Patterns and Determinants of Weight Gain among People Who Use Drugs Undergoing Treatment for Recovery in Lebanon
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
2.2. Collected Data Included
2.3. Statistical Analysis
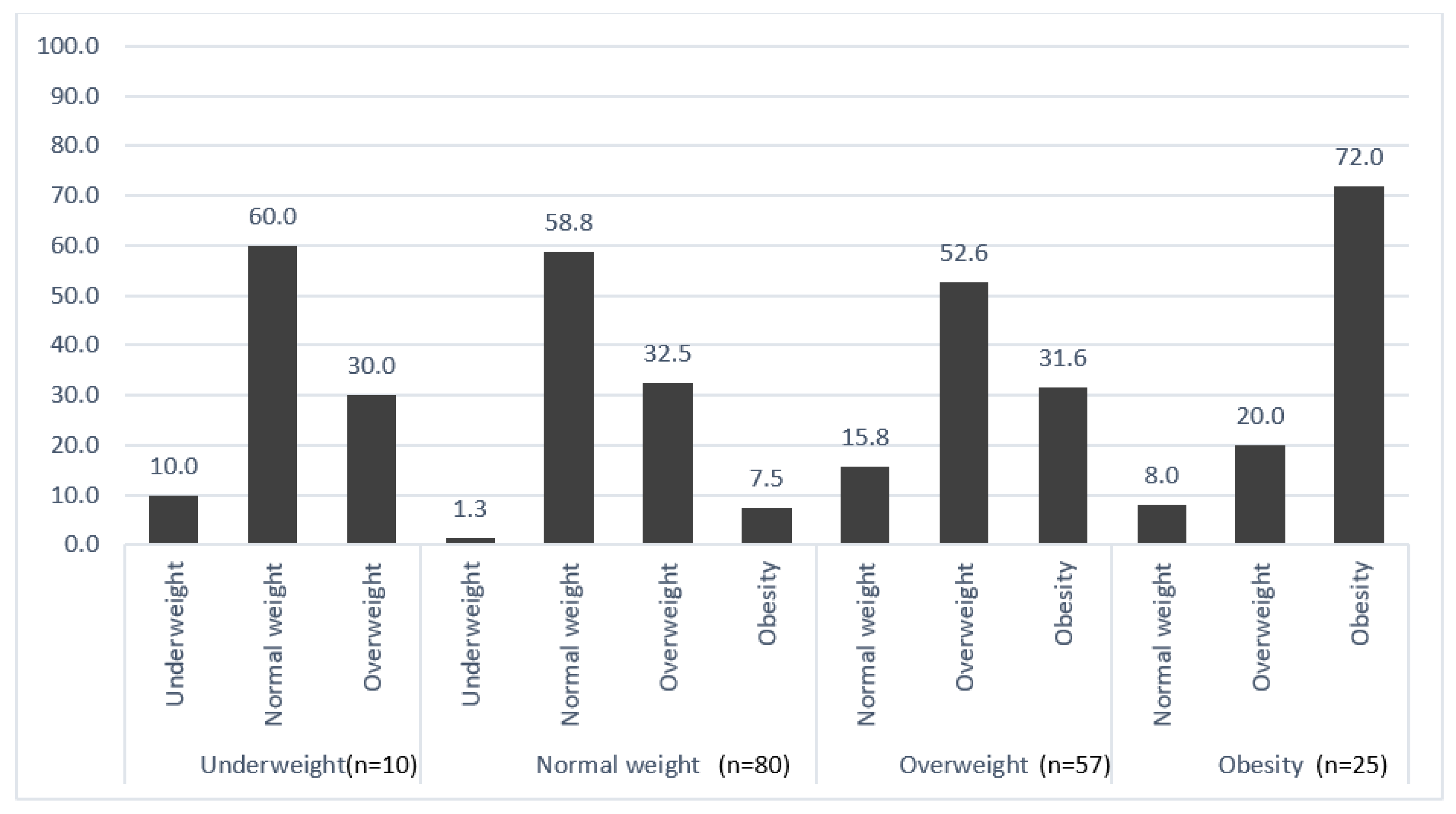
3. Results
4. Discussion
4.1. Strengths and Limitations
4.2. Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neale, J.; Nettleton, S.; Pickering, L.; Fischer, J. Eating patterns among heroin users: A qualitative study with implications for nutritional interventions. Addiction 2012, 107, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.M.; Bhatnagar, T.; Ramachandran, R.; Dong, K.; Skinner, S.; Kumar, M.S.; Wanke, C.A. Malnutrition in a population of HIV-positive and HIV-negative drug users living in Chennai, South India. Drug Alcohol Depend. 2011, 118, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Mahboub, N.; Rizk, R.; Karavetian, M.; de Vries, N. Nutritional status and eating habits of people who use drugs and/or are undergoing treatment for recovery: A narrative review. Nutr. Rev. 2020, 79, 627–635. [Google Scholar] [CrossRef] [PubMed]
- White, R. Drugs and nutrition: How side effects can influence nutritional intake. Proc. Nutr. Soc. 2010, 69, 558–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeynes, K.D.; Gibson, E.L. The importance of nutrition in aiding recovery from substance use disorders: A review. Drug Alcohol Depend. 2017, 179, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenhardt, L.; Hall, W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012, 379, 55–70. [Google Scholar] [CrossRef]
- Ersche, K.D.; Stochl, J.; Woodward, J.M.; Fletcher, P.C. The skinny on cocaine: Insights into eating behavior and body weight in cocaine-dependent men. Appetite 2013, 71, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Himmelgreen, D.A.; Perez-Escamilla, R.; Segura-Millan, S.; Romero-Daza, N.; Tanasescu, M.; Singer, M. A comparison of the nutritional status and food security of drug-using and non-drug-using Hispanic women in Hartford, Connecticut. Am. J. Phys. Anthr. 1998, 107, 351–361. [Google Scholar] [CrossRef]
- Mysels, D.J.; Sullivan, M.A. The relationship between opioid and sugar intake: Review of evidence and clinical applications. J. Opioid. Manag. 2010, 6, 445–452. [Google Scholar] [CrossRef]
- Quach, L.A.; Wanke, C.A.; Schmid, C.H.; Gorbach, S.L.; Mwamburi, D.M.; Mayer, K.H.; Spiegelman, D.; Tang, A.M. Drug use and other risk factors related to lower body mass index among HIV-infected individuals. Drug Alcohol Depend. 2008, 95, 30–36. [Google Scholar] [CrossRef] [Green Version]
- United Nations Office on Drugs and Crime (UNODC). World Drug Report 2019; Sales No. E. 19. XI. 8; United Nations Publication: New York, NY, USA, 2019. [Google Scholar]
