Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review
Abstract
:1. Introduction
2. Botanical Aspects
3. Phytochemical Profiles
3.1. Fruit
3.2. Oil
3.3. Pulp and Seed
3.4. Leaf and Root
4. Biological and Pharmacological Effects
4.1. Methodology for Literature Search and Included Studies
4.2. Preclinical Studies
4.2.1. Antioxidant Activity
4.2.2. Anti-Inflammatory Activity
4.2.3. Antinociceptive and Analgesic Activity
4.2.4. Antimicrobial Activity
4.2.5. Antiulcer Activity
4.2.6. Neuroprotective Activity
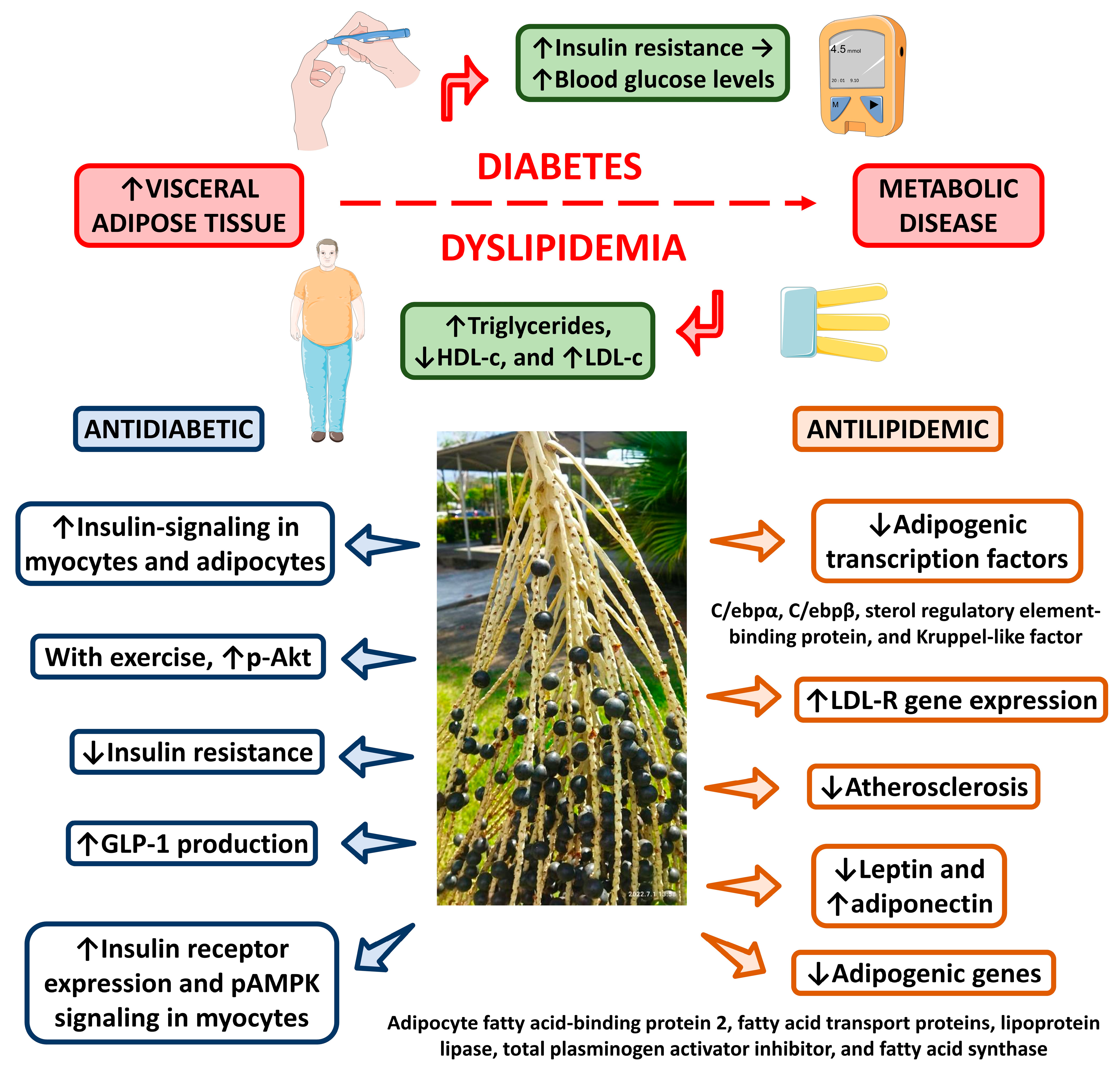
4.2.7. Antilipidemic Activity
4.2.8. Hepatoprotective Activity
4.2.9. Antidiabetic Activity
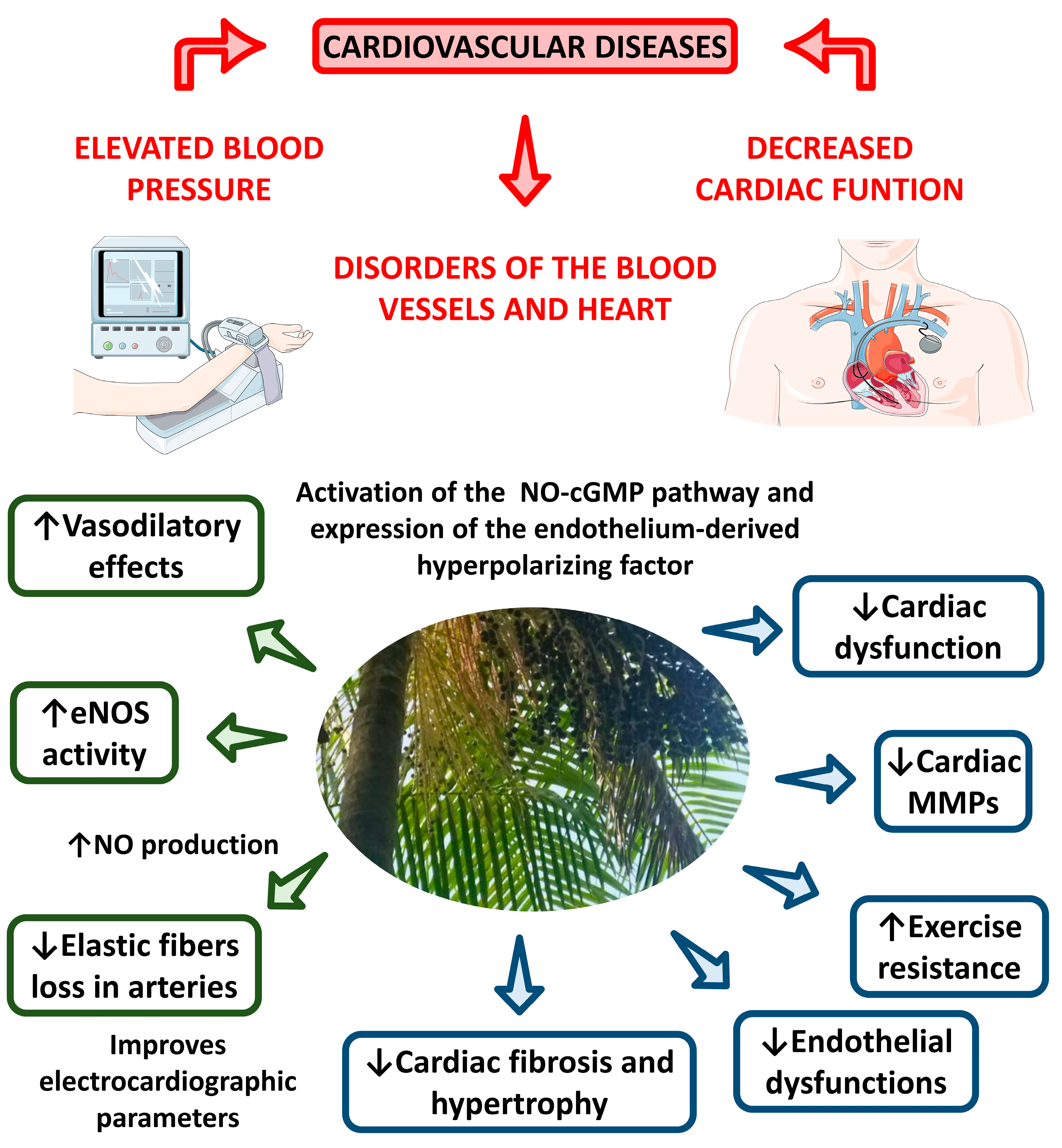
4.2.10. Antihypertensive Activity
4.2.11. Cardioprotective Effects
4.2.12. Renoprotective Effects
4.2.13. Antineoplastic Activity
4.3. Clinical Studies on Açaí and Human Health
4.3.1. Miscellaneous Effects in Healthy Subjects
4.3.2. Auditory Disorder
4.3.3. Effects on Bodyweight, Dyslipidemia and Metabolic Syndrome
4.3.4. Effect on Prostate Cancer
5. Toxicity and Safety Studies
6. Economic Importance
7. Conclusions, Limitations, and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, A.; Ribeiro, A.; Oliveira, É.; Garcia, M.; Soares Júnior, M.; Caliari, M. Structural and physicochemical properties of freeze-dried açaí pulp (Euterpe oleracea Mart.). Food Sci. Technol. 2019, 40, 282–289. [Google Scholar] [CrossRef] [Green Version]
- de Lima Yamaguchi, K.K.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Cruz Gasparini, K.A.; Fonseca, M.; Pastro, M.; Lacerda, L.; Santos, A. Agroclimatic zoning of acai crop (Euterpe oleracea Mart.) for the state of Espírito Santo. Rev. Ciência Agronômica 2015, 46, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Matta, F.V.; Xiong, J.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods 2020, 9, 1481. [Google Scholar] [CrossRef]
- de Souza Silva, A.P.; de Camargo, A.C.; Lazarini, J.G.; Franchin, M.; Sardi, J.d.C.O.; Rosalen, P.L.; de Alencar, S.M. Phenolic Profile and the Antioxidant, Anti-Inflammatory, and Antimicrobial Properties of Açaí (Euterpe oleracea) Meal: A Prospective Study. Foods 2023, 12, 86. [Google Scholar]
- Figueiredo, A.M.; Cardoso, A.C.; Pereira, B.L.B.; Silva, R.A.C.; Ripa, A.; Pinelli, T.F.B.; Oliveira, B.C.; Rafacho, B.P.M.; Ishikawa, L.L.W.; Azevedo, P.S.; et al. Açai supplementation (Euterpe oleracea Mart.) attenuates cardiac remodeling after myocardial infarction in rats through different mechanistic pathways. PLoS ONE 2022, 17, e0264854. [Google Scholar] [CrossRef]
- Martins, G.R.; Guedes, D.; Marques de Paula, U.L.; de Oliveira, M.; Lutterbach, M.T.S.; Reznik, L.Y.; Sérvulo, E.F.C.; Alviano, C.S.; Ribeiro da Silva, A.J.; Alviano, D.S. Açaí (Euterpe oleracea Mart.) Seed Extracts from Different Varieties: A Source of Proanthocyanidins and Eco-Friendly Corrosion Inhibition Activity. Molecules 2021, 26, 3433. [Google Scholar] [CrossRef]
- Torres, T.; Farah, A. Coffee, maté, açaí and beans are the main contributors to the antioxidant capacity of Brazilian’s diet. Eur. J. Nutr. 2017, 56, 1523–1533. [Google Scholar] [CrossRef]
- Nogueira, A.K.M.; Santana, A.C.D.; Garcia, W.S. A dinâmica do mercado de açaí fruto no Estado do Pará: De 1994 a 2009. Rev. Ceres 2013, 60, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, C.; Brigham, A.; Burke, D.; Costa, D.; Giese, N.; Iovin, R.; Grimes Serrano, J.M.; Tanguay-Colucci, S.; Weissner, W.; Windsor, R. An evidence-based systematic review of acai (Euterpe oleracea) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2012, 9, 128–147. [Google Scholar] [CrossRef]
- Lima, A.; Bastos, D.; Alzamora, M.; Bon, E.; Cammarota, M.; Teixeira, R.; Gutarra, M. Physicochemical characterization of residual biomass (seed and fiber) from açaí (Euterpe oleracea) processing and assessment of the potential for energy production and bioproducts. Biomass Convers. Biorefinery 2021, 11, 925–935. [Google Scholar] [CrossRef]
- Monteiro, C.; Filho, H.; Silva, F.G.O.; de Souza, M.F.F.; Sousa, J.A.O.; Franco, Á.X.; Resende, Â.C.; de Moura, R.S.; de Souza, M.H.L.; Soares, P.M.G.; et al. Euterpe oleracea Mart. (Açaí) attenuates experimental colitis in rats: Involvement of TLR4/COX-2/NF-ĸB. Inflammopharmacology 2021, 29, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, D.R.; Silva, E.M.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.B.; Lichtenthäler, R.; Zimmermann, B.F.; Papagiannopoulos, M.; Fabricius, H.; Marx, F.; Maia, J.G.; Almeida, O. Total oxidant scavenging capacity of Euterpe oleracea Mart. (açaí) seeds and identification of their polyphenolic compounds. J. Agric. Food Chem. 2006, 54, 4162–4167. [Google Scholar] [CrossRef]
- da Silva, C.d.M.S.; de Castro, D.A.R.; Santos, M.C.; Almeida, H.d.S.; Schultze, M.; Lüder, U.; Hoffmann, T.; Machado, N.T. Process Analysis of Main Organic Compounds Dissolved in Aqueous Phase by Hydrothermal Processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of Process Temperature, Biomass-to-Water Ratio, and Production Scales. Energies 2021, 14, 5608. [Google Scholar] [CrossRef]
- de Oliveira, M.d.S.P.; Schwartz, G. Açaí—Euterpe oleracea. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–5. [Google Scholar]
- Lorene Simioni, Y.; Acácio Antonio Ferreira, Z.; Aline, A.; Paulo Ricardo, L.; Ivo Mottin, D.; Deise Rosana Silva, S.; Alessandro, N. An overview of Brazilian smoothies: From consumer profile to evaluation of their physicochemical composition, bioactive compounds, antioxidant activity and sensory description. J. Food Bioact. 2020, 10, 9–19. [Google Scholar] [CrossRef]
- Marques, E.S.; Tsuboy, M.S.F.; Carvalho, J.C.T.; Rosa, P.C.P.; Perazzo, F.F.; Gaivão, I.O.M.; Maistro, E.L. First cytotoxic, genotoxic, and antigenotoxic assessment of Euterpe oleracea fruit oil (açaí) in cultured human cells. Genet. Mol. Res. 2017, 16, gmr16039700. [Google Scholar] [CrossRef]
- Favacho, H.; Oliveira, B.; Santos, K.; Medeiros, B.; Sousa, P.; Perazzo, F.; Carvalho, J.C. Anti-inflammatory and antinociceptive activities of Euterpe oleracea oil. Rev. Bras. De Farmacogn. 2011, 21, 105–114. [Google Scholar] [CrossRef]
- de Moraes Arnoso, B.J.; Magliaccio, F.M.; de Araújo, C.A.; de Andrade Soares, R.; Santos, I.B.; de Bem, G.F.; Fernandes-Santos, C.; Ognibene, D.T.; de Moura, R.S.; Resende, A.C.; et al. Açaí seed extract (ASE) rich in proanthocyanidins improves cardiovascular remodeling by increasing antioxidant response in obese high-fat diet-fed mice. Chem.-Biol. Interact. 2022, 351, 109721. [Google Scholar] [CrossRef]
- de Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Converti, A.; Neves de Lima, Á.A. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules 2020, 10, 813. [Google Scholar] [CrossRef]
- da Silva, A.S.; Nunes, D.V.Q.; Carvalho, L.; Santos, I.B.; de Menezes, M.P.; de Bem, G.F.; Costa, C.A.D.; Moura, R.S.; Resende, A.C.; Ognibene, D.T. Açaí (Euterpe oleracea Mart) seed extract protects against maternal vascular dysfunction, hypertension, and fetal growth restriction in experimental preeclampsia. Hypertens Pregnancy 2020, 39, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Souza-Monteiro, J.R.; Hamoy, M.; Santana-Coelho, D.; Arrifano, G.P.; Paraense, R.S.; Costa-Malaquias, A.; Mendonça, J.R.; da Silva, R.F.; Monteiro, W.S.; Rogez, H.; et al. Anticonvulsant properties of Euterpe oleracea in mice. Neurochem. Int. 2015, 90, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.J.M.; Souza-Monteiro, J.R.; Rogez, H.; Crespo-López, M.E.; Do Nascimento, J.L.M.; Silva, E.O. Selective effects of Euterpe oleracea (açai) on Leishmania (Leishmania) amazonensis and Leishmania infantum. Biomed. Pharmacother. 2018, 97, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Illiano, A.; Del Giudice, R.; Raiola, A.; Amoresano, A.; Rigano, M.M.; Piccoli, R.; Monti, D.M. Malvidin and cyanidin derivatives from açai fruit (Euterpe oleracea Mart.) counteract UV-A-induced oxidative stress in immortalized fibroblasts. J. Photochem. Photobiol. B 2017, 172, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Alessandra-Perini, J.; Rodrigues-Baptista, K.C.; Machado, D.E.; Nasciutti, L.E.; Perini, J.A. Anticancer potential, molecular mechanisms and toxicity of Euterpe oleracea extract (açaí): A systematic review. PLoS ONE 2018, 13, e0200101. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Henderson, A. The Genus Euterpe in Brazil-Sellowia Anais Botânicos do Herbário Barbosa Rodrigues; Fundação O Boticário de Proteção à Natureza: Itajaí, Brazil, 2000; Volume 49–52, p. 350. [Google Scholar]
- Rogez, H.; Pompeu, D.R.; Akwie, S.N.T.; Larondelle, Y. Sigmoidal kinetics of anthocyanin accumulation during fruit ripening: A comparison between açai fruits (Euterpe oleracea) and other anthocyanin-rich fruits. J. Food Compos. Anal. 2011, 24, 796–800. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.D.; Mochiutti, S.; Do Nascimento, W.M.O.; De Mattietto, R.A.; Pereira, J.E.S. Açaí-do-Pará, “Palmeiras nativas do Brasil”; Embrapa Amazônia Ocidental: Distrito Federal, Brazil, 2015; Volume 1. [Google Scholar]
- Oppitz, S.J.; Garcia, M.V.; Bruno, R.S.; Zemolin, C.M.; Baptista, B.O.; Turra, B.O.; Barbisan, F.; Cruz, I.; Silveira, A.F.D. Supplementation with açaí (Euterpe Oleracea Martius) for the treatment of chronic tinnitus: Effects on perception, anxiety levels and oxidative metabolism biomarkers. CoDAS 2022, 34, e20210076. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M.; Szulc, M.; Wielgus, K.; Kujawski, R.; Wolski, H.; Seremak-Mrozikiewicz, A. Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials-Review of Perspectives for Novel Therapies. Pharmaceuticals 2021, 14, 269. [Google Scholar] [CrossRef]
- Belmonte-Herrera, B.H.; Domínguez-Avila, J.A.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Preciado-Saldaña, A.M.; Salazar-López, N.J.; López-Martínez, L.X.; Yahia, E.M.; Robles-Sánchez, R.M.; González-Aguilar, G.A. Lesser-Consumed Tropical Fruits and Their by-Products: Phytochemical Content and Their Antioxidant and Anti-Inflammatory Potential. Nutrients 2022, 14, 3663. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Talcott, S.T.; Safe, S.; Mertens-Talcott, S. Absorption and biological activity of phytochemical-rich extracts from acai (Euterpe oleracea Mart.) pulp and oil in vitro. J. Agric. Food Chem. 2008, 56, 3593–3600. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.R.; da Costa, C.A.; de Bem, G.F.; Cordeiro, V.S.; Santos, I.B.; de Carvalho, L.C.; da Conceição, E.P.; Lisboa, P.C.; Ognibene, D.T.; Sousa, P.J.; et al. Euterpe oleracea Mart.-Derived Polyphenols Protect Mice from Diet-Induced Obesity and Fatty Liver by Regulating Hepatic Lipogenesis and Cholesterol Excretion. PLoS ONE 2015, 10, e0143721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, M.O.; Silva, M.; Silva, M.E.; Oliveira Rde, P.; Pedrosa, M.L. Diet supplementation with acai (Euterpe oleracea Mart.) pulp improves biomarkers of oxidative stress and the serum lipid profile in rats. Nutrition 2010, 26, 804–810. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Rios, J.; Jilma-Stohlawetz, P.; Pacheco-Palencia, L.A.; Meibohm, B.; Talcott, S.T.; Derendorf, H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J. Agric. Food Chem. 2008, 56, 7796–7802. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Patel, D.; Huang, D.; Kababick, J.P. Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart.(Acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.V.; Ferreira Ferreira da Silveira, T.; Mattietto, R.D.A.; Padilha de Oliveira, M.D.S.; Godoy, H.T. Chemical composition and antioxidant capacity of açaí (Euterpe oleracea) genotypes and commercial pulps. J. Sci. Food Agric. 2017, 97, 1467–1474. [Google Scholar] [CrossRef]
- ALNasser, M.N.; Mellor, I.R.; Carter, W.G. A Preliminary Assessment of the Nutraceutical Potential of Acai Berry (Euterpe sp.) as a Potential Natural Treatment for Alzheimer’s Disease. Molecules 2022, 27, 4891. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Costa, B.E.; Ferreira, W.H.; Goycoolea, F.M.; Murray, B.S.; Andrade, C.T. Improved Antioxidant and Mechanical Properties of Food Packaging Films Based on Chitosan/Deep Eutectic Solvent, Containing Açaí-Filled Microcapsules. Molecules 2023, 28, 1507. [Google Scholar] [CrossRef] [PubMed]
- Brunschwig, C.; Leba, L.-J.; Saout, M.; Martial, K.; Bereau, D.; Robinson, J.-C. Chemical Composition and Antioxidant Activity of Euterpe oleracea Roots and Leaflets. Int. J. Mol. Sci. 2016, 18, 61. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; da Costa, É.C.P.; de Aragão Tavares, E.; da Silva, V.C.; Guerra, G.C.B.; Pereira, J.R.; de Araújo Moura Lemos, T.M.; de Negreiros, M.M.F.; de Oliveira Rocha, H.A.; et al. Increase in the Antioxidant and Anti-Inflammatory Activity of Euterpe oleracea Martius Oil Complexed in β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin. Int. J. Mol. Sci. 2021, 22, 11524. [Google Scholar] [CrossRef] [PubMed]
