C.; Schloss, P.D. Diagnostic potential and interactive dynamics of the colorectal cancer virome. mBio 2018, 9, e02248-18. [Google Scholar] [CrossRef]
- Nakatsu, G.; Zhou, H.; Wu, W.K.K.; Wong, S.H.; Coker, O.O.; Dai, Z.; Li, X.; Szeto, C.H.; Sugimura, N.; Lam, T.Y.T.; et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 2018, 155, 529–541.e5. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, L.; Landry, J.J.M.; Rausch, T.; Abba, M.L.; Delecluse, S.; Delecluse, H.J.; Allgayer, H. Metagenomic analysis of primary colorectal carcinomas and their metastases identifies potential microbial risk factors. Mol. Oncol. 2021, 15, 3363–3384. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Dong, X.; Pan, P.; Chen, K.W.; Fan, J.X.; Cheng, S.X.; Zhang, X.Z. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 2019, 3, 717–728. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018, 67, 634–643. [Google Scholar] [CrossRef]
- Haro, C.; Garcia-Carpintero, S.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Delgado-Lista, J.; Perez-Martinez, P.; Rangel Zuñiga, O.A.; Quintana-Navarro, G.M.; Landa, B.B.; Clemente, J.C.; et al. The gut microbial community in metabolic syndrome patients is modified by diet. J. Nutr. Biochem. 2016, 27, 27–31. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, Y.; Kong, C.; Xia, K.; Li, H.; Zhu, Y.; Zhang, X.; Liu, Y.; Zhong, H.; Yang, R.; et al. Alterations, interactions, and diagnostic potential of gut bacteria and viruses in colorectal cancer. Front. Cell. Infect. Microbiol. 2021, 11, 657867. [Google Scholar] [CrossRef]
- Lang, S.; Demir, M.; Martin, A.; Jiang, L.; Zhang, X.; Duan, Y.; Gao, B.; Wisplinghoff, H.; Kasper, P.; Roderburg, C.; et al. Intestinal virome signature associated with severity of nonalcoholic fatty liver disease. Gastroenterology 2020, 159, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Conrad, M.A.; Kelsen, J.R.; Kessler, L.R.; Breton, J.; Albenberg, L.G.; Marakos, S.; Galgano, A.; Devas, N.; Erlichman, J.; et al. Dynamics of the stool virome in very early onset inflammatory bowel disease. J. Crohn’s Colitis 2020, 14, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Alexander, L.M.; Pan, M.; Schueler, K.L.; Keller, M.P.; Attie, A.D.; Walter, J.; van Pijkeren, J.P. Dietary fructose and microbiota-derived short-chain fatty acids promote bacteriophage production in the gut symbiont Lactobacillus reuteri. Cell Host Microbe 2019, 25, 273–284.e6. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Blanton, L.V.; Cao, S.; Zhao, G.; Manary, M.; Trehan, I.; Smith, M.I.; Wang, D.; Virgin, H.W.; Rohwer, F.; et al. Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc. Natl. Acad. Sci. USA 2015, 112, 11941–11946. [Google Scholar] [CrossRef]
- Schulfer, A.; Santiago-Rodriguez, T.M.; Ly, M.; Borin, J.M.; Chopyk, J.; Blaser, M.J.; Pride, D.T. Fecal viral community responses to high-fat diet in mice. mSphere 2020, 5, e00833-19. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Govoni, S.; Coppola, A.; Gazzaruso, C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: New insights into old diseases. Curr. Opin. Pharmacol. 2019, 49, 1–5. [Google Scholar] [CrossRef]