- Edge, P.J.; Gold, M.S. Drug withdrawal and hyperphagia: Lessons from tobacco and other drugs. Curr. Pharm. Des. 2011, 17, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.; Devine, C. Food, eating, and weight concerns of men in recovery from substance addiction. Appetite 2008, 50, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zahari, Z.; Siong, L.C.; Musa, N.; Mohd Yasin, M.A.; Choon, T.S.; Mohamad, N.; Ismail, R. Report: Demographic profiles and sleep quality among patients on methadone maintenance therapy (MMT) in Malaysia. Pak. J. Pharm. Sci. 2016, 29, 239–246. [Google Scholar] [PubMed]
- Peles, E.; Schreiber, S.; Adelson, M. Documented poor sleep among methadone-maintained patients is associated with chronic pain and benzodiazepine abuse, but not with methadone dose. Eur. Neuropsychopharmacol. 2009, 19, 581–588. [Google Scholar] [CrossRef]
- Sason, A.; Adelson, M.; Herzman-Harari, S.; Peles, E. Knowledge about nutrition, eating habits and weight reduction intervention among methadone maintenance treatment patients. J. Subst. Abus. Treat. 2018, 86, 52–59. [Google Scholar] [CrossRef]
- Beswick, T.; Best, D.; Rees, S.; Bearn, J.; Gossop, M.; Strang, J. Major disruptions of sleep during treatment of the opiate withdrawal syndrome: Differences between methadone and lofexidine detoxification treatments. Addict. Biol. 2003, 8, 49–57. [Google Scholar] [CrossRef]
- Roessler, K.K. Exercise treatment for drug abuse—A Danish pilot study. Scand. J. Public Health 2010, 38, 664–669. [Google Scholar] [CrossRef]
- Gimenez-Meseguer, J.; Tortosa-Martinez, J.; de los Remedios Fernandez-Valenciano, M. Benefits of exercise for the quality of life of drug-dependent patients. J. Psychoact. Drugs 2015, 47, 409–416. [Google Scholar] [CrossRef]
- Van Strien, T.; Herman, C.P.; Verheijden, M.W. Eating style, overeating, and overweight in a representative Dutch sample. Does external eating play a role? Appetite 2009, 52, 380–387. [Google Scholar] [CrossRef]
- Meule, A.; Gearhardt, A.N. Food addiction in the light of DSM-5. Nutrients 2014, 6, 3653–3671. [Google Scholar] [CrossRef]
- Lee, N.M.; Hall, W.D.; Lucke, J.; Forlini, C.; Carter, A. Food addiction and its impact on weight-based stigma and the treatment of obese individuals in the US and Australia. Nutrients 2014, 6, 5312–5326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerma-Cabrera, J.M.; Carvajal, F.; Lopez-Legarrea, P. Food addiction as a new piece of the obesity framework. Nutr. J. 2015, 15, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meguid, M.M.; Fetissov, S.O.; Varma, M.; Sato, T.; Zhang, L.; Laviano, A.; Rossi-Fanelli, F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 2000, 16, 843–857. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Müller, D.J.; Muglia, P.; Fortune, T.; Kennedy, J.L. Pharmacogenetics of antipsychotic-induced weight gain. Pharmacol. Res. 2004, 49, 309–329. [Google Scholar] [CrossRef]
- Wetterling, T.; Müssigbrodt, H.E. Weight gain: Side effect of atypical neuroleptics? J. Clin. Psychopharmacol. 1999, 19, 316–321. [Google Scholar] [CrossRef]