- Xavier, G.S.; Teles, A.M.; Moragas-Tellis, C.J.; Chagas, M.; Behrens, M.D.; Moreira, W.F.F.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Nascimento, M.; Almeida-Souza, F. Inhibitory Effect of Catechin-Rich Açaí Seed Extract on LPS-Stimulated RAW 264.7 Cells and Carrageenan-Induced Paw Edema. Foods 2021, 10, 1014. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Machado, A.K.; Assmann, C.E.; Andrade, E.N.; Azzolin, V.F.; Duarte, M.; Prado-Lima, P.; Riffel, R.T.; Maia-Ribeiro, E.A.; Cadoná, F.C.; et al. Açaí (Euterpe oleracea Mart.) reduces the inflammatory response triggered in vitro by the antipsychotic drug olanzapine in RAW 264.7 macrophage cells. Acta Sci. Pol. Technol. Aliment. 2021, 20, 149–163. [Google Scholar] [CrossRef]
- Melo, P.S.; Massarioli, A.P.; Lazarini, J.G.; Soares, J.C.; Franchin, M.; Rosalen, P.L.; Alencar, S.M. Simulated gastrointestinal digestion of Brazilian açaí seeds affects the content of flavan-3-ol derivatives, and their antioxidant and anti-inflammatory activities. Heliyon 2020, 6, e05214. [Google Scholar] [CrossRef]
- Kandagatla, S.K.; Uhl, R.T.; Graf, T.N.; Oberlies, N.H.; Raner, G.M. Pheophorbide Derivatives Isolated from Açaí Berries (Euterpea oleracea) Activate an Antioxidant Response Element In Vitro. Nat. Prod. Commun. 2019, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Soares, E.R.; Monteiro, E.B.; de Bem, G.F.; Inada, K.O.P.; Torres, A.G.; Perrone, D.; Soulage, C.O.; Monteiro, M.C.; Resende, A.C.; Moura-Nunes, N.; et al. Up-regulation of Nrf2-antioxidant signaling by Açaí (Euterpe oleracea Mart.) extract prevents oxidative stress in human endothelial cells. J. Funct. Foods 2017, 37, 107–115. [Google Scholar] [CrossRef]
- Vrbovská, H.; Babincová, M. Comparative analysis of synthetic and nutraceutical antioxidants as possible neuroprotective agents. Pharmazie 2016, 71, 724–726. [Google Scholar] [CrossRef]
- Barros, L.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Santos, E.A.; Regis, W.C.B.; Ferreira, I.C.F.R. The powerful in vitro bioactivity of Euterpe oleracea Mart. seeds and related phenolic compounds. Ind. Crops Prod. 2015, 76, 318–322. [Google Scholar] [CrossRef] [Green Version]
- Bonomo, L.D.F.; Silva, D.N.; Boasquivis, P.F.; Paiva, F.A.; Guerra, J.F.D.C.; Martins, T.A.F.; de Jesus Torres, Á.G.; de Paula, I.T.B.R.; Caneschi, W.L.; Jacolot, P.; et al. Açaí (Euterpe oleracea Mart.) Modulates Oxidative Stress Resistance in Caenorhabditis elegans by Direct and Indirect Mechanisms. PLoS ONE 2014, 9, e89933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Wu, X.; Patterson, K.M.; Barnes, J.; Carter, S.G.; Scherwitz, L.; Beaman, R.; Endres, J.R.; Schauss, A.G. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J. Agric. Food Chem. 2008, 56, 8326–8333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, Y.W.; Chai, H.B.; Keller, W.J.; Kinghorn, A.D. Lignans and other constituents of the fruits of Euterpe oleracea (Acai) with antioxidant and cytoprotective activities. J. Agric. Food Chem. 2008, 56, 7759–7764. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.S.; Hart, A.N.; Shanbrom, E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae mart. (acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef]
- Martins, G.R.; do Amaral, F.R.L.; Brum, F.L.; Mohana-Borges, R.; de Moura, S.S.T.; Ferreira, F.A.; Sangenito, L.S.; Santos, A.L.S.; Figueiredo, N.G.; Silva, A.S.A.D. Chemical characterization, antioxidant and antimicrobial activities of açaí seed (Euterpe oleracea Mart.) extracts containing A- and B-type procyanidins. LWT 2020, 132, 109830. [Google Scholar] [CrossRef]
- Ferreira, L.T.; Venancio, V.P.; Kawano, T.; Abrão, L.C.C.; Tavella, T.A.; Almeida, L.D.; Pires, G.S.; Bilsland, E.; Sunnerhagen, P.; Azevedo, L.; et al. Chemical Genomic Profiling Unveils the in Vitro and in Vivo Antiplasmodial Mechanism of Açaí (Euterpe oleracea Mart.) Polyphenols. ACS Omega 2019, 4, 15628–15635. [Google Scholar] [CrossRef] [Green Version]
- Dias-Souza, M.V.; dos Santos, R.M.; Cerávolo, I.P.; Cosenza, G.; Ferreira Marçal, P.H.; Figueiredo, F.J.B. Euterpe oleracea pulp extract: Chemical analyses, antibiofilm activity against Staphylococcus aureus, cytotoxicity and interference on the activity of antimicrobial drugs. Microb. Pathog. 2018, 114, 29–35. [Google Scholar] [CrossRef]
- Sprenger, L.K.; Giese, E.G.; dos Santos, J.N.; Molento, M.B. In vitro antibacterial effect of Euterpe oleracea Mart. and Theobroma grandiflorum hydroalcoholic extracts. Arch. Veterian Sci. 2016, 21, 2. [Google Scholar] [CrossRef] [Green Version]
- Cadoná, F.C.; de Souza, D.V.; Fontana, T.; Bodenstein, D.F.; Ramos, A.P.; Sagrillo, M.R.; Salvador, M.; Mota, K.; Davidson, C.B.; Ribeiro, E.E.; et al. Açaí (Euterpe oleracea Mart.) as a Potential Anti-neuroinflammatory Agent: NLRP3 Priming and Activating Signal Pathway Modulation. Mol. Neurobiol. 2021, 58, 4460–4476. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Lichtenstein, M.P.; Souza-Monteiro, J.R.; Farina, M.; Rogez, H.; Carvalho, J.C.T.; Suñol, C.; Crespo-López, M.E. Clarified Açaí (Euterpe oleracea) Juice as an Anticonvulsant Agent: In Vitro Mechanistic Study of GABAergic Targets. Oxidative Med. Cell Longev. 2018, 2018, 2678089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torma, P.D.; Brasil, A.V.; Carvalho, A.V.; Jablonski, A.; Rabelo, T.K.; Moreira, J.C.; Gelain, D.P.; Flôres, S.H.; Augusti, P.R.; Rios, A.O. Hydroethanolic extracts from different genotypes of açaí (Euterpe oleracea) presented antioxidant potential and protected human neuron-like cells (SH-SY5Y). Food Chem. 2017, 222, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.K.; Andreazza, A.C.; da Silva, T.M.; Boligon, A.A.; do Nascimento, V.; Scola, G.; Duong, A.; Cadoná, F.C.; Ribeiro, E.E.; da Cruz, I.B. Neuroprotective Effects of Açaí (Euterpe oleracea Mart.) against Rotenone In Vitro Exposure. Oxidative Med. Cell. Longev. 2016, 2016, 8940850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajit, D.; Simonyi, A.; Li, R.; Chen, Z.; Hannink, M.; Fritsche, K.L.; Mossine, V.V.; Smith, R.E.; Dobbs, T.K.; Luo, R.; et al. Phytochemicals and botanical extracts regulate NF-κB and Nrf2/ARE reporter activities in DI TNC1 astrocytes. Neurochem. Int. 2016, 97, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Poulose, S.M.; Fisher, D.R.; Bielinski, D.F.; Gomes, S.M.; Rimando, A.M.; Schauss, A.G.; Shukitt-Hale, B. Restoration of stressor-induced calcium dysregulation and autophagy inhibition by polyphenol-rich açaí (Euterpe spp.) fruit pulp extracts in rodent brain cells in vitro. Nutrition 2014, 30, 853–862. [Google Scholar] [CrossRef]
- Wong, D.Y.; Musgrave, I.F.; Harvey, B.S.; Smid, S.D. Açaí (Euterpe oleraceae Mart.) berry extract exerts neuroprotective effects against β-amyloid exposure in vitro. Neurosci. Lett. 2013, 556, 221–226. [Google Scholar] [CrossRef]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef]
- Spada, P.D.; Dani, C.; Bortolini, G.V.; Funchal, C.; Henriques, J.A.; Salvador, M. Frozen fruit pulp of Euterpe oleraceae Mart. (Acai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J. Med. Food 2009, 12, 1084–1088. [Google Scholar] [CrossRef]
- Trindade, P.L.; Soares, E.D.R.; Monteiro, E.B.; Resende, Â.C.; Moura-Nunes, N.; Souza-Mello, V.; Ferraz, D.C.; Daleprane, J.B. Antiadipogenic effects of açai seed extract on high fat diet-fed mice and 3T3-L1 adipocytes: A potential mechanism of action. Life Sci. 2019, 228, 316–322. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Dias, M.M.D.S.; Noratto, G.; Talcott, S.; Mertens-Talcott, S.U. Anti-lipidaemic and anti-inflammatory effect of açai (Euterpe oleracea Martius) polyphenols on 3T3-L1 adipocytes. J. Funct. Foods 2016, 23, 432–443. [Google Scholar] [CrossRef]
- Costa, R.; Azevedo, D.; Barata, P.; Soares, R.; Guido, L.F.; Carvalho, D.O. Antiangiogenic and Antioxidant In Vitro Properties of Hydroethanolic Extract from açaí (Euterpe oleracea) Dietary Powder Supplement. Molecules 2021, 26, 2011. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, M.F.; Romualdo, G.R.; Vanderveer, L.A.; Franco-Barraza, J.; Cukierman, E.; Clapper, M.L.; Carvalho, R.F.; Barbisan, L.F. Lyophilized açaí pulp (Euterpe oleracea Mart) attenuates colitis-associated colon carcinogenesis while its main anthocyanin has the potential to affect the motility of colon cancer cells. Food Chem. Toxicol. 2018, 121, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibuyi, N.R.S.; Thipe, V.C.; Panjtan-Amiri, K.; Meyer, M.; Katti, K.V. Green synthesis of gold nanoparticles using Acai berry and Elderberry extracts and investigation of their effect on prostate and pancreatic cancer cells. Nanobiomedicine 2021, 8, 1849543521995310. [Google Scholar] [CrossRef] [PubMed]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.; Silva, J.R.; Fascineli, M.L.; de Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; Carneiro, F.P.; et al. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J. Photochem. Photobiol. B 2017, 166, 301–310. [Google Scholar] [CrossRef]
- Freitas, D.D.S.; Morgado-Díaz, J.A.; Gehren, A.S.; Vidal, F.C.B.; Fernandes, R.M.T.; Romão, W.; Tose, L.V.; Frazão, F.N.S.; Costa, M.C.P.; Silva, D.F.; et al. Cytotoxic analysis and chemical characterization of fractions of the hydroalcoholic extract of the Euterpe oleracea Mart. seed in the MCF-7 cell line. J. Pharm. Pharmacol. 2017, 69, 714–721. [Google Scholar] [CrossRef]
- Silva, D.F.; Vidal, F.C.B.; Santos, D.; Costa, M.C.P.; Morgado-Díaz, J.A.; do Desterro Soares Brandão Nascimento, M.; de Moura, R.S. Cytotoxic effects of Euterpe oleracea Mart. in malignant cell lines. BMC Complement. Altern. Med. 2014, 14, 175. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.M.D.S.; Noratto, G.; Martino, H.S.D.; Arbizu, S.; Peluzio, M.D.C.G.; Talcott, S.; Ramos, A.M.; Mertens-Talcott, S.U. Pro-Apoptotic Activities of Polyphenolics From Açai (Euterpe oleracea Martius) in Human SW-480 Colon Cancer Cells. Nutr. Cancer 2014, 66, 1394–1405. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.U.; Talcott, S.T. In vitro absorption and antiproliferative activities of monomeric and polymeric anthocyanin fractions from açai fruit (Euterpe oleracea Mart.). Food Chem. 2010, 119, 1071–1078. [Google Scholar] [CrossRef]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Spada, P.D.; de Souza, G.G.; Bortolini, G.V.; Henriques, J.A.; Salvador, M. Antioxidant, mutagenic, and antimutagenic activity of frozen fruits. J. Med. Food 2008, 11, 144–151. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Percival, S.S.; Talcott, S.T. Açai (Euterpe oleracea Mart.) Polyphenolics in Their Glycoside and Aglycone Forms Induce Apoptosis of HL-60 Leukemia Cells. J. Agric. Food Chem. 2006, 54, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Alqurashi, R.M.; Alarifi, S.N.; Walton, G.E.; Costabile, A.F.; Rowland, I.R.; Commane, D.M. In vitro approaches to assess the effects of açai (Euterpe oleracea) digestion on polyphenol availability and the subsequent impact on the faecal microbiota. Food Chem. 2017, 234, 190–198. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Interdonato, L.; Marino, Y.; Crupi, R.; Gugliandolo, E.; Macrì, F.; Di Paola, D.; et al. Complex Interplay between Autophagy and Oxidative Stress in the Development of Endometriosis. Antioxidants 2022, 11, 2484. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Stavroullakis, A.; Oliveira, T.; Prakki, A. Cytotoxicity and potential anti-inflammatory activity of velutin on RAW 264.7 cell line differentiation: Implications in periodontal bone loss. Arch. Oral Biol. 2017, 83, 348–356. [Google Scholar] [CrossRef]
- Brito, C.; Stavroullakis, A.T.; Ferreira, A.C.; Li, K.; Oliveira, T.; Nogueira-Filho, G.; Prakki, A. Extract of acai-berry inhibits osteoclast differentiation and activity. Arch. Oral Biol. 2016, 68, 29–34. [Google Scholar] [CrossRef]
- Barbosa, P.O.; Souza, M.O.; Silva, M.P.S.; Santos, G.T.; Silva, M.E.; Bermano, G.; Freitas, R.N. Açaí (Euterpe oleracea Martius) supplementation improves oxidative stress biomarkers in liver tissue of dams fed a high-fat diet and increases antioxidant enzymes’ gene expression in offspring. Biomed. Pharmacother. 2021, 139, 111627. [Google Scholar] [CrossRef]