- Rasmussen, T.S.; Mentzel, C.M.J.; Kot, W.; Castro-Mejía, J.L.; Zuffa, S.; Swann, J.R.; Hansen, L.H.; Vogensen, F.K.; Hansen, A.K.; Nielsen, D.S. Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut 2020, 69, 2122–2130. [Google Scholar] [CrossRef]
- Khaliq, A.; Wraith, D.; Nambiar, S.; Miller, Y. A review of the prevalence, trends, and determinants of coexisting forms of malnutrition in neonates, infants, and children. BMC Public Health 2022, 22, 879. [Google Scholar] [CrossRef]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; Destefano, J.; Meier, M.F.; Muegge, B.D.; et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Low, S.J.; Džunková, M.; Chaumeil, P.A.; Parks, D.H.; Hugenholtz, P. Evaluation of a concatenated protein phylogeny for classification of tailed double-stranded DNA viruses belonging to the order Caudovirales. Nat. Microbiol. 2019, 4, 1306–1315. [Google Scholar] [CrossRef]
- Jegatheesan, P.; De Bandt, J.P. Fructose and NAFLD: The multifaceted aspects of fructose metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Tetz, G.; Tetz, V. Bacteriophage infections of microbiota can lead to leaky gut in an experimental rodent model. Gut Pathog. 2016, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Dejea, C.M.; Edler, D.; Hoang, L.T.; Santidrian, A.F.; Felding, B.H.; Ivanisevic, J.; Cho, K.; Wick, E.C.; Hechenbleikner, E.M.; et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015, 21, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Cepko, L.C.S.; Garling, E.E.; Dinsdale, M.J.; Scott, W.P.; Bandy, L.; Nice, T.; Faber-Hammond, J.; Mellies, J.L. Myoviridae phage PDX kills enteroaggregative Escherichia coli without human microbiome dysbiosis. J. Med. Microbiol. 2020, 69, 309–323. [Google Scholar] [CrossRef]
- Massimino, L.; Lovisa, S.; Antonio Lamparelli, L.; Danese, S.; Ungaro, F. Gut eukaryotic virome in colorectal carcinogenesis: Is that a trigger? Comput. Struct. Biotechnol. J. 2021, 19, 16–28. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Ritz, N.L.; Lin, D.; Carroll-Portillo, A.; Lin, H.C. Transplanting fecal virus-like particles reduces high-fat diet-induced small intestinal bacterial overgrowth in mice. Front. Cell. Infect. Microbiol. 2019, 9, 348. [Google Scholar] [CrossRef]
- Basic, M.; Keubler, L.M.; Buettner, M.; Achard, M.; Breves, G.; Schröder, B.; Smoczek, A.; Jörns, A.; Wedekind, D.; Zschemisch, N.H.; et al. Norovirus triggered microbiota-driven mucosal inflammation in interleukin 10-deficient mice. Inflamm. Bowel Dis. 2014, 20, 431–443. [Google Scholar] [CrossRef]
- Ott, S.J.; Waetzig, G.H.; Rehman, A.; Moltzau-Anderson, J.; Bharti, R.; Grasis, J.A.; Cassidy, L.; Tholey, A.; Fickenscher, H.; Seegert, D.; et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 2017, 152, 799–811.e7. [Google Scholar] [CrossRef]
- Draper, L.A.; Ryan, F.J.; Dalmasso, M.; Casey, P.G.; McCann, A.; Velayudhan, V.; Ross, R.P.; Hill, C. Autochthonous faecal viral transfer (FVT) impacts the murine microbiome after antibiotic perturbation. BMC Biol. 2020, 18, 173. [Google Scholar] [CrossRef]