- Nolan, L.J.; Scagnelli, L.M. Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Subst. Use Misuse 2007, 42, 1555–1566. [Google Scholar] [CrossRef]
- Schlienz, N.J.; Huhn, A.S.; Speed, T.J.; Sweeney, M.M.; Antoine, D.G. Double jeopardy: A review of weight gain and weight management strategies for psychotropic medication prescribing during methadone maintenance treatment. Int. Rev. Psychiatry 2018, 30, 147–154. [Google Scholar] [CrossRef]
- Mysels, D.J.; Vosburg, S.; Benga, I.; Levin, F.R.; Sullivan, M.A. Course of weight change during naltrexone vs. methadone maintenance for opioid-dependent patients. J. Opioid Manag. 2011, 7, 47–53. [Google Scholar] [CrossRef]
- Romelsjö, A.; Leifman, A. Association between alcohol consumption and mortality, myocardial infarction, and stroke in 25 year follow up of 49 618 young Swedish men. BMJ 1999, 319, 821–822. [Google Scholar] [CrossRef] [Green Version]
- Wansink, B.; Cheney, M.M.; Chan, N. Exploring comfort food preferences across age and gender. Physiol. Behav. 2003, 79, 739–747. [Google Scholar] [CrossRef]
- Sobal, J.; Nelson, M.K. Commensal eating patterns: A community study. Appetite 2003, 41, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.M.; Antoine, D.G.; Nanda, L.; Géniaux, H.; Lofwall, M.R.; Bigelow, G.E.; Umbricht, A. Increases in body mass index and cardiovascular risk factors during methadone maintenance treatment. J. Opioid Manag. 2019, 15, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Gottfredson, N.C.; Sokol, R.L. Explaining excessive weight gain during early recovery from addiction. Subst. Use Misuse 2019, 54, 769–778. [Google Scholar] [CrossRef]
- Warren, C.S.; Lindsay, A.R.; White, E.K.; Claudat, K.; Velasquez, S.C. Weight-related concerns related to drug use for women in substance abuse treatment: Prevalence and relationships with eating pathology. J. Subst. Abus. Treat. 2013, 44, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Mahboub, N.; Honein-AbouHaidar, G.; Rizk, R.; de Vries, N. People who use drugs in rehabilitation, from chaos to discipline: Advantages and pitfalls: A qualitative study. PLoS ONE 2021, 16, e0245346. [Google Scholar] [CrossRef]
- Glasner-Edwards, S.; Mooney, L.J.; Marinelli-Casey, P.; Hillhouse, M.; Ang, A.; Rawson, R. Bulimia nervosa among methamphetamine dependent adults: Association with outcomes three years after treatment. Eat. Disord. 2011, 19, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Mahboub, N.; Rizk, R.; de Vries, N. Nutritional parameters and lifestyle practices of people who use drugs undergoing treatment for recovery in Lebanon: A descriptive study. J. Nutr. Sci. 2021, 10, e16. [Google Scholar] [CrossRef]
- Ministry of Public Health; Ministry of Education and Higher Education; Ministry of Interior and Municipalities; Ministry of Justice; Ministry of Social Affairs. Inter-Ministerial Substance Use Response Strategy for Lebanon 2016–2021; Republic of Lebanon Ministry of Public Health: Beirut, Lebanon, 2016.