- Alegre, P.; Mathias, L.; Lourenço, M.A.; Santos, P.P.D.; Gonçalves, A.; Fernandes, A.A.; Gaiolla, P.S.A.; Minicucci, M.F.; Zornoff, L.; Paiva, S.A.R.; et al. Euterpe Oleracea Mart. (Açaí) Reduces Oxidative Stress and Improves Energetic Metabolism in Myocardial Ischemia-Reperfusion Injury in Rats. Arq. Bras. Cardiol. 2020, 114, 78–86. [Google Scholar] [CrossRef]
- Nascimento, V.H.; Lima, C.D.; Paixão, J.T.; Freitas, J.J.; Kietzer, K.S. Antioxidant effects of açaí seed (Euterpe oleracea) in anorexia-cachexia syndrome induced by Walker-256 tumor. Acta Cir. Bras. 2016, 31, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Guerra, J.F.; Magalhães, C.L.; Costa, D.C.; Silva, M.E.; Pedrosa, M.L. Dietary açai modulates ROS production by neutrophils and gene expression of liver antioxidant enzymes in rats. J. Clin. Biochem. Nutr. 2011, 49, 188–194. [Google Scholar] [CrossRef] [Green Version]
- de Moura, R.S.; Ferreira, T.S.; Lopes, A.A.; Pires, K.M.P.; Nesi, R.T.; Resende, A.C.; Souza, P.J.C.; da Silva, A.J.R.; Borges, R.M.; Porto, L.C.; et al. Effects of Euterpe oleracea Mart. (AÇAÍ) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine 2012, 19, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Sudo, R.T.; Neto, M.L.; Monteiro, C.E.; Amaral, R.V.; Resende, Â.C.; Souza, P.J.; Zapata-Sudo, G.; Moura, R.S. Antinociceptive effects of hydroalcoholic extract from Euterpe oleracea Mart. (Açaí) in a rodent model of acute and neuropathic pain. BMC Complement. Altern. Med. 2015, 15, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cury, B.J.; Boeing, T.; Somensi, L.B.; Mariano, L.N.B.; de Andrade, S.F.; Breviglieri, E.; Klein-Junior, L.C.; de Souza, P.; da Silva, L.M. Açaí berries (Euterpe oleracea Mart.) dried extract improves ethanol-induced ulcer in rats. J. Pharm. Pharmacol. 2020, 72, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Interdonato, L.; Marino, Y.; Franco, G.A.; Arangia, A.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Impellizzeri, D.; Fusco, R.; Cuzzocrea, S.; et al. Açai Berry Administration Promotes Wound Healing through Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2023, 24, 834. [Google Scholar] [PubMed]
- de Bem, G.F.; Okinga, A.; Ognibene, D.T.; da Costa, C.A.; Santos, I.B.; Soares, R.A.; Silva, D.L.B.; da Rocha, A.P.M.; Isnardo Fernandes, J.; Fraga, M.C.; et al. Anxiolytic and antioxidant effects of Euterpe oleracea Mart. (açaí) seed extract in adult rat offspring submitted to periodic maternal separation. Appl. Physiol. Nutr. Metab. 2020, 45, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, C.; Aydin, S.; Donertas, B.; Oner, S.; Kilic, F.S. Effects of Euterpe oleracea to Enhance Learning and Memory in a Conditioned Nicotinic and Muscarinic Receptor Response Paradigm by Modulation of Cholinergic Mechanisms in Rats. J. Med. Food 2020, 23, 388–394. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, F.; Kuo, J.; Wohlenberg, M.F.; da Rocha Frusciante, M.; Freitas, M.; Oliveira, A.S.; Andrade, R.B.; Wannmacher, C.M.; Dani, C.; Funchal, C. Subchronic treatment with acai frozen pulp prevents the brain oxidative damage in rats with acute liver failure. Metab. Brain Dis. 2016, 31, 1427–1434. [Google Scholar] [CrossRef]
- Carey, A.N.; Miller, M.G.; Fisher, D.R.; Bielinski, D.F.; Gilman, C.K.; Poulose, S.M.; Shukitt-Hale, B. Dietary supplementation with the polyphenol-rich açaí pulps (Euterpe oleracea Mart. and Euterpe precatoria Mart.) improves cognition in aged rats and attenuates inflammatory signaling in BV-2 microglial cells. Nutr. Neurosci. 2017, 20, 238–245. [Google Scholar] [CrossRef]
- de Souza Machado, F.; Marinho, J.P.; Abujamra, A.L.; Dani, C.; Quincozes-Santos, A.; Funchal, C. Carbon Tetrachloride Increases the Pro-inflammatory Cytokines Levels in Different Brain Areas of Wistar Rats: The Protective Effect of Acai Frozen Pulp. Neurochem. Res. 2015, 40, 1976–1983. [Google Scholar] [CrossRef]
- Poulose, S.M.; Bielinski, D.F.; Carey, A.; Schauss, A.G.; Shukitt-Hale, B. Modulation of oxidative stress, inflammation, autophagy and expression of Nrf2 in hippocampus and frontal cortex of rats fed with açaí-enriched diets. Nutr. Neurosci. 2017, 20, 305–315. [Google Scholar] [CrossRef]
- Souza-Monteiro, J.R.; Arrifano, G.P.F.; Queiroz, A.I.D.G.; Mello, B.S.F.; Custódio, C.S.; Macêdo, D.S.; Hamoy, M.; Paraense, R.S.O.; Bittencourt, L.O.; Lima, R.R.; et al. Antidepressant and Antiaging Effects of Açaí (Euterpe oleracea Mart.) in Mice. Oxidative Med. Cell. Longev. 2019, 2019, 3614960. [Google Scholar] [CrossRef] [Green Version]
- Faria, E.S.B.S.; Carvalho, H.O.; Taglialegna, T.; Barros, A.S.A.; da Cunha, E.L.; Ferreira, I.M.; Keita, H.; Navarrete, A.; Carvalho, J.C.T. Effect of Euterpe oleracea Mart. (Açaí) Oil on Dyslipidemia Caused by Cocos nucifera L. Saturated Fat in Wistar Rats. J. Med. Food 2017, 20, 830–837. [Google Scholar] [CrossRef]
- e Souza, B.S.F.; Carvalho, H.O.; Ferreira, I.M.; da Cunha, E.L.; Barros, A.S.; Taglialegna, T.; Carvalho, J.C.T. Effect of the treatment with Euterpe oleracea Mart. oil in rats with Triton-induced dyslipidemia. Biomed. Pharmacother. 2017, 90, 542–547. [Google Scholar] [CrossRef]
- de Souza, M.O.; Souza, E.S.L.; de Brito Magalhães, C.L.; de Figueiredo, B.B.; Costa, D.C.; Silva, M.E.; Pedrosa, M.L. The hypocholesterolemic activity of açaí (Euterpe oleracea Mart.) is mediated by the enhanced expression of the ATP-binding cassette, subfamily G transporters 5 and 8 and low-density lipoprotein receptor genes in the rat. Nutr. Res. 2012, 32, 976–984. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.C.; Batista, A.; Costa, D.; Moura-Nunes, N.; Koury, J.C.; da Costa, C.A.; Resende, Â.C.; Daleprane, J.B. Açai (Euterpe oleracea Mart.) seed flour prevents obesity-induced hepatic steatosis regulating lipid metabolism by increasing cholesterol excretion in high-fat diet-fed mice. Food Res. Int. (Ott. Ont.) 2018, 111, 408–415. [Google Scholar] [CrossRef]
- Feio, C.A.; Izar, M.C.; Ihara, S.S.; Kasmas, S.H.; Martins, C.M.; Feio, M.N.; Maués, L.A.; Borges, N.C.; Moreno, R.A.; Póvoa, R.M.; et al. Euterpe Oleracea (açai) Modifies Sterol Metabolism and Attenuates Experimentally-Induced Atherosclerosis. J. Atheroscler. Thromb. 2012, 19, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, P.O.; de Souza, M.O.; Paiva, D.P.D.; Silva, M.E.; Lima, W.G.; Bermano, G.; Freitas, R.N. Açaí (Euterpe oleracea Martius) supplementation in the diet during gestation and lactation attenuates liver steatosis in dams and protects offspring. Eur. J. Nutr. 2020, 59, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.M.F.; Reis, L.L.T.; Lopes, J.M.M.; Lage, N.N.; Guerra, J.; Zago, H.P.; Bonomo, L.F.; Pereira, R.R.; Lima, W.G.; Silva, M.E.; et al. Açai improves non-alcoholic fatty liver disease (NAFLD) induced by fructose. Nutr. Hosp. 2018, 35, 318–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhang, J.; Wang, C.; Qu, S.; Zhu, Y.; Yang, Z.; Wang, L. Açaí (Euterpe oleracea Mart.) attenuates alcohol-induced liver injury in rats by alleviating oxidative stress and inflammatory response. Exp. Ther. Med. 2018, 15, 166–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bem, G.F.; da Costa, C.A.; da Silva Cristino Cordeiro, V.; Santos, I.B.; de Carvalho, L.; de Andrade Soares, R.; Ribeiro, J.H.; de Souza, M.A.V.; da Cunha Sousa, P.J.; Ognibene, D.T.; et al. Euterpe oleracea Mart. (açaí) seed extract associated with exercise training reduces hepatic steatosis in type 2 diabetic male rats. J. Nutr. Biochem. 2018, 52, 70–81. [Google Scholar] [CrossRef]
- Pereira, R.R.; de Abreu, I.C.; Guerra, J.F.; Lage, N.N.; Lopes, J.M.; Silva, M.; de Lima, W.G.; Silva, M.E.; Pedrosa, M.L. Açai (Euterpe oleracea Mart.) Upregulates Paraoxonase 1 Gene Expression and Activity with Concomitant Reduction of Hepatic Steatosis in High-Fat Diet-Fed Rats. Oxidative Med. Cell. Longev. 2016, 2016, 8379105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Freitas Carvalho, M.M.; Lage, N.N.; de Souza Paulino, A.H.; Pereira, R.R.; de Almeida, L.T.; da Silva, T.F.; de Brito Magalhães, C.L.; de Lima, W.G.; Silva, M.E.; Pedrosa, M.L.; et al. Effects of açai on oxidative stress, ER stress, and inflammation-related parameters in mice with high fat diet-fed induced NAFLD. Sci. Rep. 2019, 9, 8107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, J.F.D.C.; Maciel, P.S.; de Abreu, I.C.M.E.; Pereira, R.R.; Silva, M.; Cardoso, L.D.M.; Pinheiro-Sant’Ana, H.M.; Lima, W.G.D.; Silva, M.E.; Pedrosa, M.L. Dietary açai attenuates hepatic steatosis via adiponectin-mediated effects on lipid metabolism in high-fat diet mice. J. Funct. Foods 2015, 14, 192–202. [Google Scholar] [CrossRef]
- de Bem, G.F.; Costa, C.A.; Santos, I.B.; Cristino Cordeiro, V.D.S.; de Carvalho, L.; de Souza, M.A.V.; Soares, R.A.; Sousa, P.; Ognibene, D.T.; Resende, A.C.; et al. Antidiabetic effect of Euterpe oleracea Mart. (açaí) extract and exercise training on high-fat diet and streptozotocin-induced diabetic rats: A positive interaction. PLoS ONE 2018, 13, e0199207. [Google Scholar] [CrossRef] [Green Version]
- da Costa, C.A.; de Oliveira, P.R.; de Bem, G.F.; de Cavalho, L.C.; Ognibene, D.T.; da Silva, A.F.; Dos Santos Valença, S.; Pires, K.M.; da Cunha Sousa, P.J.; de Moura, R.S.; et al. Euterpe oleracea Mart.-derived polyphenols prevent endothelial dysfunction and vascular structural changes in renovascular hypertensive rats: Role of oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 1199–1209. [Google Scholar] [CrossRef]
- de Bem, G.F.; da Costa, C.A.; de Oliveira, P.R.; Cordeiro, V.S.; Santos, I.B.; de Carvalho, L.C.; Souza, M.A.; Ognibene, D.T.; Daleprane, J.B.; Sousa, P.J.; et al. Protective effect of Euterpe oleracea Mart (açaí) extract on programmed changes in the adult rat offspring caused by maternal protein restriction during pregnancy. J. Pharm. Pharmacol. 2014, 66, 1328–1338. [Google Scholar] [CrossRef]
- Vilhena, J.C.; Lopes de Melo Cunha, L.; Jorge, T.M.; de Lucena Machado, M.; de Andrade Soares, R.; Santos, I.B.; Freitas de Bem, G.; Fernandes-Santos, C.; Ognibene, D.T.; Soares de Moura, R.; et al. Açaí Reverses Adverse Cardiovascular Remodeling in Renovascular Hypertension: A Comparative Effect With Enalapril. J. Cardiovasc. Pharmacol. 2021, 77, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, V.N.; Miranda, D.C.; Isoldi, M.C.; Drummond, F.R.; Soares, L.L.; Reis, E.C.C.; Pelúzio, M.; Pedrosa, M.L.; Silva, M.E.; Natali, A.J. Effects of aerobic exercise training and açai supplementation on cardiac structure and function in rats submitted to a high-fat diet. Food Res. Int. (Ott. Ont.) 2021, 141, 110168. [Google Scholar] [CrossRef]
- Pontes, V.C.B.; Tavares, J.; Rosenstock, T.R.; Rodrigues, D.S.; Yudi, M.I.; Soares, J.P.M.; Ribeiro, S.C.; Sutti, R.; Torres, L.M.B.; de Melo, F.H.M.; et al. Increased acute blood flow induced by the aqueous extract of Euterpe oleracea Mart. fruit pulp in rats in vivo is not related to the direct activation of endothelial cells. J. Ethnopharmacol. 2021, 271, 113885. [Google Scholar] [CrossRef] [PubMed]
- Mathias, L.; Alegre, P.H.C.; Dos Santos, I.O.F.; Bachiega, T.; Figueiredo, A.M.; Chiuso-Minicucci, F.; Fernandes, A.A.; Bazan, S.G.Z.; Minicucci, M.F.; Azevedo, P.S.; et al. Euterpe oleracea Mart. (Açai) Supplementation Attenuates Acute Doxorubicin-Induced Cardiotoxicity in Rats. Cell Physiol. Biochem. 2019, 53, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Zapata-Sudo, G.; da Silva, J.S.; Pereira, S.L.; Souza, P.J.; de Moura, R.S.; Sudo, R.T. Oral treatment with Euterpe oleracea Mart. (açaí) extract improves cardiac dysfunction and exercise intolerance in rats subjected to myocardial infarction. BMC Complement. Altern. Med. 2014, 14, 227. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, V.; Carvalho, L.C.R.M.; Bem, G.; da Costa, C.; Souza, M.; Sousa, P.; Souza, M.; Rocha, V.; Carvalho, J.; Moura, R.; et al. Euterpe oleracea Mart. extract prevents vascular remodeling and endothelial dysfunction in spontaneously hypertensive rats. Int. J. Appl. Res. Nat. Prod. 2015, 8, 6–16. [Google Scholar]
- da Costa, C.A.; Ognibene, D.T.; Cordeiro, V.S.C.; de Bem, G.F.; Santos, I.B.; Soares, R.A.; de Melo Cunha, L.L.; Carvalho, L.; de Moura, R.S.; Resende, A.C. Effect of Euterpe oleracea Mart. Seeds Extract on Chronic Ischemic Renal Injury in Renovascular Hypertensive Rats. J. Med. Food 2017, 20, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- da Silva Cristino Cordeiro, V.; de Bem, G.F.; da Costa, C.A.; Santos, I.B.; de Carvalho, L.C.R.M.; Ognibene, D.T.; da Rocha, A.P.M.; de Carvalho, J.J.; de Moura, R.S.; Resende, A.C. Euterpe oleracea Mart. seed extract protects against renal injury in diabetic and spontaneously hypertensive rats: Role of inflammation and oxidative stress. Eur. J. Nutr. 2018, 57, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Unis, A. Açai berry extract attenuates glycerol-induced acute renal failure in rats. Ren. Fail. 2015, 37, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Morsy, E.M.; Ahmed, M.A.; Ahmed, A.A. Attenuation of renal ischemia/reperfusion injury by açaí extract preconditioning in a rat model. Life Sci. 2015, 123, 35–42. [Google Scholar] [CrossRef]