- Brunse, A.; Deng, L.; Pan, X.; Hui, Y.; Castro-Mejía, J.L.; Kot, W.; Nguyen, D.N.; Secher, J.B.M.; Nielsen, D.S.; Thymann, T. Fecal filtrate transplantation protects against necrotizing enterocolitis. ISME J. 2022, 16, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.S.W.; Jayasinghe, T.N.; Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; et al. Effects of fecal microbiome transfer in adolescents with obesity: The gut bugs randomized controlled trial. JAMA Netw. Open 2020, 3, e2030415. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage adhering to mucus provide a nonhost-derived immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Xu, Z.; Mak, J.W.Y.; Yang, K.; Liu, Q.; Zuo, T.; Tang, W.; Lau, L.; Lui, R.N.; Wong, S.H.; et al. Microbiota engraftment after fecal microbiota transplantation in obese subjects with type 2 diabetes: A 24-week, double-blind, randomised controlled trial. Gut 2022, 71, 716–723. [Google Scholar] [CrossRef]
- Schwartz, M.; Gluck, M.; Koon, S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am. J. Gastroenterol. 2013, 108, 1367. [Google Scholar] [CrossRef]
- Ianiro, G.; Gasbarrini, A.; Cammarota, G. Autologous faecal microbiota transplantation for type 1 diabetes: A potential mindshift in therapeutic microbiome manipulation? Gut 2021, 70, 2–3. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Segal, J.P.; Carding, S.R.; Hart, A.L.; Hold, G.L. The gut virome: The ‘missing link’ between gut bacteria and host immunity? Ther. Adv. Gastroenterol. 2019, 12, 1756284819836620. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioral changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
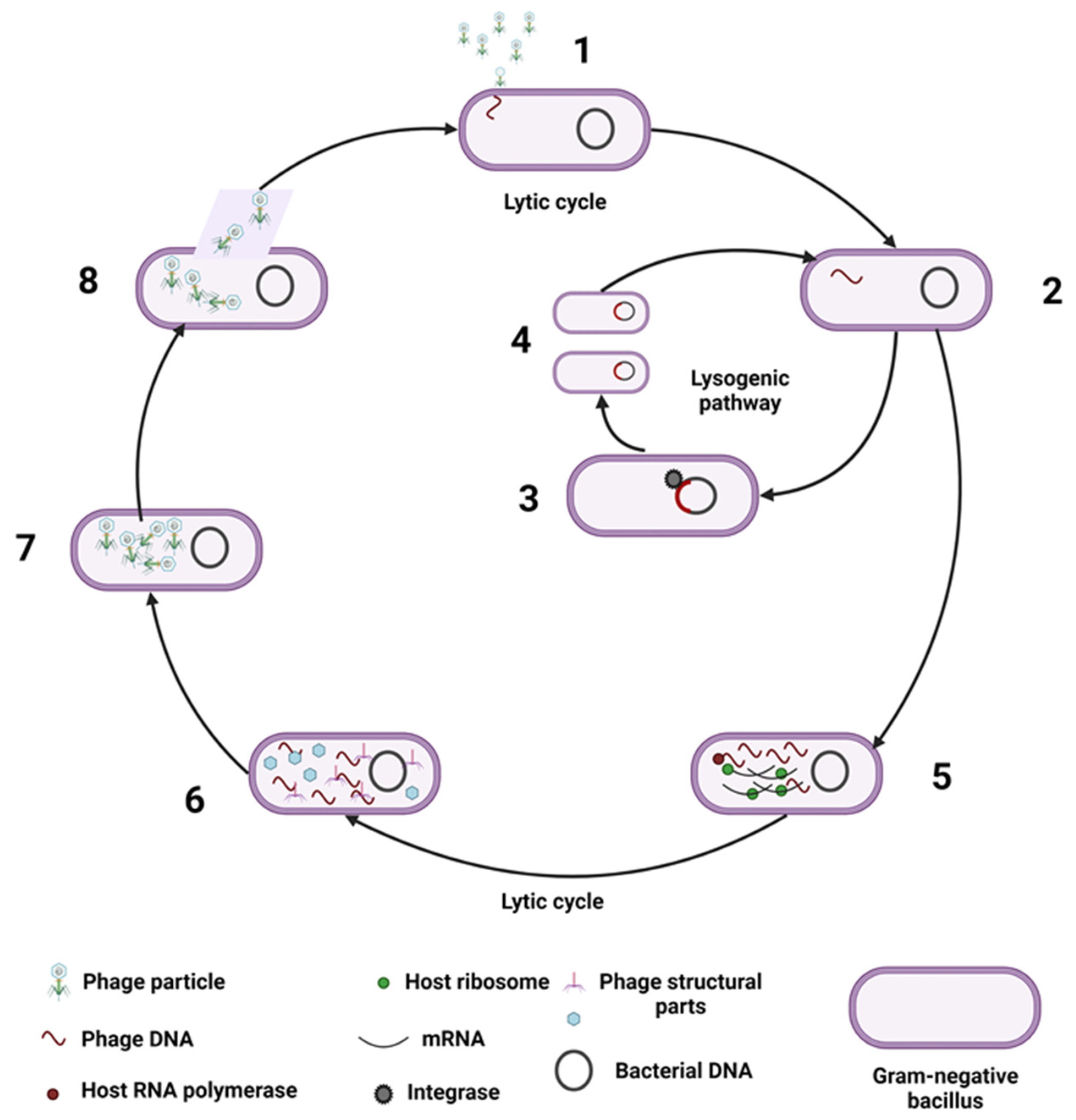
| Model | Subjects | Determination | Main Findings | References |
|---|---|---|---|---|
| Humans (twin kids) | 12 personal fecal samples (4 twins and his/her mothers) | Metagenomic sequencing of bacterial and viral content from fecal bulk | -The composition of the intestinal phageome has similarities in people from different parts of the world. -Ignorance of sequences in viral metagenomes can lead to errors in determining the diversity of the human virome. -This study identified and validated the genome sequence of CrAssphage. | [11] |
| Humans (healthy adults) | 1986 individuals representing 16 countries | Metagenomic sequencing of the viral content of fecal bulk | -The variability in gut virome (GV) among studies due to technical deficiencies is greater than the effect of any disease. -Gut viral richness increases from birth to median age and declines in elderly individuals. | [13] |
| Human (1-year-old child) | 662 paired samples obtained at 1 year from an unselected childhood cohort | Fecal sample metagenomics and comparison with metaviromics datasets | -An important part of the GV may be lost during the viral enrichment stage or may not be correctly detected as it is in the form of bacteria containing dormant phages (prophages). -Most viral populations in the metavirome were not found in the metagenome datasets. | [1] |