- World Health Organization, Regional office for the Eastern Mediterranean. MENAHRA the Middle East and North Africa Harm Reduction Association Best Practices in Strengthening Civil Society’s Role in Delivering Harm Reduction Services; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- UNOCD. World Drug Report; United Nations Office on Drugs and Crime: Vienna, Austria, 2017. [Google Scholar]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An overview of USDA’s Dietary Intake Data System. J. Food Compos. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
- Pellet, P.; Shadarevian, S. Food Composition Tables for Use in the Middle East; American University of Beirut: Beirut, Lebanon, 1970. [Google Scholar]
- Dickson-Spillmann, M.; Siegrist, M.; Keller, C. Development and validation of a short, consumer-oriented nutrition knowledge questionnaire. Appetite 2011, 56, 617–620. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidar, S.A.; de Vries, N.K.; Papandreou, D.; Rizk, R.; Karavetian, M. The Freshman Weight Gain Phenomenon: Does It Apply To. Open Access Maced. J. Med. Sci. 2018, 6, 2214–2220. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Process of Translation and Adaptation of Instruments. Available online: https://www.mhinnovation.net/sites/default/files/files/WHO%20Guidelines%20on%20Translation%20and%20Adaptation%20of%20Instruments.docx (accessed on 15 April 2022).
- Zolala, F.; Mahdavian, M.; Haghdoost, A.A.; Karamouzian, M. Pathways to addiction: A gender-based study on drug use in a triangular clinic and drop-in center, Kerman, Iran. Int. J. High Risk Behav. Addict. 2016, 5, e22320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumtaz, G. Estimating the Prevalence of Injecting Drug Use in the Middle East and North Africa. In Proceedings of the Qatar Foundation Annual Research Forum, Doha, Qatar, 19–20 March 2018; Volume 2013, Issue 1. p. BIOP–031. [Google Scholar]
- Montazerifar, F.; Karajibani, M.; Lashkaripour, K. Effect of methadone maintenance therapy on anthropometric indices in opioid dependent patients. Int. J. High Risk Behav. Addict. 2012, 1, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Peles, E.; Schreiber, S.; Sason, A.; Adelson, M. Risk factors for weight gain during methadone maintenance treatment. Subst. Abus. 2016, 37, 613–618. [Google Scholar] [CrossRef]
- Emerson, M.H.; Glovsky, E.; Amaro, H.; Nieves, R. Unhealthy weight gain during treatment for alcohol and drug use in four residential programs for Latina and African American women. Subst. Use Misuse 2009, 44, 1553–1565. [Google Scholar] [CrossRef]
- Chavez, M.N.; Rigg, K.K. Nutritional implications of opioid use disorder: A guide for drug treatment providers. Psychol. Addict. Behav. 2020, 34, 699–707. [Google Scholar] [CrossRef]
- Cowan, J.A.; Devine, C.M. Process evaluation of an environmental and educational nutrition intervention in residential drug-treatment facilities. Public Health Nutr. 2012, 15, 1159–1167. [Google Scholar] [CrossRef]
- Fenn, J.M.; Laurent, J.S.; Sigmon, S.C. Increases in body mass index following initiation of methadone treatment. J. Subst. Abus. Treat. 2015, 51, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigson, P.S. Like drugs for chocolate: Separate rewards modulated by common mechanisms? Physiol. Behav. 2002, 76, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sarwer, D.B.; von Sydow Green, A.; Vetter, M.L.; Wadden, T.A. Behavior therapy for obesity: Where are we now? Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Leibel, R.L.; Rosenbaum, M.; Hirsch, J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 1995, 332, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Adam, T.C.; Jocken, J.; Westerterp-Plantenga, M.S. Decreased glucagon-like peptide 1 release after weight loss in overweight/obese subjects. Obes. Res. 2005, 13, 710–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdich, C.; Lysgård Madsen, J.; Toubro, S.; Buemann, B.; Holst, J.; Astrup, A. Effect of obesity and major weight reduction on gastric emptying. Int. J. Obes. 2000, 24, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumithran, P.; Proietto, J. The defence of body weight: A physiological basis for weight regain after weight loss. Clin. Sci. 2013, 124, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Hodgkins, C.C.; Cahill, K.S.; Seraphine, A.E.; Frostpineda, K.; Gold, M.S. Adolescent Drug Addiction Treatment and Weight Gain. J. Addict. Dis. 2004, 23, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Falahi, E.; Rad, A.H.K.; Roosta, S. What is the best biomarker for metabolic syndrome diagnosis? Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 366–372. [Google Scholar] [CrossRef]
- Fareed, A.; Casarella, J.; Amar, R.; Vayalapalli, S.; Drexler, K. Benefits of retention in methadone maintenance and chronic medical conditions as risk factors for premature death among older heroin addicts. J. Psychiatr. Pract. 2009, 15, 227–234. [Google Scholar] [CrossRef]