- Alessandra-Perini, J.; Perini, J.A.; Rodrigues-Baptista, K.C.; de Moura, R.S.; Junior, A.P.; Dos Santos, T.A.; Souza, P.J.C.; Nasciutti, L.E.; Machado, D.E. Euterpe oleracea extract inhibits tumorigenesis effect of the chemical carcinogen DMBA in breast experimental cancer. BMC Complement. Altern. Med. 2018, 18, 116. [Google Scholar] [CrossRef] [Green Version]
- Fragoso, M.F.; Romualdo, G.R.; Ribeiro, D.A.; Barbisan, L.F. Açai (Euterpe oleracea Mart.) feeding attenuates dimethylhydrazine-induced rat colon carcinogenesis. Food Chem. Toxicol. 2013, 58, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Choi, Y.J.; Kim, N.; Nam, R.H.; Lee, S.; Lee, H.S.; Lee, H.N.; Surh, Y.J.; Lee, D.H. Açaí Berries Inhibit Colon Tumorigenesis in Azoxymethane/Dextran Sulfate Sodium-Treated Mice. Gut Liver 2017, 11, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Romualdo, G.R.; Fragoso, M.F.; Borguini, R.G.; de Araújo Santiago, M.C.P.; Fernandes, A.A.H.; Barbisan, L.F. Protective effects of spray-dried açaí (Euterpe oleracea Mart) fruit pulp against initiation step of colon carcinogenesis. Food Res. Int. 2015, 77, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Stoner, G.D.; Wang, L.S.; Seguin, C.; Rocha, C.; Stoner, K.; Chiu, S.; Kinghorn, A.D. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm. Res. 2010, 27, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.H.; Choi, S.; Kim, B.-H. Skin Wound Healing Effects and Action Mechanism of Acai Berry Water Extracts. Toxicol. Res. 2017, 33, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.H.; Kim, B.-H. Oral Wound Healing Effects of Acai Berry Water Extracts in Rat Oral Mucosa. Toxicol. Res. 2018, 34, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muto, N.A.; Hamoy, M.; Rodrigues Lucas, D.C.; Teixeira, B.B.; Santos Almeida, A.F.; de Castro Navegantes, T.; de Sousa Ferreira de Sá, V.S.; de Moraes, B.P.; do Vale Medeiros, J.P.; Dos Santos, Y.A.; et al. Myorelaxation, respiratory depression and electrocardiographic changes caused by the administration of extract of açai (Euterpe oleracea Mart.) stone in rats. Toxicol. Rep. 2021, 8, 829–838. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Soares, R.; de Oliveira, B.C.; de Bem, G.F.; de Menezes, M.P.; Romão, M.H.; Santos, I.B.; da Costa, C.A.; de Carvalho, L.; Nascimento, A.L.R.; de Carvalho, J.J.; et al. Açaí (Euterpe oleracea Mart.) seed extract improves aerobic exercise performance in rats. Food Res. Int. 2020, 136, 109549. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.E.; Rodrigues-Baptista, K.C.; Alessandra-Perini, J.; Soares de Moura, R.; Santos, T.A.; Pereira, K.G.; Marinho da Silva, Y.; Souza, P.J.; Nasciutti, L.E.; Perini, J.A. Euterpe oleracea Extract (Açaí) Is a Promising Novel Pharmacological Therapeutic Treatment for Experimental Endometriosis. PLoS ONE 2016, 11, e0166059. [Google Scholar] [CrossRef] [Green Version]
- Brasil, A.; Rocha, F.A.F.; Gomes, B.D.; Oliveira, K.R.M.; de Carvalho, T.S.; Batista, E.J.O.; Borges, R.D.S.; Kremers, J.; Herculano, A.M. Diet enriched with the Amazon fruit açaí (Euterpe oleracea) prevents electrophysiological deficits and oxidative stress induced by methyl-mercury in the rat retina. Nutr. Neurosci. 2017, 20, 265–272. [Google Scholar] [CrossRef]
- Shibuya, S.; Toda, T.; Ozawa, Y.; Yata, M.J.V.; Shimizu, T. Acai Extract Transiently Upregulates Erythropoietin by Inducing a Renal Hypoxic Condition in Mice. Nutrients 2020, 12, 533. [Google Scholar] [CrossRef] [Green Version]
- Aranha, L.N.; Silva, M.G.; Uehara, S.K.; Luiz, R.R.; Nogueira Neto, J.F.; Rosa, G.; Moraes de Oliveira, G.M. Effects of a hypoenergetic diet associated with açaí (Euterpe oleracea Mart.) pulp consumption on antioxidant status, oxidative stress and inflammatory biomarkers in overweight, dyslipidemic individuals. Clin. Nutr. (Edinb. Scotl.) 2020, 39, 1464–1469. [Google Scholar] [CrossRef]
- Silva, I.C.d.; Conceição, E.O.A.; Pereira, D.S.; Rogez, H.; Muto, N.A. Evaluation of the Antimicrobial Capacity of Bacteria Isolated from Stingless Bee (Scaptotrigona aff. postica) Honey Cultivated in Açai (Euterpe oleracea) Monoculture. Antibiotics 2023, 12, 223. [Google Scholar] [CrossRef]
- Sanches, S.C.d.C.; Ré, M.I.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Organogel of Acai Oil in Cosmetics: Microstructure, Stability, Rheology and Mechanical Properties. Gels 2023, 9, 150. [Google Scholar] [CrossRef]
- Earling, M.; Beadle, T.; Niemeyer, E.D. Açai Berry (Euterpe oleracea) Dietary Supplements: Variations in Anthocyanin and Flavonoid Concentrations, Phenolic Contents, and Antioxidant Properties. Plant Foods Hum. Nutr. 2019, 74, 421–429. [Google Scholar] [CrossRef]
- Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Cordaro, M.; Cuzzocrea, S.; et al. Açai Berry Attenuates Cyclophosphamide-Induced Damage in Genitourinary Axis-Modulating Nrf-2/HO-1 Pathways. Antioxidants 2022, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.M.; Martino, H.S.; Noratto, G.; Roque-Andrade, A.; Stringheta, P.C.; Talcott, S.; Ramos, A.M.; Mertens-Talcott, S.U. Anti-inflammatory activity of polyphenolics from açai (Euterpe oleracea Martius) in intestinal myofibroblasts CCD-18Co cells. Food Funct. 2015, 6, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.K.; Cadoná, F.C.; Assmann, C.E.; Andreazza, A.C.; Duarte, M.M.M.F.; dos Santos Branco, C.; Zhou, X.; de Souza, D.V.; Ribeiro, E.E.; da Cruz, I.B.M. Açaí (Euterpe oleracea Mart.) has anti-inflammatory potential through NLRP3-inflammasome modulation. J. Funct. Foods 2019, 56, 364–371. [Google Scholar] [CrossRef]
- Rocha, A.P.; Carvalho, L.C.; Sousa, M.A.; Madeira, S.V.; Sousa, P.J.; Tano, T.; Schini-Kerth, V.B.; Resende, A.C.; Soares de Moura, R. Endothelium-dependent vasodilator effect of Euterpe oleracea Mart. (Açaí) extracts in mesenteric vascular bed of the rat. Vasc. Pharmacol. 2007, 46, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Marinho, B.G.; Herdy, S.A.; Sá, A.C.; Santos, G.B.; Matheus, M.E.; Menezes, F.S.; Fernandes, P.D. Atividade antinociceptiva de extratos de açaí (Euterpe oleraceae Mart.). Rev. Bras. Farmacogn. 2002, 12, 52–53. [Google Scholar] [CrossRef] [Green Version]
- de Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Kawashima Pacheco, S.Y.; Silva, S.S.D.; Barbosa, E.G.; Pereira, R.R.; Costa, R.M.R.; Silva Junior, J.O.C.; da Silva Ferreira, M.A.; de Almeida, J.C.; et al. Development and Evaluation of Antimicrobial and Modulatory Activity of Inclusion Complex of Euterpe oleracea Mart Oil and β-Cyclodextrin or HP-β-Cyclodextrin. Int. J. Mol. Sci. 2020, 21, 942. [Google Scholar] [CrossRef] [Green Version]
- Crespo-López, M.E.; Soares, E.S.; Macchi, B.d.M.; Santos-Sacramento, L.; Takeda, P.Y.; Lopes-Araújo, A.; Paraense, R.S.d.O.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Luz, D.A.; et al. Towards Therapeutic Alternatives for Mercury Neurotoxicity in the Amazon: Unraveling the Pre-Clinical Effects of the Superfruit Açaí (Euterpe oleracea, Mart.) as Juice for Human Consumption. Nutrients 2019, 11, 2585. [Google Scholar] [CrossRef] [Green Version]
- Afonso, S.R. Innovation Perspectives for the Bioeconomy of Non-Timber Forest Products in Brazil. Forests 2022, 13, 2046. [Google Scholar] [CrossRef]
- Muto, N.A.; Hamoy, M.; da Silva Ferreira, C.B.; Hamoy, A.O.; Lucas, D.C.R.; de Mello, V.J.; Rogez, H. Extract of Euterpe oleracea Martius Stone Presents Anticonvulsive Activity via the GABAA Receptor. Front. Cell Neurosci. 2022, 16, 872743. [Google Scholar] [CrossRef]
- de Souza, D.V.; Pappis, L.; Bandeira, T.T.; Sangoi, G.G.; Fontana, T.; Rissi, V.B.; Sagrillo, M.R.; Duarte, M.M.; Duarte, T.; Bodenstein, D.F.; et al. Açaí (Euterpe oleracea Mart.) presents anti-neuroinflammatory capacity in LPS-activated microglia cells. Nutr. Neurosci. 2022, 25, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Seeberger, J.; Alberico, T.; Wang, C.; Wheeler, C.T.; Schauss, A.G.; Zou, S. Açai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Exp. Gerontol. 2010, 45, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babazadeh, A.; Vahed, F.M.; Liu, Q.; Siddiqui, S.A.; Kharazmi, M.S.; Jafari, S.M. Natural Bioactive Molecules as Neuromedicines for the Treatment/Prevention of Neurodegenerative Diseases. ACS Omega 2023, 8, 3667–3683. [Google Scholar] [CrossRef] [PubMed]
- Medina Dos Santos, N.; Batista Â, G.; Padilha Mendonça, M.C.; Figueiredo Angolini, C.F.; Grimaldi, R.; Pastore, G.M.; Sartori, C.R.; Alice da Cruz-Höfling, M.; Maróstica Júnior, M.R. Açai pulp improves cognition and insulin sensitivity in obese mice. Nutr. Neurosci. 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- de Liz, S.; Cardoso, A.L.; Copetti, C.L.K.; Hinnig, P.D.F.; Vieira, F.G.K.; da Silva, E.L.; Schulz, M.; Fett, R.; Micke, G.A.; Di Pietro, P.F. Açaí (Euterpe oleracea Mart.) and juçara (Euterpe edulis Mart.) juices improved HDL-c levels and antioxidant defense of healthy adults in a 4-week randomized cross-over study. Clin. Nutr. 2020, 39, 3629–3636. [Google Scholar] [CrossRef] [PubMed]
- Sofiullah, S.S.M.; Murugan, D.D.; Muid, S.A.; Seng, W.Y.; Kadir, S.Z.S.A.; Abas, R.; Ridzuan, N.R.A.; Zamakshshari, N.H.; Woon, C.K. Natural Bioactive Compounds Targeting NADPH Oxidase Pathway in Cardiovascular Diseases. Molecules 2023, 28, 1047. [Google Scholar] [CrossRef] [PubMed]
- Sena, I.S.; Ferreira, A.M.; Marinho, V.H.; e Holanda, F.H.; Borges, S.F.; de Souza, A.A.; de Carvalho, R.; Koga, R.; Lima, A.L.; Florentino, A.C.; et al. Euterpe oleracea Mart (Açaizeiro) from the Brazilian Amazon: A Novel Font of Fungi for Lipase Production. Microorganisms 2022, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Krępa, E.; Kłapcińska, B.; Podgórski, T.; Szade, B.; Tyl, K.; Hadzik, A. Effects of supplementation with acai (Euterpe oleracea Mart.) berry-based juice blend on the blood antioxidant defence capacity and lipid profile in junior hurdlers. A pilot study. Biol. Sport 2015, 32, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Shen, X.; Deng, R.; Zhang, Y.; Zheng, X. Dietary anthocyanin-rich extract of açai protects from diet-induced obesity, liver steatosis, and insulin resistance with modulation of gut microbiota in mice. Nutrition 2021, 86, 111176. [Google Scholar] [CrossRef]
- Martins, G.R.; Mattos, M.M.G.; Nascimento, F.M.; Brum, F.L.; Mohana-Borges, R.; Figueiredo, N.G.; Neto, D.F.M.; Domont, G.B.; Nogueira, F.C.S.; de Paiva Campos, F.A.; et al. Phenolic Profile and Antioxidant Properties in Extracts of Developing Açaí (Euterpe oleracea Mart.) Seeds. J. Agric. Food Chem. 2022, 70, 16218–16228. [Google Scholar] [CrossRef]
- Ji, L.; Deng, H.; Xue, H.; Wang, J.; Hong, K.; Gao, Y.; Kang, X.; Fan, G.; Huang, W.; Zhan, J.; et al. Research progress regarding the effect and mechanism of dietary phenolic acids for improving nonalcoholic fatty liver disease via gut microbiota. Compr. Rev. Food Sci. Food Saf. 2023. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.B.; de Bem, G.F.; da Costa, C.A.; de Carvalho, L.; de Medeiros, A.F.; Silva, D.L.B.; Romão, M.H.; de Andrade Soares, R.; Ognibene, D.T.; de Moura, R.S.; et al. Açaí seed extract prevents the renin-angiotensin system activation, oxidative stress and inflammation in white adipose tissue of high-fat diet-fed mice. Nutr. Res. 2020, 79, 35–49. [Google Scholar] [CrossRef]
- Shin, G.C.; Lee, H.M.; Kim, N.; Yoo, S.K.; Park, H.S.; Choi, L.S.; Kim, K.P.; Lee, A.R.; Seo, S.U.; Kim, K.H. Paraoxonase-2 contributes to promoting lipid metabolism and mitochondrial function via autophagy activation. Sci. Rep. 2022, 12, 21483. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Kampangkaew, J.; Nambi, V. Prevention of Cardiovascular Disease in Women. Methodist Debakey Cardiovasc. J. 2017, 13, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nitsa, A.; Toutouza, M.; Machairas, N.; Mariolis, A.; Philippou, A.; Koutsilieris, M. Vitamin D in Cardiovascular Disease. In Vivo 2018, 32, 977–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaub, J.A.; Hamidi, H.; Subramanian, L.; Kretzler, M. Systems Biology and Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 695–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.; Costa, J.H.; Pacheco-Fill, T.; Ruiz, A.; Vidal, F.C.B.; Borges, K.R.A.; Guimarães, S.J.A.; Azevedo-Santos, A.P.S.; Buglio, K.E.; Foglio, M.A.; et al. Açai (Euterpe oleracea Mart.) Seed Extract Induces ROS Production and Cell Death in MCF-7 Breast Cancer Cell Line. Molecules 2021, 26, 3546. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Nascimento, R.P.D.; Machado, A.; Marostica Junior, M.R. Signaling pathways and the potential anticarcinogenic effect of native Brazilian fruits on breast cancer. Food Res. Int. (Ott. Ont.) 2022, 155, 111117. [Google Scholar] [CrossRef]