| Human (healthy children) | 60 samples from 18 girls and 12 boys | Isolation and quantification of phages and bacteria from stool, metagenome assembly, and analysis | -Phages can regulate bacterial abundance and composition in an age-specific manner. -Phages could be related to the gut microbiota (GM) changes observed in child stunting. | [14] |
| Humans (twin kids) | 8 infants (4 twin pairs) | Bulk virome characterization | -Both eukaryotic virome and bacterial microbiome expanded from birth to 2 years of age, whereas phageome composition decreased. -The infant microbiome is highly dynamic with respect to bacteria, viruses, and bacteriophages, whereas, in adults, it is more stable. | [7] |
| Humans (dietary intervention) | Purified virus-like particles from stool samples collected longitudinally from six healthy volunteers | Dietary intervention high-fat/low-fiber diet and comparison of the GV for 8 days | -Viral contigs were rich in functions required in lytic and lysogenic growth, as well as viral CRISPR arrays and genes for antibiotic resistance. -The largest source of variance among GV samples was interpersonal variation. -Dietary intervention caused a change in the GV community, causing convergence of GV in individuals with similar diets. | [15] |
| Mice (gnotobiotic) | 5 Germfree C57BL/6 mice | Gnotobiotic mice subjected to predation by cognate lytic phages | -Shifts in the microbiome caused by phage predation alter the gut metabolome. | [16] |
| Humans (healthy adults) | 10 healthy volunteers | Fecal samples were collected monthly and synchronously over a 12-month period | -Several groups of CrAss-like and Microviridae bacteriophages were identified as the most stable colonizers of the human gut. -There are stable, numerically predominant individual-specific persistent viromes typical of each subject. | [10] |
| Humans (healthy adults) | 930 healthy adult subjects | Bulk DNA virome characterization | -Factors associated with urbanization and geography factors were the top covariates of GV variation. -GV showed more heterogeneity than the bacterial microbiome in the investigated samples. | [12] |
| Indication/Model | Subjects | Dosage and Time of Exposition | Main Findings | Reference |
|---|---|---|---|---|
| Inflammatory bowel disease (IBD)/humans | 5 patients with symptomatic chronic-relapsing Clostridium difficile infection | Stool collection and characterization according to fecal microbiota transplantation (FMT) standards | -Fecal filtrate transfer (FFT) eliminated symptoms of C. difficile infection for a minimum period of 6 months. -Bacterial components, metabolites, or phages mediate many of the effects of FMT, and FFT might be an alternative approach, particularly for immunocompromised patients. | [79] |
| IBD/piglets | 16 piglets were used to obtain FFT, transferred to 14 piglets by rectal transfer and to 13 by oro-gastric administration | FMT administration by cognate rectal FFT, oro-gastric FFT administration, and saline solutions | -FFT increased viral diversity and reduced Proteobacteria abundance in the ileal mucosa of FFT receiver piglets relative to controls. | [81] |