- Howard, A.A.; Hoover, D.R.; Anastos, K.; Wu, X.; Shi, Q.; Strickler, H.D.; Cole, S.R.; Cohen, M.H.; Kovacs, A.; Augenbraun, M. The effects of opiate use and hepatitis C virus infection on risk of diabetes mellitus in the Women’s Interagency HIV Study. J. Acquir. Immune Defic. Syndr. 1999 2010, 54, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, G.B. Do obese individuals gain weight more easily than nonobese individuals? Am. J. Clin. Nutr. 1990, 52, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, G.B. Lean body mass-body fat interrelationships in humans. Nutr. Rev. 1987, 45, 225–231. [Google Scholar] [CrossRef]
- Wang, G.J.; Volkow, N.D.; Thanos, P.K.; Fowler, J.S. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: A concept review. J. Addict. Dis. 2004, 23, 39–53. [Google Scholar] [CrossRef] [PubMed]
- O’brien, G.; Davies, M. Nutrition knowledge and body mass index. Health Educ. Res. 2007, 22, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Steptoe, A.; Perkins-Porras, L.; McKay, C.; Rink, E.; Hilton, S.; Cappuccio, F.P. Psychological factors associated with fruit and vegetable intake and with biomarkers in adults from a low-income neighborhood. Health Psychol. 2003, 22, 148–155. [Google Scholar] [CrossRef]
- Wardle, J.; Parmenter, K.; Waller, J. Nutrition knowledge and food intake. Appetite 2000, 34, 269–275. [Google Scholar] [CrossRef]
- Masheb, R.M.; Ruser, C.B.; Min, K.M.; Bullock, A.J.; Dorflinger, L.M. Does food addiction contribute to excess weight among clinic patients seeking weight reduction? Examination of the Modified Yale Food Addiction Survey. Compr. Psychiatry 2018, 84, 1–6. [Google Scholar] [CrossRef]
- Pedram, P.; Wadden, D.; Amini, P.; Gulliver, W.; Randell, E.; Cahill, F.; Vasdev, S.; Goodridge, A.; Carter, J.C.; Zhai, G.; et al. Food addiction: Its prevalence and significant association with obesity in the general population. PLoS ONE 2013, 8, e74832. [Google Scholar] [CrossRef] [Green Version]
- Pursey, K.M.; Stanwell, P.; Gearhardt, A.N.; Collins, C.E.; Burrows, T.L. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: A systematic review. Nutrients 2014, 6, 4552–4590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stice, E.; Figlewicz, D.P.; Gosnell, B.A.; Levine, A.S.; Pratt, W.E. The contribution of brain reward circuits to the obesity epidemic. Neurosci. Biobehav. Rev. 2013, 37, 2047–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, E.M.; Grilo, C.M.; Gearhardt, A.N. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin. Psychol. Rev. 2016, 44, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, R.W.; Epstein, L.H.; Wilson, G.T.; Drewnowski, A.; Stunkard, A.J.; Wing, R.R. Long-term maintenance of weight loss: Current status. Health Psychol. 2000, 19, 5–16. [Google Scholar] [CrossRef]
- Wing, R.R. Physical activity in the treatment of the adulthood overweight and obesity: Current evidence and research issues. Med. Sci. Sport. Exerc. 1999, 31, S547–S552. [Google Scholar] [CrossRef]
- Bell, A.C.; Ge, K.; Popkin, B.M. Weight gain and its predictors in Chinese adults. Int. J. Obes. 2001, 25, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- Ravussin, E.; Gautier, J. Metabolic predictors of weight gain. Int. J. Obes. 1999, 23, S37–S41. [Google Scholar] [CrossRef] [Green Version]
- Williamson, D.F. Dietary intake and physical activity as ”predictors” of weight gain in observational, prospective studies of adults. Nutr. Rev. 1996, 54, S101. [Google Scholar] [CrossRef]
- Fogelholm, M.; Kukkonen-Harjula, K. Does physical activity prevent weight gain–a systematic review. Obes. Rev. 2000, 1, 95–111. [Google Scholar] [CrossRef]
- Stein, M.D.; Herman, D.S.; Bishop, S.; Lassor, J.A.; Weinstock, M.; Anthony, J.; Anderson, B.J. Sleep disturbances among methadone maintained patients. J. Subst. Abus. Treat. 2004, 26, 175–180. [Google Scholar] [CrossRef]
- Chaput, J.-P.; Després, J.-P.; Bouchard, C.; Tremblay, A. The association between sleep duration and weight gain in adults: A 6-year prospective study from the Quebec Family Study. Sleep 2008, 31, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.R.; Hu, F.B. Short sleep duration and weight gain: A systematic review. Obes. Silver Spring Md. 2008, 16, 643–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangwisch, J.E.; Malaspina, D.; Boden-Albala, B.; Heymsfield, S.B. Inadequate sleep as a risk factor for obesity: Analyses of the NHANES I. Sleep 2005, 28, 1289–1296. [Google Scholar] [CrossRef] [Green Version]
- Magee, L.; Hale, L. Longitudinal associations between sleep duration and subsequent weight gain: A systematic review. Sleep Med. Rev. 2012, 16, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiss, D.A. A biopsychosocial overview of the opioid crisis: Considering nutrition and gastrointestinal health. Front. Public Health 2019, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.K. Dietary intake--how do we measure what people are really eating? Obesity 2002, 10, 63S. [Google Scholar] [CrossRef]
- Genell, A.; Nemes, S.; Steineck, G.; Dickman, P.W. Model selection in medical research: A simulation study comparing Bayesian model averaging and stepwise regression. BMC Med. Res. Methodol. 2010, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M. Stepwise Versus Hierarchical Regression: Pros and Cons. Available online: https://files.eric.ed.gov/fulltext/ED534385.pdf (accessed on 15 June 2021).