- Alavarsa-Cascales, D.; Aliaño-González, M.J.; Palma, M.; Barbero, G.F.; Carrera, C. Optimization of an Enzyme-Assisted Extraction Method for the Anthocyanins Present in Açai (Euterpe oleracea Mart.). Agronomy 2022, 12, 2327. [Google Scholar] [CrossRef]
- Martinez, R.M.; Guimarães, D.A.B.; Berniz, C.R.; Abreu, J.P.; Rocha, A.; Moura, R.S.; Resende, A.C.; Teodoro, A.J. Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods 2018, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Fragoso, M.F.; Prado, M.G.; Barbosa, L.; Rocha, N.S.; Barbisan, L.F. Inhibition of mouse urinary bladder carcinogenesis by açai fruit (Euterpe oleraceae Martius) intake. Plant Foods Hum. Nutr. 2012, 67, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, N.; Choi, Y.J.; Nam, R.H.; Lee, S.; Ham, M.H.; Suh, J.H.; Choi, Y.J.; Lee, H.S.; Lee, D.H. Anti-inflammatory and Anti-tumorigenic Effects of Açai Berry in Helicobacter felis-infected mice. J. Cancer Prev. 2016, 21, 48–54. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.A.C.N.; do Desterro Soares Brandão Nascimento, M.; de Carvalho, J.E. Traditional Uses, Phytochemistry, Pharmacology and Anticancer Activity of Açaí (Euterpe oleracea Mart): A Narrative Review. Curr. Tradit. Med. 2021, 7, 41–62. [Google Scholar] [CrossRef]
- Pala, D.; Barbosa, P.O.; Silva, C.T.; de Souza, M.O.; Freitas, F.R.; Volp, A.C.P.; Maranhão, R.C.; Freitas, R.N. Açai (Euterpe oleracea Mart.) dietary intake affects plasma lipids, apolipoproteins, cholesteryl ester transfer to high-density lipoprotein and redox metabolism: A prospective study in women. Clin. Nutr. (Edinb. Scotl.) 2018, 37, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Alqurashi, R.M.; Galante, L.A.; Rowland, I.R.; Spencer, J.P.; Commane, D.M. Consumption of a flavonoid-rich açai meal is associated with acute improvements in vascular function and a reduction in total oxidative status in healthy overweight men. Am. J. Clin. Nutr. 2016, 104, 1227–1235. [Google Scholar] [CrossRef] [Green Version]
- Carvalho-Peixoto, J.; Moura, M.R.; Cunha, F.A.; Lollo, P.C.; Monteiro, W.D.; Carvalho, L.M.; Farinatti Pde, T. Consumption of açai (Euterpe oleracea Mart.) functional beverage reduces muscle stress and improves effort tolerance in elite athletes: A randomized controlled intervention study. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 725–733. [Google Scholar] [CrossRef]
- Gale, A.M.; Kaur, R.; Baker, W.L. Hemodynamic and electrocardiographic effects of açaí berry in healthy volunteers: A randomized controlled trial. Int. J. Cardiol. 2014, 174, 421–423. [Google Scholar] [CrossRef]
- Kim, H.; Simbo, S.Y.; Fang, C.; McAlister, L.; Roque, A.; Banerjee, N.; Talcott, S.T.; Zhao, H.; Kreider, R.B.; Mertens-Talcott, S.U. Açaí (Euterpe oleracea Mart.) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food Funct. 2018, 9, 3097–3103. [Google Scholar] [CrossRef]
- Udani, J.K.; Singh, B.B.; Singh, V.J.; Barrett, M.L. Effects of Açai (Euterpe oleracea Mart.) berry preparation on metabolic parameters in a healthy overweight population: A pilot study. Nutr. J. 2011, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Kessler, E.R.; Su, L.J.; Gao, D.; Torkko, K.C.; Wacker, M.; Anduha, M.; Chronister, N.; Maroni, P.; Crawford, E.D.; Flaig, T.W.; et al. Phase II Trial of Acai Juice Product in Biochemically Recurrent Prostate Cancer. Integr. Cancer Ther. 2018, 17, 1103–1108. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.C.; Antunes, L.M.G.; Aissa, A.F.; Darin, J.D.A.C.; De Rosso, V.V.; Mercadante, A.Z.; Bianchi, M.D.L.P. Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açai pulp (Euterpe oleracea Mart.) on mice using the erythrocytes micronucleus test and the comet assay. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2010, 695, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.D.S.; Froder, J.G.; Oliveira, P.R.D.; Perazzo, F.F.; Rosa, P.C.P.; Gaivão, I.O.N.D.M.; Mathias, M.I.C.; Maistro, E.L. Cytotoxic effects of Euterpe oleraceae fruit oil (açaí) in rat liver and thyroid tissues. Rev. Bras. Farmacogn. 2019, 29, 54–61. [Google Scholar] [CrossRef]
- Caiado, R.R.; Peris, C.S.; Lima-Filho, A.A.S.; Urushima, J.G.P.; Novais, E.; Badaró, E.; Maia, A.; Sinigaglia-Coimbra, R.; Watanabe, S.E.S.; Rodrigues, E.B.; et al. Retinal Toxicity of Acai Fruit (Euterpe oleracea) Dye Concentrations in Rabbits: Basic Principles of a New Dye for Chromovitrectomy in Humans. Curr. Eye Res. 2017, 42, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, B.G.; Martinez, R.C.R.; de Castro, I.A.; Saad, S.M.I. Innovative açaí (Euterpe oleracea, Mart., Arecaceae) functional frozen dessert exhibits high probiotic viability throughout shelf-life and supplementation with inulin improves sensory acceptance. Food Sci. Biotechnol. 2014, 23, 1843–1849. [Google Scholar] [CrossRef]
- Freitas, H.V.; Dos Santos Filho, A.L.; Rodrigues, S.; Abreu, V.K.G.; Narain, N.; Lemos, T.D.O.; Gomes, W.F.; Pereira, A.L.F. Synbiotic açaí juice (Euterpe oleracea) containing sucralose as noncaloric sweetener: Processing optimization, bioactive compounds, and acceptance during storage. J. Food Sci. 2021, 86, 730–739. [Google Scholar] [CrossRef]
- Costa, M.G.; Ooki, G.N.; Vieira, A.D.; Bedani, R.; Saad, S.M. Synbiotic Amazonian palm berry (açai, Euterpe oleracea Mart.) ice cream improved Lactobacillus rhamnosus GG survival to simulated gastrointestinal stress. Food Funct. 2017, 8, 731–740. [Google Scholar] [CrossRef]
- Coïsson, J.D.; Travaglia, F.; Piana, G.; Capasso, M.; Arlorio, M. Euterpe oleracea juice as a functional pigment for yogurt. Food Res. Int. 2005, 38, 893–897. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Villaño, D.; Moreno, D.A.; García-Viguera, C. New isotonic drinks with antioxidant and biological capacities from berries (maqui, açaí and blackthorn) and lemon juice. Int. J. Food Sci. Nutr. 2013, 64, 897–906. [Google Scholar] [CrossRef]
- Garbossa, W.A.C.; Maia Campos, P.M.B.G. Euterpe oleracea, Matricaria chamomilla, and Camellia sinensis as promising ingredients for development of skin care formulations. Ind. Crops Prod. 2016, 83, 1–10. [Google Scholar] [CrossRef]
- Ferrari, M.; Rocha-filho, P. Multiple emulsions containing amazon oil: Açaí oil (Euterpe oleracea). Rev. Bras. Farmacogn. 2011, 21, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Coelho da Mota, D.S.; Sicuro, F.L.; Resende, A.C.; De Moura, R.S.; Bottino, D.A.; Bouskela, E. Effects of açaí and cilostazol on skin microcirculation and viability of TRAM flaps in hamsters. J. Surg. Res. 2018, 228, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, S.; Xue, Y.; Dong, Y. Evaluation and Development of Novel Thermo Reversible Gel of Acai Extract (Euterpe oleracea) for the Treatment of Keratoconus: Preclinical Study in Rabbit Model. Nanosci. Nanotechnol. Lett. 2020, 12, 702–708. [Google Scholar] [CrossRef]
- Silva, C.K.D.; Mastrantonio, D.J.D.S.; Costa, J.A.V.; Morais, M.G.D. Innovative pH sensors developed from ultrafine fibers containing açaí (Euterpe oleracea) extract. Food Chem. 2019, 294, 397–404. [Google Scholar] [CrossRef]
- Affonso Celso Gonçalves, J.; Daniel, S.; Elio Conradi, J.; Juliano, Z.; Gustavo Ferreira, C. Adsorption of Cd (II), Pb (II) and Cr (III) on chemically modified Euterpe Oleracea biomass for the remediation of water pollution. Acta Sci. Technol. 2020, 43. [Google Scholar] [CrossRef]
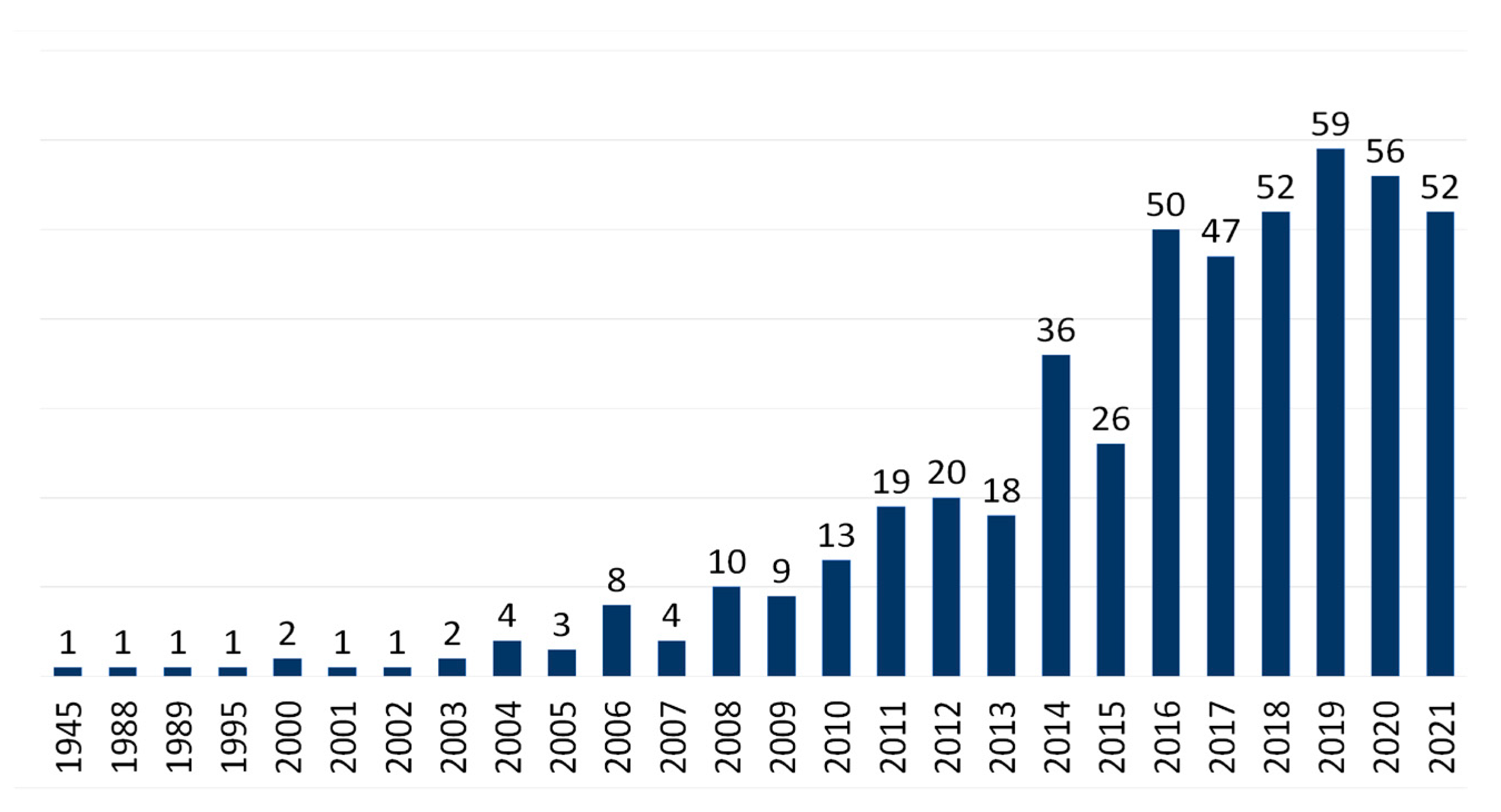

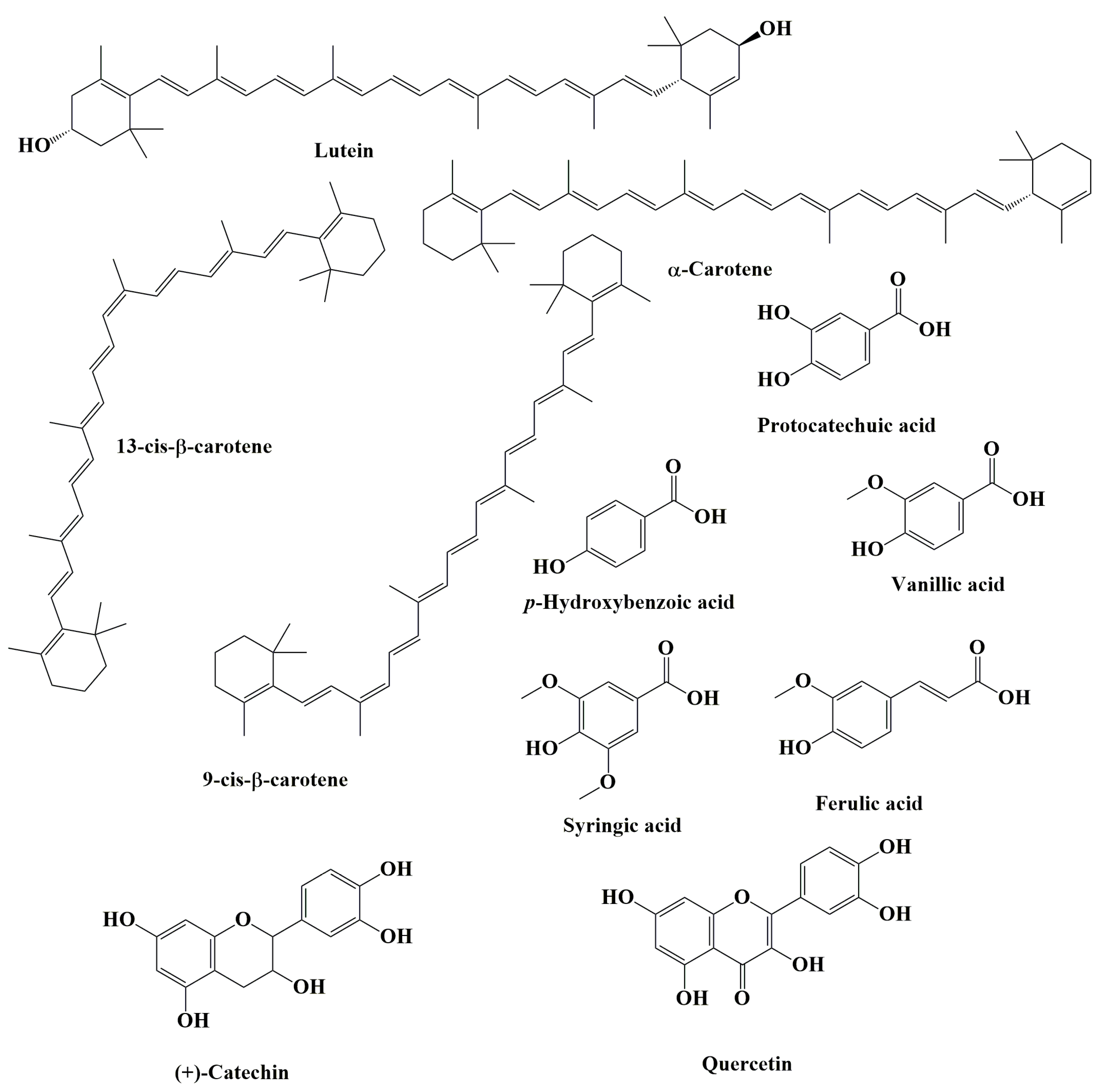


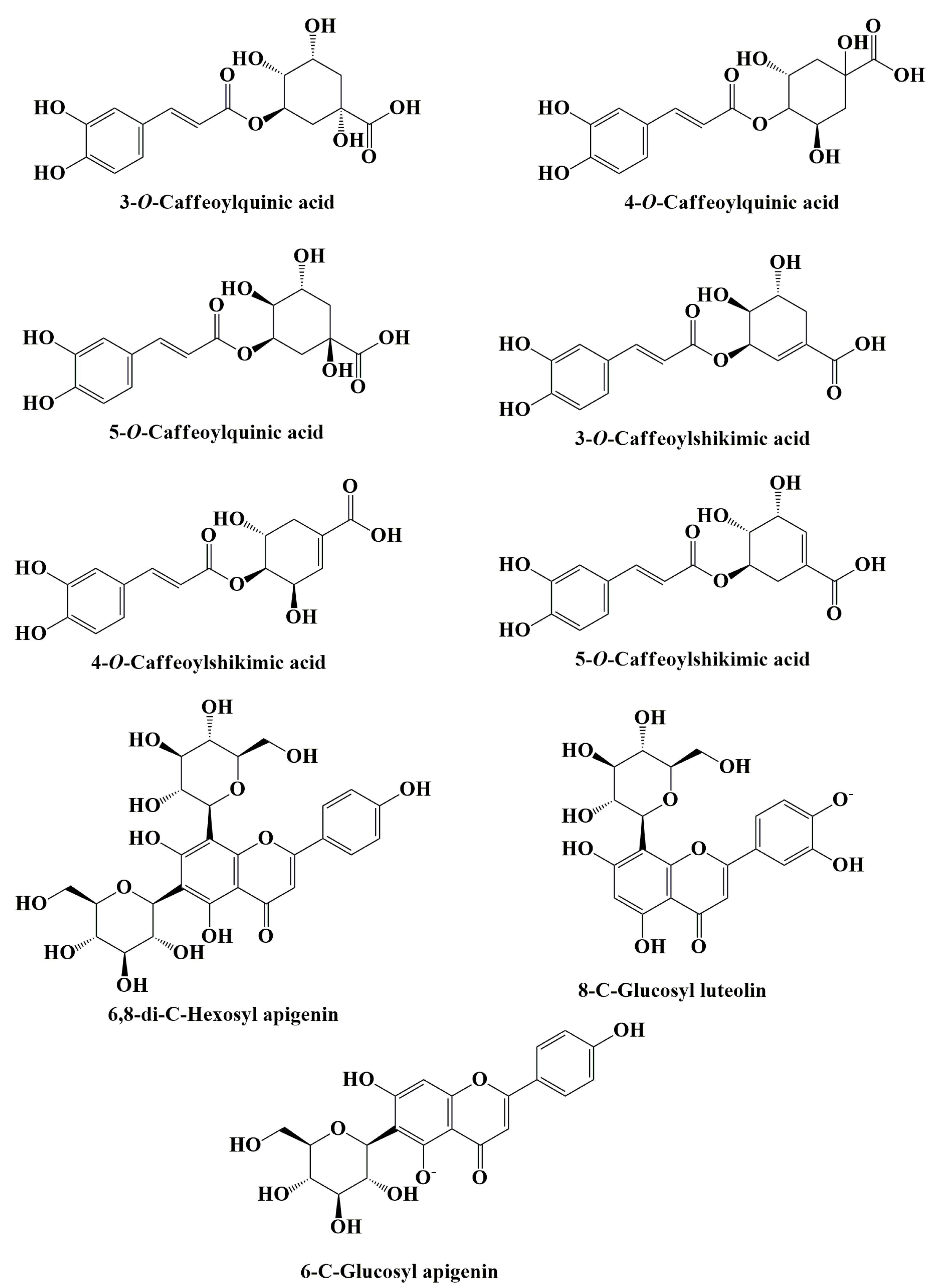


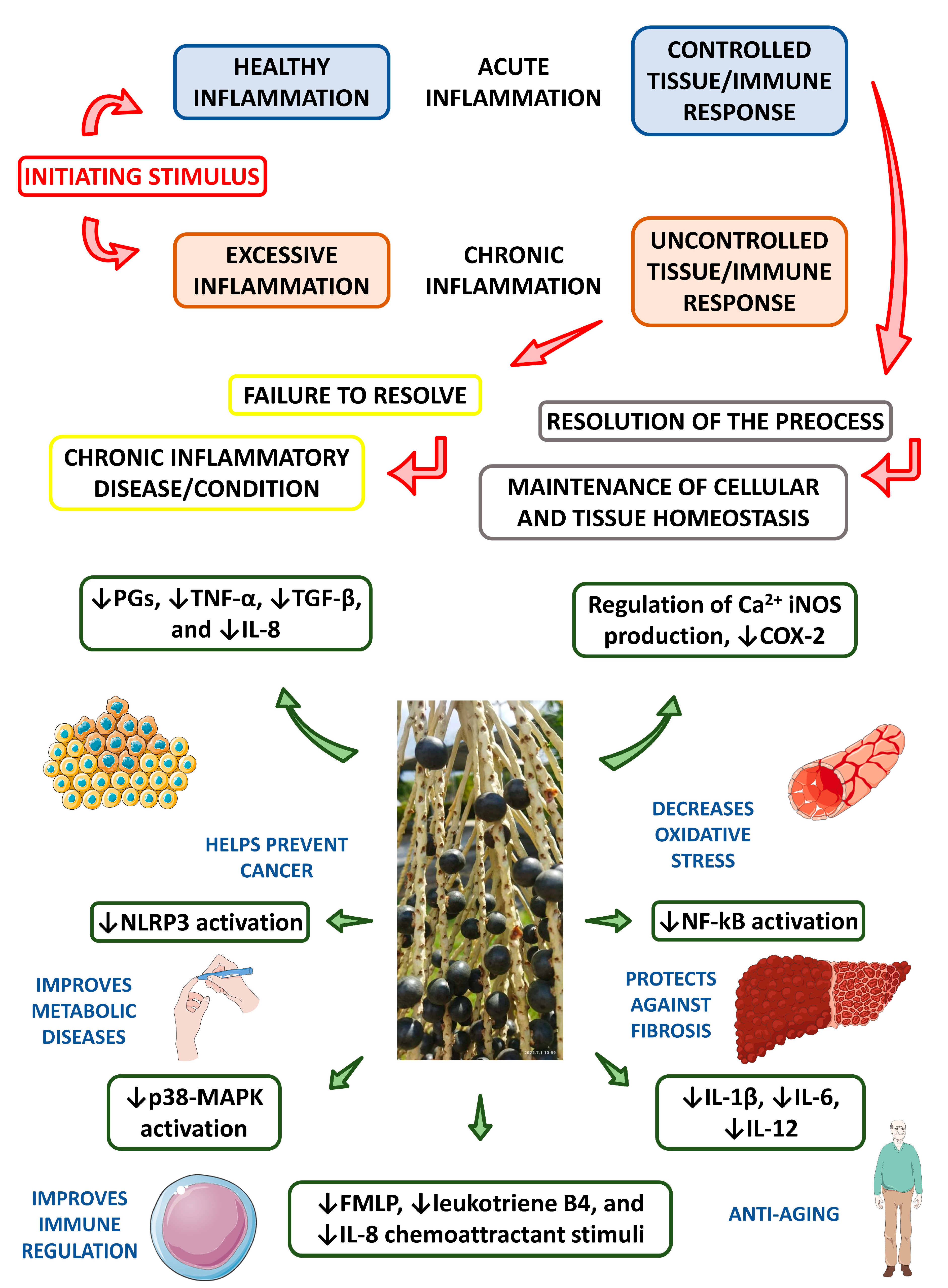

| Properties | Plant Part Used or Compounds | Models | Type of Extract | Concentrations | Observations | Reference |
|---|---|---|---|---|---|---|
| Antioxidant and anti-inflammatory | Açaí oil | Carrageenan-induced edematic mice paws, carrageenan-induced mice air pouches | EOO, EOO-βCD, and EOO-HPβCD | 0.25, 0.5, 1.0, and 1.5 mg/mL | EOO-HPβCD achieved antioxidant activity 47% greater than that of the pure EOO | [47] |
| Açaí seed extract | LPS-stimulated RAW 264.7 macrophages | Catechin-rich ethyl acetate açaí seed extract | 125, 250, and 500 µg/mL | EO-ACET did not exert cytotoxic effects; the RAW macrophages showed lower levels of nitrite, IL-1β, IL-6, and IL-12 | [48] | |
| Açaí extract made from skin and pulp fractions | RAW 264.7 macrophages treated with pro-inflammatory doses of OLZ | Hydroalcoholic | 0.01, 0.05, 0.1, 1.0, 5.0, and 10 µg/mL | Açaí extract at 5 µg/mL showed reduction of NO, IL-1β, IL-6, TNF-α, and IFN-γ | [49] | |
| Açaí seed extract | RAW 264.7 macrophages | Açaí seed extract rich in flavan-3-ols | 10, 30, 100, and 300 μg/mL | Açaí-treated macrophages presented lower NF-κB activation, TNF-α production, and oxidative stress | [50] | |
| Açaí berry freeze-dried extract | HepG2 cells | Hexane fraction, dried chloroform, dried butanol, and aqueous extracts rich in pheophorbides | 50 μg/mL, 200 μg/mL, and 8.2 and 16.9 μM for pheophorbide A methyl ester and pheophorbide A, respectively | The methyl and ethyl esters of the common pheophorbide A parent demonstrated ARE-activation at 8.2 μM and 16.9 μM for pheophorbide A methyl ester and pheophorbide A, respectively | [51] | |
| Açaí seed extracts rich in phenolic bioactive compounds, especially (−)-epicatechin (497 mg/100 g), and (+)-catechin (403 mg/100 g) | HUVEC cells stressed by H2O2 | Lyophilized | 0.1–100 mg/mL for oxidative stress assays and 10 mg/mL for endothelial cell migration assays | Açaí prevented H2O2-cytotoxicity, oxidative stress, and migratory function loss, and stimulated the upregulation of Nrf2 antioxidant pathways via ERK | [52] | |
| Açaí berry extract | A liposome-rich environment with induced oxidation | Aqueous | 50 mL | Açaí treatment protected the structures of a lipid-rich environment of liposomes from oxidative damage | [53] | |
| Açaí seed extract rich in a B-type (epi) catechin tetramer and procyanidin trimers | Human breast adenocarcinoma MCF-7 cells, non-small NCI-H460 cells lung cancer, cervical HeLa carcinoma cells, HepG2 cells, and non-tumor freshly porcine harvested cells | Aqueous | 8 mg/mL for cytotoxicity screening and 1 mg/mL for antioxidant activity evaluation | Açaí aqueous seed extract had potent antioxidant capacity and exerted cytotoxic actions against HeLa cells | [54] | |
| Açaí fruit extract composed of 31.0 ± 2.4 mg/100 g of total anthocyanins | HUVEC cells and an E. coli bacteria strain | Aqueous | 2.5 mg/mL for HUVEC cells and 100 mg/mL for E. coli bacteria | Açaí treatment blocked bacterial growth significantly; ROS production was limited and conferred protection against oxidative damage | [55] | |
| Freeze-dried açaí pulp powder rich in five different flavonoids: (2S,3S) dihyrokaempferol 3-O b-D-glucoside, (2R,3R) dihydrokaempferol 3 O-b-D-glucoside, isovitexin, velutin, and 5,40-dihydroxy-7,30,50 -trimethoxyflavone | RAW-blue cells induced by LPS | Flavonoid extracts/isolates | Velutin: 0.625, 1.25, 2.5, 5 μM; luteolin: 2.5, 5, 10, and 20 μM | Velutin exerted significant anti-inflammatory activities in SEAP assays; 5,40-dihydroxy-7,30,50 trimethoxyflavone demonstrated more potent antioxidant capacity compared to its isomer | [56] | |
| Antioxidant-rich fruit and berry juice blend of açaí as the predominant ingredient and other fruits and berries (white and purple grape, Nashi pear, acerola, aronia, cranberry, passionfruit, apricot, prune, kiwifruit, blueberry, wolfberry, pomegranate, lychee, camu camu, pear, banana), and bilberry with anthocyanins, predominantly cyanidin 3-rutoside, cyanidin 3-diglycoside, and cyanidin 3-glucoside | PMN cells, polymorphonuclear cells, and erythrocytes | MonaVie Active juice blend | Approximately 7.2 g of dissolved material | The blend protected erythrocytes from oxidative damage, prevented ROS production by polymorphonuclear cells, and reduced leukocyte migration through inhibition of FMLP | [57] | |