| Obesity and type 2 diabetes (T2D) induced by diet/mice | 40 C57Bl/&NTac mice | Mice with a high-fat diet plus fecal viral transplantation (FVT) and high-fat diet plus ampicillin plus FVT were compared to controls | -At both 4 and 6 weeks after the first FVT, a significantly lower body weight gain was observed in the high-fat diet + FVT mice and compared to high-fat diet mice. -FVT normalized the blood glucose tolerance in the high-fat + FVT mice. -FVT strongly influences and partly reshapes the gut microbiota composition both with and without ampicillin treatment. | [67] |
| Phage adherence/in vitro | Bacteriophage T4 | T4 phages were serially diluted and used to inoculate plates | -Phage adherence to the mucus model provides immunity applicable to mucosal surfaces. -The symbiotic relationship between phage and hosts provides protection for mucosal surfaces. | [83] |
| IBD/mice | C57BL/6J and C3H/HeJBir wild-type and Il10 mice kept under special pathogen-free conditions | Norovirus infection and investigation in changes induced in structural and functional intestinal barrier changes | -Norovirus caused epithelial barrier disruption in Il10 mice. -Norovirus might trigger individuals with a nonsymptomatic predisposition for IBD by impairment of the intestinal mucosa. | [78] |
| Antibiotic disturbance/mice | 16 BALB/c mice | Administration of antibiotic treatment in the drinking water for 2 days | -Mice showed a perturbed microbiome because of antibiotic treatment, which was reverted over time similar to the pretreatment one. -Mice that had received FVT maintained gut microbiota (GM) more similar to the original before antibiotic treatment compared to mice that had received nonviable phages. | [80] |
| Obesity/humans | A total of 87 individuals took part-565 individuals responded to advertisements | Fecal microbiome transfer | -There was no effect of FMT on weight loss in adolescents with obesity, although a reduction in abdominal adiposity was observed. | [82] |
| Clostridium difficile infection/mice | 26 C57BL/6 mice | Comparison of effects of the fecal VLP fraction against conventional FMT on the ileal microbiome | -VLP fraction played a potential role in modifying the gut microbiome during dysbiosis. -In both recipient groups, transplantation of the fecal VLP fraction alone produced the same outcome as that of the whole FMT. | [7] |
| Melanoma/humans | 10 patients with anti-PD-1-refractory metastatic melanoma | Fecal transfer by FMT | -FMT showed favorable effects in immune cell infiltrates and gene expression profiles in both the gut lamina propia and tumor microenvironment. -There were two partial responses and one complete response in melanoma patients after FMT. | [56] |
| Colorectal cancer/humans | 72 patients with colorectal cancer and 52 healthy subjects | Elimination of F. nucleatum by phages | -Oral administration of the phage-guided irinotecan-loaded nanoparticles in piglets led to negligible changes in hemocyte counts, immunoglobulin, and histamine levels, as well as liver and renal functions. -Phage-guided nanotechnology for the modulation of the GM might inspire new approaches for the treatment of colorectal cancer. | [55] |