| OST (n = 94) | Rehabilitation (n = 78) | p-Value | Total (n = 172) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 33.7 | 8.3 | 30.5 | 8.3 | 0.007 ** | 33.0 | 8.6 |
| N | % | N | % | N | % | ||
| Educational level | |||||||
| Illiterate | 8.0 | 8.5 | 2.0 | 2.6 | 0.446 | 10.0 | 5.8 |
| Elementary/intermediate | 35.0 | 37.2 | 31.0 | 39.7 | 66.0 | 38.4 | |
| Secondary | 26.0 | 27.7 | 22.0 | 28.2 | 48.0 | 27.9 | |
| University | 25.0 | 26.6 | 23.0 | 29.5 | 48.0 | 27.9 | |
| Occupation | |||||||
| Unemployed/Retired | 38.0 | 40.4 | 38.0 | 48.7 | 0.07 | 76.0 | 44.2 |
| Employed | 27.0 | 28.7 | 15.0 | 19.2 | 42.0 | 24.4 | |
| Self-employed | 29.0 | 30.9 | 20.0 | 25.6 | 49.0 | 28.5 | |
| Student | 0.0 | 0.0 | 4.0 | 5.1 | 4.0 | 2.3 | |
| Other | 0.0 | 0.0 | 1.0 | 1.3 | 1.0 | 0.6 | |
| Marital status | |||||||
| Single | 63.0 | 67.0 | 61.0 | 78.2 | 0.221 | 124.0 | 72.1 |
| Married | 23.0 | 24.5 | 11.0 | 14.1 | 34.0 | 19.8 | |
| Divorced/separated | 8.0 | 8.5 | 6.0 | 7.7 | 14.0 | 8.1 | |
| Current housing | |||||||
| Residence | 94.0 | 100.0 | 24.0 | 30.8 | <0.001 | 118.0 | 68.6 |
| Rehabilitation | 0.0 | 0.0 | 54.0 | 69.2 | 54.0 | 31.4 | |
| People with whom the participant stays: pre-treatment (rehabilitation) and currently (OST) | |||||||
| Alone | 7.0 | 7.4 | 4.0 | 5.1 | <0.001 | 11.0 | 6.4 |
| Spouse/partner | 26.0 | 27.7 | 2.0 | 2.6 | 28.0 | 16.3 | |
| Parents | 59.0 | 62.8 | 13.0 | 16.7 | 72.0 | 41.9 | |
| Relative/colleagues | 2.0 | 2.1 | 56.0 | 71.8 | 58.0 | 33.7 | |
| No response | 0.0 | 0.0 | 3.0 | 3.8 | 3.0 | 1.7 | |
| Medications used | |||||||
| Antidepressants | 16.0 | 17.0 | 23.0 | 29.5 | 0.067 | 39.0 | 22.7 |
| Antipsychotic | 30.0 | 31.9 | 31.0 | 39.7 | 0.337 | 61.0 | 35.5 |
| Epilepsy-bipolar | 11.0 | 11.7 | 24.0 | 30.8 | 0.002 | 35.0 | 20.3 |
| Previous treatment | |||||||
| None | 35.0 | 37.2 | 41.0 | 52.6 | 0.002 | 76.0 | 44.2 |
| OST | 6.0 | 6.4 | 4.0 | 5.1 | 10.0 | 5.8 | |
| Rehabilitation | 22.0 | 23.4 | 27.0 | 34.6 | 49.0 | 28.5 | |
| Rehabilitation and OST | 10.0 | 10.6 | 3.0 | 3.8 | 13.0 | 7.6 | |
| Hospital detoxification | 17.0 | 18.1 | 2.0 | 2.6 | 19.0 | 11.0 | |
| Hospital detoxification and rehabilitation | 4.0 | 4.3 | 0.0 | 0.0 | 4.0 | 2.3 | |
| No response | 0.0 | 0.0 | 1.0 | 1.3 | 1.0 | 0.6 | |
| Other addiction | |||||||
| None | 87.0 | 92.6 | 46.0 | 59.0 | <0.001 | 133.0 | 77.3 |
| Alcohol | 1.0 | 1.1 | 25.0 | 32.1 | 26.0 | 15.1 | |
| Other | 6.0 | 6.4 | 7.0 | 9.0 | 13.0 | 7.6 | |
| Mean | SD | Mean | SD | Mean | SD | ||
| Duration of drug use (years) | 11.5 | 7.2 | 11.4 | 7.5 | 0.782 | 12.8 | 7.7 |
| Duration of drug injection (years) (among those who reported drug injection) | 7.4 | 6.3 | 8.6 | 5.7 | 0.352 | 7.7 | 6.2 |