| Açaí berry pulp bioactive compounds | Human MCF-7 breast cancer cells stressed by H2O2 | All bioactive compounds were extracted and isolated for the research procedures and analyzed by the results | Different concentrations of the isolated bioactive compounds | Açaí berry pulp bioactive compounds demonstrated high values in the OH radical scavenging assays | [58] | |
| Açaí fruit pulp and skin powder with 13.9 mg GAE/g of total polyphenolics | Human PMN cells | Acetone, water, and acetic acid extracts | 5, 12.5, 25, 50, 125, 250, 500, and 1000 μg/mL | Açaí promoted high antioxidant capacity against the peroxyl radical and mild activity against peroxynitrite and hydroxyl radicals; inhibited COX-1 and COX-2 | [59] | |
| Antimicrobial | Açaí seed extracts rich in A- and B-type procyanidins | Human THP1 monocyte cells, monkey LLC-MK2 kidney epithelial cells, and HepG2 cells; Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa, and Candida albicans | Hydroalcoholic and aqueous extracts | Mammalian THP1, LLC-MK2, and HepG2 cells were treated at 15.6–1000 μg/mL; microbial cells were treated at 2 mg/mL | Açaí extract exerted antimicrobial effects against Gram-positive bacteria and Candida albicans strains and was not cytotoxic to THP1 and LLC-MK2 mammalian cells; açaí also protected macrophages from ROS | [60] |
| Dried açaí pulp powder extract | Erythrocytes from O+ individuals infected by chloroquine-sensitive and multidrug-resistant strains of P. falciparum and RAW 264.7 cells | Polyphenol-rich extracts: (1) rich in phenolic compounds, (2) rich in non-anthocyanin phenolics, and (3) rich in anthocyanins | Doses at concentrations ranging from 1.0 to 20.0 mg/L GAE | The açaí fraction rich in non-anthocyanin phenolics inhibited the growth of the parasites, and none of the fractions exerted cytotoxic effects in the cells | [61] | |
| Açaí pulp extract | HepG2 cells, planktonic cells, Staphylococcus aureus, and other Gram-positive bacteria | Methanolic extract of açaí pulp | The HepG2 cells were treated with 20 µL of the extract at 500-7.81 µg/mL; microbes were treated with açaí extract at concentrations ranging from 1 to 7.8 µg/mL | Açaí extract decreased the proliferation of cancerous HepG2 cells and inhibited biofilm production by planktonic cells and Staphylococcus aureus strains | [62] | |
| Açaí pulp, seed, and leaf extracts | Clostridium perfringens, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa | Hydroalcoholic | 10 μL of açaí extracts at concentrations ranging from 10 to 2.560 μg/mL | Açaí seed and pulp extracts showed significant inhibition against the proliferation of all investigated microorganisms | [63] | |
| Neuroprotective | Açaí fresh fruits extract | Microglia EOC 13.31 cells line | Hydroalcoholic | The cells were treated with final concentrations ranging 0.001–1000 μg/mL | Açaí exposure reverted LPS-induced inflammation and ROS production and reduced cell proliferation induced by the LPS stress; reduced NLRP3, caspase-1, and IL-1β expression levels | [64] |
| Açaí juice rich in orientin, homoorientin, taxifolin deoxyhexose, cyanidin 3-glucoside, and cyanidin 3-rutinoside | Neurons and astrocytes | Clarified | 0–25% EO in Hank’s buffer at a final volume of 250 μL | Low concentrations of clarified açaí juice improved GABAergic neurotransmission by modulating GABA uptake | [65] | |
| Açaí fruit extract rich in anthocyanins (cyanidin 3-glucoside and cyanidin 3-rutinoside) and carotenoids (lutein, zeaxanthin, a-carotene, and b-carotene | Human neuroblastoma SH-SY5Y cells line | Hydroethanolic | 0.5, 5.0, and 50 μg/mL | Açaí extract protected cells from 13% to 62% of the SY5Y cells from H2O2-related oxidative damage | [66] | |
| Freeze-dried açaí extracts rich in gallic acid, catechin, chlorogenic acid, caffeic acid, p-coumaric acid, epicatechin, orientin, vitexin, cyanidin-3-O-glucoside, luteolin, apigenin, and chrysin | Neuronal-like cells (SH-SY5Y) with mitochondrial complex I deficiency | Hydroalcoholic | The cells were treated with final concentrations ranging 0.001–1000 g/mL | Açaí significantly potentialized the expression of NDUFS7 and NDUFS8, augmenting the protein amount and enzyme activity of mitochondrial complex I and diminishing ROS production and lipid peroxidation | [67] | |
| Açaí berry extract | Immortalized DI TNC1 rat astrocytes stimulated by an Nrf2-ARE or an LPS-insulated NF-κB response element | Not reported | The cells were treated with final concentrations ranging from 6.25-50 μg/mL | Açaí inhibited the LPS-induced NF-κB reporter activity, as well as enhanced the antioxidant Nrf2/ARE response alone and also the Nrf2/ARE in the presence of the LPS-related stress | [68] | |
| Polyphenol-rich pulp extracts of açaí rich in cyanidin 3-O-glucoside, cyanidin 3-rutinoside, and delphinidin 3-glucoside | Sprague–Dawley rat embryonic hippocampal neuronal E18 cells and HT22 hippocampal cells | Aqueous | The cells were treated with final concentrations ranging 1–5 µg/mL | The treatment significantly caused a rapid recovery of the depolarized dopamine-(DA-)-induced Ca2+ influx neurons; there was attenuation in the inhibitor-induced autophagy dysfunction in the neurons | [69] | |
| Açaí fresh extract | Rat PC12 pheochromocytoma cells | Aqueous | The cells were treated with final concentrations ranging 0.5–50 µg/mL | The use of açaí was effective in preventing β-amyloid deposition in neuronal-like cells and further aggregation | [70] | |
| Pasteurized, freeze-dried açaí pulp extract rich in anthocyanins and other phenolic compounds. The study evaluated different fractions, such as ETOH, MEOH, ETAC, and ACE | Murine BV-2 microglial cells stressed by LPS treatment | Not reported | The cells were treated with final concentrations ranging 50 μg to 10 mg/mL | The treatment decreased nitrite production and iNOS expression by the ferulic acid content among the fractions. The MEOH, ETOH, and ACE fractions primarily exerted anti-inflammatory effects by downregulating COX-2, p38-MAPK, TNF-α, and NF-κB expressions | [71] | |
| Açaí fruit extract | Dissected cerebral cortex, cerebellum, and hippocampus of pretreated with H2O2 rats | Aqueous | The cells were treated with açaí pulp at a final concentration of 40% wt/vol | A negative correlation was observed between the açaí polyphenol content and the lipid and protein oxidative-related damage in the brain tissues | [72] | |
| Antiadipogenic | Açaí seed extract rich in catechin and polymeric proanthocyanidins | 3T3-L1 adipocytes | Not reported | 0, 10, 25, 50, and 100 μg/mL | The extract inhibited adipogenesis by decreasing adipocyte differentiation through the decreasing expression of many adipogenic proteins and transcription factors of PPARɣ, SREBP-1, and FAS. Additionally, the extract suppressed lipid accumulation | [73] |
| Frozen, concentrated, açaí juice rich in anthocyanins (cyanidin 3-glucoside and cyanidin-3-rutinoside) and flavonoids C glycosides (orientin, homoorientin, isovitexin, taxifolin deoxyhexose, and flavan-3-ol monomers) | 3T3-L1 adipocytes | Not reported | The cells were differentiated with and without açaí polyphenols at concentrations of 2.5, 5, and 10 μg GAE/mL | The polyphenolic compounds reduced the intracellular lipid accumulation of adipocytes; downregulated PPARγ2 expression; and decreased the expression of adipogenic transcription factors, such as C/EPBα, C/EPBβ, Klf5, and SREBP-1c, and adipogenic genes, such as aP2, LPL, FATP1, and FAS | [74] | |
| Cardiovascular protective | Açaí dietary powder supplement extract rich in anthocyanins (cyanidin-3-O-rutinoside) and flavonoids | HMEC-1 cells | Hydroethanolic | The cells were treated with final concentrations of 1–75 mg/L | Açaí powder exerted antiangiogenic effects without being cytotoxic and decreased the migration and invasion potentials of HMEC-1 cells, as well as the formation of capillary-like structures | [75] |
| Anticancer | Açaí pulp rich in anthocyanin cyanidin 3-rutinoside, (C3R, 214.09 ± 17.32 mg/100 g) | RKO human colon adenocarcinoma cells | Lyophilized | C3R at concentrations of 25, 50, and 100 μM. | C3R at concentrations of about 25 μM inhibited RKO cell motility, possibly exerting an anticancer potential | [76] |
| Gold nanoparticles of açaí berries | Pancreatic (Panc-1) and prostate (PC-3) cancer cell lines | Aqueous | 50-200 mg/mL of açaí berries extract and 0.0–0.4 mg/mL of açaí gold nanoparticles | The açaí gold nanoparticles showed potent anticancer activity against pancreatic and prostate cancer cell lines | [77] | |
| Kinetically stable açaí oil at a concentration of 50 mg oil/mL | Murine fibroblast NIH/3T3 normal cells and murine B16F10 melanoma cell lines | Nanoemulsion | PDT with the açaí oil nanoemulsion at 50 mg oil/mL concentration | Treated cells presented 85% of B16F10 melanoma cell lines death by apoptosis while preserving NIH/3T3 normal cell viability | [78] | |
| Açaí seed hexane, chloroform, and ethyl acetate extract fractions | Human MCF-7 breast adenocarcinoma-derived cells | Hydroalcoholic | The cells were treated with final concentrations ranging from 10, 20, 40, and 60 μg/mL | The results showed that the ethyl acetate fraction most effectively reduced MCF-7 cell viability by causing necroptosis | [79] | |
| Bark, seed, and total açaí fruit extracts | Human Caco-2 and HT-29 colon adenocarcinoma cells and human MDA-MB-468 and MCF-7 mammary adenocarcinoma cells | Hydroalcoholic | 10, 20, and 40 μg/mL | Only MCF-7 cells responded to the açaí treatment; the extracts reduced cell viability and altered cell morphology | [80] | |
| Frozen, concentrated, clarified açaí juice | Nonmalignant CCD-18 colon fibroblast cells and malignant colon cancer HT-29 and SW-480 cells | Polyphenolic extract | Doses ranging from 5–20 mg/L | Açaí inhibited the growth of SW-480 cells with no cytotoxic effects against CCD-18 cells. Prooncogenic proteins were downregulated, as well as Sp-targets Bcl-2, the vascular endothelial growth factor, and the factor survivin | [81] | |
| Monomeric (cyanidin-3-rutinoside and cyanidin-3-glucoside) and polymeric (mixture of anthocyanin adducts) anthocyanin fractions from açaí fruit | Human HT-29 colon adenocarcinoma cells and colon Caco-2 carcinoma cells | Anthocyanin extracts | Doses ranging from 0.5 to 100 μg cyanidin-3 glucoside equivalents/ml | Açaí anthocyanins inhibited colon HT-29 cancer cell proliferation (95.2%) | [82] | |
| Anthocyanin-rich extract from açaí (312 mg of GAE/g, 124 mg RE (flavonoid content), and 100 mg CGE (anthocyanin content)) | Rat C-6 brain glioma cells and human MDA-468 breast cancer cells | Lyophilized | 50, 100, and 200 μg/mL | Açaí suppressed the proliferation of rat C-6 brain glioma cells but did not affect human MDA-468 breast cancer cells | [83] | |
| Açaí juice | XV 185-14c strain of Saccharomyces cerevisiae | Not reported | 5%, 10%, and 15% wt/vol | The use of the açaí in higher concentrations demonstrated mutagenic effects | [84] | |
| Açaí pulp extract divided into whole pulp fraction, lipophilic fraction, C18 bound phenolics and anthocyanins fraction, ethyl acetate soluble polyphenolics, isolated anthocyanins fraction, C18 non-retained fraction, C18 bound phenolics and anthocyanins fraction, hydrolyzed anthocyanins fraction, and hydrolyzed ethyl acetate soluble polyphenolics fraction | Human HL-60 leukemia cells | Not reported | Cells were treated with all açaí fractions at concentrations ranging from 0.0–10.7 µM | The polyphenolic fraction decreased cell proliferation from 56 to 86% and increased cell apoptosis due to the caspase-3 activation pathway | [85] | |
| Freeze-dried açaí pulp | Not reported | In vitro digested freeze-dried açaí pulp | 1 g of the digested açaí pulp | In the feces examination, the pulp decreased the number of the Bacteroides–Prevotella spp. and Clostridium histolyticum colonies | [86] | |
| Açaí berry pulp and oil extract rich in phenolic acids (protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, ferulic acid, catechin, and epicatechin), flavonoids, and procyanidins | Human HT-29 colon adenocarcinoma cells | Polyphenolic extracts | The cells were treated with polyphenolics ranging the final concentrations of 0.04–12 µg of GAE/mL | The treatment could effectively inhibit cellular proliferation by up to 90.7% | [87] | |
| Musculoskeletal health | Velutin, a bioactive compound of açaí | RAW 264.7 osteoclast precursor cell line stimulated with RANKL | Not reported | The cells were treated with final concentrations ranging 0.5–2.0 μM | Velutin was not cytotoxic to RAW 264.7 osteoclast or undifferentiated cells, reduced osteoclast differentiation, and exerted potential anti-inflammatory effects downregulating the HIF-1α production | [88] |