| Stunting/humans | 15 nonstunted and 15 stunted children | Isolation of gut phages, sterilization, and cross-infection of gut bacteria community belonging to other children | -Gut phages can regulate gut bacterial abundance and composition in an age-specific manner. -Proteobacteria from non-stunted children increased in the presence of phages from younger stunted children. | [14] |
| Leaky gut/rats | 5 Wistar rats | Phage cocktail was given to rats for 10 days | -Increased intestinal permeability may be induced by phages that affect GM. | [73] |
| Obesity and T2D/humans | 61 patients | Fecal transfer by FMT from healthy donors | -FMT achieved ≥20% of lean-associated microbiota in obese with T2D patients | [84] |
| Clostridium difficile infection/humans | 24 subjects with Clostridium difficile infection and 20 healthy controls | Ultradeep metagenomic sequencing of virus-like particle preparations and bacterial 16S rRNA sequencing | -Subjects with Clostridium difficile infection showed a significantly higher abundance of Caudovirales and lower Caudovirales diversity, richness, and evenness compared with healthy controls. -FMT decreased the abundance of Caudovirales in Clostridium difficile infection. Symptoms of infections decreased when most Caudovirales came from the donor and not from the recipient. | [42] |
| Intestinal inflammation and colitis/humans and mice | 58 C57Bl/6 and Swiss Webster germfree mice 20 Patients with active ulcerative colitis | Three independent experiments with a total of n = 23 for vehicle-treated animals and n = 21 for bacteriophage-treated animals | -Treating germ-free mice with bacteriophages led to immune cell expansion in the gut. -Increasing bacteriophage levels exacerbated colitis via toll-like receptors 9 and IFN-γ stimulation. -Phages from active ulcerative colitis patients induced more IFN-γ compared to healthy individuals. | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezzatpour, S.; Mondragon Portocarrero, A.d.C.; Cardelle-Cobas, A.; Lamas, A.; López-Santamarina, A.; Miranda, J.M.; Aguilar, H.C. The Human Gut Virome and Its Relationship with Nontransmissible Chronic Diseases. Nutrients 2023, 15, 977. https://doi.org/10.3390/nu15040977
Ezzatpour S, Mondragon Portocarrero AdC, Cardelle-Cobas A, Lamas A, López-Santamarina A, Miranda JM, Aguilar HC. The Human Gut Virome and Its Relationship with Nontransmissible Chronic Diseases. Nutrients. 2023; 15(4):977. https://doi.org/10.3390/nu15040977
Chicago/Turabian StyleEzzatpour, Shahrzad, Alicia del Carmen Mondragon Portocarrero, Alejandra Cardelle-Cobas, Alexandre Lamas, Aroa López-Santamarina, José Manuel Miranda, and Hector C. Aguilar. 2023. "The Human Gut Virome and Its Relationship with Nontransmissible Chronic Diseases" Nutrients 15, no. 4: 977. https://doi.org/10.3390/nu15040977
APA StyleEzzatpour, S., Mondragon Portocarrero, A. d. C., Cardelle-Cobas, A., Lamas, A., López-Santamarina, A., Miranda, J. M., & Aguilar, H. C. (2023). The Human Gut Virome and Its Relationship with Nontransmissible Chronic Diseases. Nutrients, 15(4), 977. https://doi.org/10.3390/nu15040977