| Number of previous treatment attempts | 3.7 | 4.9 | 2.0 | 2.4 | 0.052 | 3.1 | 4.4 |
| Treatment duration (months) | 31.6 | 25.6 | 5.5 | 5.5 | <0.001 ** | 24.9 | 27.9 |
| OST (n = 94) | Rehabilitation (n = 78) | p-Value | Total (n = 172) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Pre-treatment BMI (Kg/m2) | 25.9 | 4.7 | 24.1 | 5.0 | 0.016 * | 25.9 | 5.2 |
| During-treatment BMI (Kg/m2) | 26.6 | 5.3 | 27.6 | 4.6 | 0.209 | 27.4 | 5.5 |
| Energy (Kcal) | 2781.8 | 1485.4 | 2480.4 | 892.9 | 0.548 | 2641.9 | 1251.5 |
| Energy (Kcal/Kg) | 35.4 | 21.1 | 30.0 | 12.0 | 0.297 | 32.9 | 16.6 |
| N | % | N | % | N | % | ||
| Weight change | |||||||
| Weight loss | 31.0 | 33.0 | 7.0 | 9.0 | <0.001 | 38.0 | 22.1 |
| No change | 12.0 | 12.8 | 10.0 | 12.8 | 22.0 | 12.8 | |
| Weight gain | 51.0 | 54.3 | 61.0 | 78.2 | 112.0 | 65.1 | |
| Sleep quality index | |||||||
| Good sleep quality | 28.0 | 30.1 | 14.0 | 17.9 | 0.076 | 42.0 | 24.6 |
| Poor sleep quality | 65.0 | 69.9 | 64.0 | 82.1 | 129.0 | 75.4 | |
| Physical activity level | |||||||
| Low activity level | 68.0 | 72.3 | 19.0 | 24.4 | <0.001 | 87.0 | 50.6 |
| Moderate activity level | 19.0 | 20.2 | 24.0 | 30.8 | 43.0 | 25.0 | |
| High activity level | 7.0 | 7.4 | 35.0 | 44.9 | 42.0 | 24.4 | |
| Food addiction | |||||||
| No diagnosis met | 50.0 | 53.2 | 36.0 | 48.0 | 0.538 | 86.0 | 50.9 |
| Diagnosis met | 44.0 | 46.8 | 39.0 | 52.0 | 83.0 | 49.1 | |
| Nutrition knowledge | |||||||
| Poor nutrition knowledge | 69.0 | 73.4 | 49.0 | 62.8 | 0.142 | 118.0 | 68.6 |
| Good nutrition knowledge | 25.0 | 26.6 | 29.0 | 37.2 | 54.0 | 31.4 | |
| OST (n = 94) | Rehabilitation (n = 78) | Total (n = 172) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Underweight | 1.0 | 17.0 | 9.0 | 24.5 | 12.3 | 10.0 | 23.8 | 11.9 | |
| Normal | 40.0 | 1.6 | 5.5 | 40.0 | 12.4 | 10.4 | 80.0 | 7.0 | 9.9 |
| Overweight | 40.0 | 4.8 | 12.1 | 17.0 | 4.2 | 8.9 | 57.0 | 4.6 | 11.2 |
| Obese | 13.0 | −6.1 | 17.3 | 12.0 | 3.4 | 10.5 | 25.0 | −1.56 | 15.0 |
| Total | 94.0 | 2.0 | 11.3 | 78.0 | 10.6 | 12.0 | 172.0 | 5.9 | 12.4 |
| No Weight Gain (n = 60) | Weight Gain (n = 112) | p-Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age in years | 33.2 | 8.1 | 31.8 | 8.5 | 0.309 |
| Duration of drug use (years) | 12.5 | 8.3 | 10.9 | 6.6 | 0.197 |
| Number of previous treatment attempts | 2.6 | 5.0 | 0.9 | 1.8 | 0.022 * |
| Duration of current treatment (months) | 25.5 | 27.4 | 16.6 | 20.1 | 0.030 * |
| Energy (Kcal/Kg) | 36.0 | 20.3 | 31.3 | 16.0 | 0.108 |
| Protein (g/Kg) | 1.1 | 0.6 | 1.0 | 0.6 | 0.220 |
| Fiber (g) | 21.9 | 13.1 | 22.4 | 11.3 | 0.772 |
| % From total calories | SD | % From total calories | SD | ||