| Dried açaí berry powder extract | RAW 264.7 cells stimulated with RANKL | Not reported | The cells were treated with final concentrations ranging the doses 25–100 µg/mL | Açaí decreased IL-6 and TFN-α and showed inhibitory actions of osteoclastogenesis and osteoclastic activity. There was an increase in IL-3, IL-4, and IL-13 | [89] |
| Properties | Plant Part or Active Compounds | Type of Extraction | Experimental Model | Dose | Route of Administration | Observations | Reference |
|---|---|---|---|---|---|---|---|
| Antioxidant | Açaí pulp with 549.5 mg/100 g of gallic acid equivalent | The açaí pulp was purchased commercially and stored | Female Fischer rats | Diet supplemented with 2% of açaí pulp | Oral by feeding | The açaí pulp supplemented diet augmented antioxidant GPx-1, GPx-4, and SOD1 mRNA genetic expression in the liver | [90] |
| Açaí pulp | The açaí pulp was purchased commercially and stored | Male Wistar rats | Diet supplemented with 5% of açaí pulp | Oral by feeding | Açaí supplementation reduced oxidative stress and improved energetic metabolism | [91] | |
| Açaí seed extract | The açaí was acquired and the extract was made in a laboratory | Male Wistar rats | Doses at concentrations of 100 mg/mL 200 mg/mL | Intragastric gavage | The açaí seed extract did not diminish the cachectic syndrome in a rat model of tumorigenesis | [92] | |
| Pasteurized açaí pulp with a high capacity for neutralizing free radicals | The açaí pulp was purchased commercially and stored | Female Fisher rats | Diet supplemented with 2% of açaí pulp | Oral by feeding | Açaí pulp could effectively control the oxidative species production by neutrophils and increased liver antioxidant defenses | [93] | |
| Anti-inflammatory | Açaí oil | The açaí oil was purchased commercially and stored | Male Wistar albino rats and male Swiss albino rats | Doses of 500, 1000, and 1500 mg/kg | Orally | The anti-inflammatory effects were associated with prostaglandin synthesis inhibition | [19] |
| Açaí stone extract | The açaí berries were obtained, and the stone extract was made and stored | Eight-week-old male mice | 300 mg/kg/day | Intragastric gavage | The supplementation with açaí seed extract could significantly reduce inflammatory and oxidative responses | [94] | |
| Analgesic | Açaí stones extract rich in proanthocyanidins | Hydroalcoholic extract | Male Swiss mice | The açaí stone extract was dissolved in distilled water at a concentration of 10 mg/mL | Intragastric gavage | The extract exerted antinociceptive effects | [95] |
| Antimicrobial | Açaí fractions | Rich polyphenol fractions of açaí | Murine models infected with P. chabaud | Doses of 10, 15, and 20 mg/kg/day of the açaí polyphenol-rich fractions | Intragastric gavage | The higher doses of açaí fractions reduced parasitemia and increased the survival rates of infected animals | [61] |
| Gastroprotective | Açaí seed extract with considerable amounts of proanthocyanidins and lesser amounts of catechin and epicatechin | Hydroalcoholic | Male Wistar rats | Doses of 10, 30, and 100 mg/kg | Orally | A higher dose significantly reduced inflammation, oxidative stress, and macroscopy and histological parameters of the colitis | [12] |
| Açaí berries dried extract with high radical scavenger capacity | Dried extract | Female Wistar rats | Doses of 30 and 100 mg/kg (PO) and 3 mg/kg (IP) | Orally or intraperitoneally | The extract reduced inflammation and maintained oxidative balance in the gastric mucosa | [96] | |
| Neuroprotective | Clarified açaí juice containing no lipids, proteins, or fibers | Microfiltrated and centrifugated açaí juice | Male Swiss mice | Doses of 10 µL/g | Intragastric gavage | The use of açaí clarified juice effectively protected the brain against oxidative stress in specific areas related to convulsive crises | [97] |
| Açaí seeds extract 88% of proanthocyanidins | Aqueous extract | Male Wistar rats | 200 mg/kg/day | Intragastric gavage | Açaí exerted anti-anxiety effects by reducing hypothalamus–pituitary–adrenal axis reactivity to stress and increasing the NO–BDNF–TRKB pathway | [98] | |
| Fresh açaí extract | Fresh herbal capsules | Male Wistar albino rats | Doses of 100 mg/kg/day or 300 mg/kg/day | Intragastric gavage | Açaí did not improve learning and memory abilities | [99] | |
| Açaí frozen pulp | The frozen açaí pulp was purchased commercially and stored | Male Wistar rats | 7 μL/g/day | Intragastric gavage | The açaí frozen pulp exerted antioxidant effects on the brain of the rats | [100] | |
| Lyophilized açaí pulp | The lyophilized açaí pulp was purchased commercially and stored | Aged male Fischer 344 rats | A diet containing 2% of açaí pulp | Oral by feeding | The supplementation conserved the memory of rats due to the anti-inflammatory and antioxidant effects of açaí berry | [101] | |
| Açaí frozen pulp | The frozen açaí pulp was purchased commercially and stored | Male Wistar rats | Dose of 7 μL/g | Intragastric gavage | The use of açaí prevented an increase in IL-1β, IL-18, and TNF-α, while IL-6 and IL-10 levels remained unchanged | [102] | |
| Açaí frozen pulp with 1.19 ± 0.20 mg/100 g of catechin | The frozen açaí pulp was purchased commercially and stored | Wistar rats | Açaí pulp was diluted in distilled water at a concentration of 40% wt/vol | Orally | Açaí exerted antioxidant effects against neurodegenerative diseases in a rat model of hydrogen peroxide-induced nervous damage | [72] | |
| Freeze-dried açaí powder | The freeze-dried açaí powder was purchased commercially and stored | Male Fischer rats | 2% of the freeze-dried açaí powder | Oral by feeding | The freeze-dried açaí powder modulated the Nrf2 pathway and protected neuronal cells against ubiquitin–proteasomal degradation | [103] | |
| Clarified açaí juice containing no lipids, proteins, or fibers, but with >1400 mg GAE/L | Microfiltrated and centrifugated açaí juice | Male Swiss mice | 10 µL/g | Intragastric gavage | The treatment effectively abolished despair-like and anhedonia behaviors and protected the hippocampus, striatum, and prefrontal cortex from oxidative damage related to depression | [104] | |
| Antilipidemic | Açaí oil | The açaí oil was purchased commercially and stored | Male Wistar rats | 1226 mg/kg/day | Orally | The results suggested that the use of açaí oil was effective in reducing atherosclerosis in rats with dyslipidemia | [105] |
| Açaí oil | The açaí oil was purchased commercially and stored | Male Wistar rats | 1226 mg/kg/day | Intragastric gavage | The açaí oil was able to antagonize cholesterol and triglycerides increases among rats | [106] | |
| Pasteurized açaí pulp | The frozen açaí pulp was purchased commercially and stored | Female Fischer rats | Standard or a high-fat diet supplemented with 2% of açaí pulp | Orally by feeding | The supplementation promoted an anticholesterolemic effect by increasing the expression of subfamily G transporters, ATP-binding cassette, and LDL-R genes | [107] | |
| Pasteurized açaí pulp | The frozen açaí pulp was purchased commercially and stored | Female Fischer rats | Standard and a high-fat diet supplemented with 2% of açaí pulp | Oral by feeding | The açaí pulp supplementation improved antioxidant status and diminished cholesterol serum levels | [36] | |
| Açaí seed flour | The açaí flour was purchased commercially and stored | Male C57BL/6 mice | Diet supplemented with 15% or 30% of açaí flour | Oral by feeding | Açaí flour increased cholesterol excretion among mice fed a high-fat diet and prevented the development of obesity and NAFLD | [108] | |
| Fresh açaí berries extract | The açaí berries were obtained and stored for further aqueous extract production | Male New Zealand rabbits | 80 mL of fresh açaí extract was dissolved in water | Oral by drinking water | Fresh açaí berries extract significantly improved the lipid profile and the atherosclerosis statuses in an atherosclerosis-induced rabbit model | [109] | |
| Hepatoprotective | Açaí pulp with 549.5 mg GAE/100 g of polyphenols | The açaí pulp was purchased commercially and stored | Female Fisher rats | Standard and high-fat chow with 2% of açaí pulp | Oral by feeding | The supplementation had protective effects in dams against NAFLD and protected the offspring from the effects of a maternal high-fat diet with lipid excess | [110] |
| Lyophilized açaí pulp | The açaí pulp was purchased commercially and stored | Male Fischer rats | Standard chow with 2% of the lyophilized açaí pulp | Oral by feeding | The lyophilized açaí pulp diminished inflammation and reduced liver steatosis | [111] | |
| Açaí pulp with 0.035 g/100 g of procyanidin | The açaí pulp was purchased commercially and stored | Wistar rats | 1 mL/100 g | Intragastric gavage | The treatment reduced alcohol-induced liver injury in rats by diminishing inflammation and oxidative stress | [112] | |
| Açaí seed extract with 265 mg/g of polyphenols | Hydroalcoholic extract | Male Wistar rats | 200 mg/kg/day | Intragastric gavage | The extract, in conjunction with exercise training, decreased glucose and lipid serum levels, serum hepatic enzymes, and liver triglycerides | [113] | |
| Filtered açaí pulp with 458.6 mg GAE/100 g of polyphenols and 13.59 mg/100 g of monomeric anthocyanins | The açaí oil was purchased commercially and stored | Female Fischer rats | 2 g/day | Intragastric gavage | The açaí supplementation protected liver steatosis and injuries in a high-fat diet-rats | [114] | |
| Açaí water extract | The açaí pulp was obtained commercially and stored for future aqueous extract preparation | Male Swiss mice | 3 g/kg/day | Intragastric gavage | The extract prevented liver damage, attenuated inflammation, and decreased oxidative stress | [115] | |
| Açaí water extract with 118.13 mg GAE/100 g of phenolic compounds and 9.23 mg/100 g of flavonoid compounds | The açaí pulp was obtained commercially and stored for future aqueous extract preparation | Male Swiss mice | 3 g/kg/day | Intragastric gavage | The use of açaí increased the production and effectiveness of adiponectin, improving insulin sensitivity and increasing PPAR-α-mediated fatty acid oxidation | [116] | |
| Açaí seeds extract rich in catechin and epicatechin | Hydroalcoholic | Male C57BL/6 mice | 300 mg/kg/day | Intragastric gavage | The use of the extract significantly reduced obesity and hepatic steatosis | [35] | |
| Antidiabetic | Açaí seed extract with 265 mg/g of polyphenols | Hydroalcoholic extract | Male Wistar rats | 200 mg/kg/day | Intragastric gavage | The extract exerted an antidiabetic effect in the diabetic-induced rats by potentializing the insulin-signaling pathway in skeletal muscles cells and adipose tissue, increasing GLP-1 levels | [117] |
| Antihypertensive | Açaí stones extract with 265 mg/g of polyphenols | Hydroalcoholic | Male Wistar rats | 200 mg/kg/day | Orally | The supplementation with açaí protected against vascular changes and endothelial dysfunction due to antihypertensive and antioxidant effects | [118] |
| Açaí seed extract with high amounts of proanthocyanidins | Hydroalcoholic | Female Wistar rats | 200 mg/kg/day | Oral by drinking water | The açaí seed extract protected against cardiovascular changes and intrauterine growth restriction | [22] | |
| Açaí seed extract with 265 mg/g of phenolic compounds | Hydroalcoholic | Female Wistar rats | 200 mg/kg | Intragastric gavage | Açaí promoted vasodilator and antioxidant effects | [119] | |
| Cardioprotective | Açaí pulp with 170 mg/100 g of gallic acid and 15.6 mg/100 g of total anthocyanins | The açaí pulp was purchased commercially and stored at −80 °C for later use in standard chow | Male Wistar rats | Standard chow with 2% and 5% of açaí pulp | Orally by feeding | Supplementation with açaí pulp attenuated cardiac remodeling after myocardial infarction. | [6] |
| Açaí seed extract | Aqueous | Male Wistar rats | Açaí seed extract in a dose of 200 mg/kg/day | Orally by drinking water | Reduced SBP, restored of endothelial and renal functions, decreased inflammation and oxidative stress, and attenuated of the endothelial dysfunction | [120] | |
| Lyophilized açaí pulp with 3300 mg/100 g of total polyphenols and 6.45 to 31.0 mg/100 g of anthocyanins | The açaí pulp was purchased commercially and stored | Male Fischer rats | High-fat diet supplemented with 1% of the lyophilized açaí pulp | Orally by feeding | Açaí supplementation may decrease cardiac remodeling and increase cardiac function | [121] | |
| Açaí pulp extract | Aqueous extract | Male Wistar rats | 100 mg/kg and 300 mg/kg | Intravenous | There were elevations in acute blood flow induced by açaí extract | [122] | |
| Açaí pulp | The açaí pulp was purchased commercially and stored | Male Wistar rats | Standard chow with 5% of açaí pulp | Oral by feeding | The supplementation reduced left ventricular dysfunction, oxidative stress, changes in the myocardium metabolism, and MMP-2 activation | [123] | |
| Açaí seed extract | Hydroalcoholic | Male Wistar rats | 100 mg/kg/day | Intragastric gavage | Açaí prevented the development of exercise intolerance, cardiac fibrosis, cardiac dysfunction, and cardiac hypertrophy | [124] | |
| Açaí seed extract | Hydroalcoholic | Young male Wistar rats and spontaneously hypertensive rats | 200 mg/kg/day | Orally | Açaí seed extract prevented vascular remodeling and decreased the percentage of elastic fibers, media/lumen ratio, hypertension, and oxidative damage | [125] | |
| Renoprotective | Açaí seed extract with 265 mg/g of polyphenols | Hydroalcoholic lyophilized extract | Male Wistar rats | 200 mg/kg/day | Orally by drinking water | The extract significantly reduced renal injury and prevented renal dysfunction | [126] |
| Açaí seed extract with 265 mg/g of polyphenols | Lyophilized açaí seed extract | Male Wistar rats | 200 mg/kg/day | Orally | The açaí seed extract exerted renoprotective effects, diminished renal injury, and prevented renal dysfunction | [127] | |
| Açaí berry extract | Not reported | Male Wistar albino rats | Doses of 100 and 200 mg/kg/day | Orally | The extract was capable of attenuating renal damage | [128] | |
| Açaí fruit extract | Not reported | Male Wistar albino rats | Doses of 500 and 1000 mg/kg) | Intragastric gavage | The açaí fruit extract ameliorated the ischemia–reperfusion kidney-induced syndrome bilaterally in a dose-dependent manner | [129] | |
| Anticancer | Lyophilized açaí pulp with 214.09 ± 17.32 mg/100 g of cyanidin 3-rutinoside and 1908.5 ± 24.4 mg/100 g of β-carotene | The lyophilized açaí pulp was purchased commercially and stored | Male Wistar rats | Standard chow with 5% or 7.5% of the lyophilized açaí pulp | Oral by feeding | The pulp exerted potential antitumor activity | [76] |
| Açaí fruit extract | Hydroalcoholic extract | Female Wistar rats | 200 mg/kg | Intragastric gavage | The extract promoted anti-inflammatory and antiangiogenic effects | [130] | |
| Spray-dried açaí powder | Açaí pulp was purchased commercially and dried to be sprayed | Male Wistar rats | A diet containing 5% of spray-dried açaí powder | Oral by feeding | The results showed that spray-dried açaí powder could effectively reduce the development of chemically-induced carcinogenesis | [131] | |
| Açaí pulp powder with 0.5% of polyphenolic content and freeze-dried açaí powder | The açaí pulp was purchased commercially and transformed into powder, then and the freeze-dried product was stored | Azoxymethane/dextran sulfate sodium-treated mice | 0.5 g/5 mL of phosphate-buffered saline was administered as pellets containing 5% of açaí powder | Orally | The use of açaí protected the mice model of colon tumorigenesis against cancer development | [132] | |
| Spray-dried açaí fruit pulp containing high amounts of anthocyanins (cyanidin 3-glucoside and cyanidin 3-rutinoside) and carotenoids (lutein, α-carotene, β-carotene, and 9-cis β-carotene) | The açaí pulp was dried and stored | Male Swiss albino mice | A low-fat diet containing 2.5% or 5.0% of açaí fruit pulp powder | Oral by feeding | The use of açaí attenuated carcinogenesis principally by increasing antioxidant glutathione capacity and attenuating DNA damage | [133] | |
| Kinetically stable açaí oil nanoemulsion in a concentration of 50 mg oil/mL | Nanodroplets | C57BL/6 female mice | Rats were treated five times with nanodroplets containing the nanoemulsion with 50 mg of açaí oil/mL | Nanodroplets, orally | The açaí oil nanodroplets showed a significant reduction in the tumor volume | [78] | |
| Açaí flakes extract | Dehydration of açaí berries | Male F344 rats | Diet containing 5% berry flakes | Oral by feeding | The flakes exerted inhibitory effects on esophagus tumor progression | [134] | |
| Wound-healing | Açaí berry extract | Aqueous extract | Sprague–Dawley rats | Treatments with 1%, 3%, or 5% of açaí berries aqueous extract were conducted | Application on lesions | The extract was not cytotoxic and significantly increased fibroblast migration and fibronectin expression | [135] |
| Açaí berry extract | Aqueous extract | Sprague–Dawley rats | Treatments with 1%, 3%, or 5% of açaí berries aqueous extract were conducted | Application on oral lesions | The use of açaí extract significantly improved the healing progress in wounds of rats’ oral mucosa | [136] | |
| Miscellaneous effects | Extract of açaí seeds with 25.12 mg/g of polyphenols, 9.048 mg/g of CAE, 0.258 mg/g of MRE, and 9.798 mg/g of CE | Ethanol extract | Male Wistar rats | Doses of 200 mg/kg, 300 mg/kg, and 400 mg | Intraperitoneal | The açaí extract demonstrated myorelaxant activities in the animals | [137] |
| Açaí seeds extract with 265 mg/g of polyphenols | Hydroalcoholic | Male Wistar rats | 200 mg/kg/day | Intragastric gavage | The extract improved the aerobic physical performance (↑ vascular function), reduced oxidative stress, and upregulated mitochondrial biogenesis key proteins | [138] | |
| Açaí fruit extract | Hydroalcoholic | Female Sprague–Dawley rats | 200 mg/kg/day | Intragastric gavage | The extract significantly suppressed the establishment and growth of endometriosis | [139] | |
| Dried açaí | Açaí-enriched diet | Male Wistar rats | The dried açaí was mixed with the standard diet but was not calculated | Oral by feeding | The açaí-supplemented diet exerted eye protection and antioxidant effects | [140] | |
| Açaí extract | Not reported | C57BL/6NCrSlc mice | 10 mL/kg/day | Intragastric gavage | The use of açaí can stimulate erythropoietin production by inducing a hypoxic renal condition | [141] |
| Type of the Study and Patients | Interventions | Outcomes | Adverse Effects | Reference |
|---|---|---|---|---|
| Healthy subjects | ||||
| Randomized crossover study with 38 healthy adults (22♀, 16♂; 19–48 y) in Brazil | Participants received 200 mL/day of açaí (n = 19) or juçara (Euterpe edulis) juice (n = 19) for 4 weeks with a 4-week wash-out period | No modifications in glycemia or lipid profile before the treatment period but improvement of TAC, OSI, CAT, and GPx levels | Not reported by the authors | [159] |
| 40 healthy women (24 ± 3 y). | 200 g of açai pulp/day for 4 weeks | No modifications in anthropometric parameters, arterial pressure, glucose, insulin, LDL, and HDL, triglycerides, and ApoB; increase of ApoA-I and TAC | Not reported by the authors | [178] |
| Randomized, double-blind, crossover-controlled trial with 23 healthy males (30–65 y) with a BMI 25–30 in the United Kingdom | Consumption of an açaí-based smoothie (694 mg total phenolics) or a macronutrient-matched control smoothie with a high-fat breakfast meal modification | Improvement of vascular function (increases in flow-mediated dilatation compared to control (p = 0.001). A significant reduction of iAUC for total peroxide oxidative status after açaí intake. No significant modifications for heart rate, blood pressure, or postprandial glycemia | Patients did not report AE | [179] |
| Simple-blinded randomized intervention trial with fourteen male athletes (mean age of 26 y) in Brazil | Performance of 3 tests: 45 a ramp-incremental maximal test and two maximal bouts in two conditions (açaí or control) at 90% VO2 max. After the first exercise bout, subjects drank 300 mL of freeze-dried and were instructed to intake the fruit 3 consecutive days, 1 h before the exercise bout to exhaustion | Increase in time to exhaustion during short-term high-intensity (p = 0.045), attenuating the metabolic stress induced by exercise | Not reported by the authors | [180] |
| Randomized, double-blind, placebo-controlled crossover study with 20 participants (13♀, 7♂; 22.4 ± 2.50 y) in the USA | Phase 1: subjects received two capsules (500 mg of açaí or placebo). After a 7-day wash-out, subjects returned for phase 2 and consumed the opposing treatment | After the first dose, no significant differences for ECG between groups, and no differences were seen for the primary or secondary hemodynamic endpoints (except for significant lower systolic blood pressure at 6 h with açaí) | Patients did not report AE | [181] |
| Auditory disorder | ||||
| Randomized, double-blind study with 30 patients (14♀, 16♂; mean age of 50.5 y); complaint of tinnitus, hearing thresholds; annoyance score of at least four in Brazil | Patients were divided into a placebo (starch capsules) and a treated group that received an extract of dry açaí (100 mg/capsule) | Reduction in the discomfort of tinnitus evaluated by THI (p = 0.006); significant improvement for anxiety disorders symptoms (p = 0.016). No significant differences for oxidative metabolism biomarkers, but a decrease in posttreatment values for all groups | Patients did not report AE | [31] |
| Overweight, dyslipidemia, and metabolic syndrome | ||||
| Randomized, double-blind, placebo-controlled clinical trial with 69 subjects (BMI > 25 kg/m2) (46♀, 23♂; 20–59 y) in Brazil | Participants with at least one lipid profile alteration that received 200 g of açaí or placebo, and a hypo-energetic diet (calculated individually)/60 days | Reduction of oxidative stress and improvement of inflammatory status (decrease of IL-6 and INF-γ) | Not reported by the authors | [142] |
| Randomized, double-blinded, and placebo-controlled trial with 37 subjects with MetS (BMI 33.5 ± 6.7 kg/m2; 26♀, 11♂; 18–65 y) in USA | Intake of 325 mL of açaí beverage twice/day or placebo/12 weeks | No modifications on lipid and glycemic profile; significant reduction of IFN-γ and urinary levels of 8-isoprostane, compared to the placebo group (p = 0.0141 and 0.0099, respectively) | Not reported by the authors | [182] |
| Open label pilot study with 10 adults, 18–65 y (BMI ≥ 25 kg/m2 and ≤30 kg/ m2) in USA | Intake of 100 g açai pulp twice daily for 1 month | Reductions in serum blood glucose and insulin levels (p < 0.02), CT (p = 0.03), LDL. No effects on blood pressure, CRP or NO metabolites | Patients did not report AE | [183] |
| Prostate cancer | ||||
| Phase II, Simon 2-stage clinical trial in subjects showing biochemically recurrent prostate cancer (54–80 y) USA | Subjects received açaí juice product (mix of fruit juices and tea extracts, with 80% of the juice produced with açaí berry), twice daily, for 36 weeks | PSA response >50% was observed in 1/21 subjects within 30 w of the treatment. The PSA doubling time was lengthened in most patients (71%) | Patients did not report AE | [184] |
| Study | Question Focus | Appropriate Randomization | Allocation Blinding | Double-Blind | Losses (<20%) | Prognostic and Demographic Characteristics | Outcomes | Intention to Treat Analysis | Sample Calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Oppitz et al. [31] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| de Liz et al. [159] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Aranha et al. [142] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | NR | Yes |
| Kessler et al. [184] | Yes | NR | NR | NR | NR | NR | NR | NR | NR | Yes |
| Kim et al. [182] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N | Yes | Yes |
| Pala et al. [178] | Yes | No | No | No | Yes | No | Yes | No | No | Yes |
| Alqurashi et al. [179] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Carvalho-Peixoto et al. [180] | Yes | No | Yes | No | No | No | Yes | No | No | No |
| Gale et al. [181] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Udani et al. [183] | Yes | No | No | No | Yes | Yes | Yes | No | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. https://doi.org/10.3390/nu15040989
Laurindo LF, Barbalho SM, Araújo AC, Guiguer EL, Mondal A, Bachtel G, Bishayee A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients. 2023; 15(4):989. https://doi.org/10.3390/nu15040989
Chicago/Turabian StyleLaurindo, Lucas Fornari, Sandra Maria Barbalho, Adriano Cressoni Araújo, Elen Landgraf Guiguer, Arijit Mondal, Gabrielle Bachtel, and Anupam Bishayee. 2023. "Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review" Nutrients 15, no. 4: 989. https://doi.org/10.3390/nu15040989
APA StyleLaurindo, L. F., Barbalho, S. M., Araújo, A. C., Guiguer, E. L., Mondal, A., Bachtel, G., & Bishayee, A. (2023). Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients, 15(4), 989. https://doi.org/10.3390/nu15040989