| Carbohydrate | 48.6 | 9.7 | 49.6 | 10.4 | 0.578 |
| Added sugar | 2.8 | 4.0 | 2.5 | 3.3 | 0.673 |
| Fat | 38.7 | 9.4 | 38.1 | 10.1 | 0.693 |
| N | % | N | % | ||
| Educational level | |||||
| Illiterate | 2.0 | 3.3 | 8.0 | 7.1 | 0.735 |
| Elementary/intermediate | 23.0 | 38.3 | 43.0 | 38.4 | |
| Secondary | 16.0 | 26.7 | 32.0 | 28.6 | |
| University | 19.0 | 31.7 | 29.0 | 25.9 | |
| Type of treatment | |||||
| OST | 43.0 | 71.7 | 51.0 | 45.5 | 0.001 * |
| Rehabilitation | 17.0 | 28.3 | 61.0 | 54.5 | |
| Current use of antidepressants | 11.0 | 18.3 | 28.0 | 25.0 | 0.347 |
| Current use of antipsychotics | 16.0 | 26.7 | 45.0 | 40.2 | 0.095 |
| Current use of epilepsy/bipolar medications | 10.0 | 16.7 | 25.0 | 22.3 | 0.432 |
| Current use of any medications | 24.0 | 40.0 | 58.0 | 51.8 | 0.152 |
| Pre-treatment BMI (kg/m2) | |||||
| Underweight (%) | 0.0 | 0.0 | 10.0 | 8.9 | 0.003 * |
| Normal weight (%) | 22.0 | 36.7 | 58.0 | 51.8 | |
| Overweight (%) | 24.0 | 40.0 | 33.0 | 29.5 | |
| Obesity (%) | 14.0 | 23.3 | 11.0 | 9.8 | |
| Food addiction | |||||
| No diagnosis met | 30.0 | 52.6 | 56.0 | 50.0 | 0.871 |
| Diagnosis met | 27.0 | 47.4 | 56.0 | 50.0 | |
| Nutrition knowledge | |||||
| Poor knowledge | 40.0 | 66.7 | 78.0 | 69.6 | 0.732 |
| Good knowledge | 20.0 | 33.3 | 34.0 | 30.4 | |
| Sleep quality index | |||||
| Good sleep quality | 18.0 | 30.0 | 24.0 | 21.6 | 0.265 |
| Poor sleep quality | 42.0 | 70.0 | 87.0 | 78.4 | |
| Physical activity level | |||||
| Low | 33.0 | 55.0 | 54.0 | 48.2 | 0.408 |
| Moderate | 16.0 | 26.7 | 27.0 | 24.1 | |
| High | 11.0 | 18.3 | 31.0 | 27.7 | |
| Weight Gain (Reference: No) | ||||
|---|---|---|---|---|
| OR | 95% CI | p-Value | ||
| Lower | Upper | |||
| Number of previous treatments | 0.86 | 0.74 | 0.99 | 0.043 |
| Duration of current treatment (months) | 0.98 | 0.96 | 0.99 | 0.015 |
| Pre-treatment BMI (Kg/m2) | 0.86 | 0.79 | 0.95 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahboub, N.; Rizk, R.; Farsoun, C.G.; de Vries, N. Patterns and Determinants of Weight Gain among People Who Use Drugs Undergoing Treatment for Recovery in Lebanon. Nutrients 2023, 15, 990. https://doi.org/10.3390/nu15040990
Mahboub N, Rizk R, Farsoun CG, de Vries N. Patterns and Determinants of Weight Gain among People Who Use Drugs Undergoing Treatment for Recovery in Lebanon. Nutrients. 2023; 15(4):990. https://doi.org/10.3390/nu15040990
Chicago/Turabian StyleMahboub, Nadine, Rana Rizk, Cynthia George Farsoun, and Nanne de Vries. 2023. "Patterns and Determinants of Weight Gain among People Who Use Drugs Undergoing Treatment for Recovery in Lebanon" Nutrients 15, no. 4: 990. https://doi.org/10.3390/nu15040990






