Patterns of Dietary Blood Markers Are Related to Frailty Status in the FRAILOMIC Validation Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Frailty Classification and Multimorbidity
2.2. Biomarker Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Criteria | Characteristic |
|---|---|
| Slowness | Defined as the worst quintile in the three-meter walking speed test, adjusted for sex and height |
| Inactivity | Defined as the worst quintile in the PASE score |
| Shrinking, weight loss | Unintentional weight loss of ≥4.5 kg within a year |
| Weakness | Defined as the worst quintile of maximum grip strength on the dominant hand, adjusted for sex and body mass index |
| Exhaustion | Self-reported exhaustion (CES-D depression scale) |
| Total | ENRICA | TSHA | EXERNET | SardiNIA | p-Value | |
|---|---|---|---|---|---|---|
| Country | Spain | Spain | Spain | Italy | ||
| N (%) | 1927 | 498 (30.5) | 498 (30.5) | 431 (26.4) | 500 (12.6) | |
| Age (years) | 75.32 ± 5.57 | 74.66 ± 6.08 | 75.86 ± 6.64 | 76.86 ± 4.54 | 74.29 ± 4.01 | <0.001 |
| Missing, n | 52 | 1 | 0 | 51 | 0 | |
| Sex, n (%) | <0.001 | |||||
| Female | 1124 (59.9) | 285 (57.3) | 281 (56.4) | 286 (75.3) | 272 (54.4) | |
| Male | 751 (40.1) | 212 (42.7) | 217 (43.6) | 94 (24.7) | 228 (45.6) | |
| Missing, n | 52 | 1 | 0 | 51 | 0 | |
| BMI, kg/m2 | 28.60 ± 4.51 | 28.23 ± 4.56 | 29.35 ± 4.98 | 28.86 ± 3.86 | 28.04 ± 4.20 | <0.001 |
| <25 kg/m2, n (%) | 369 (19.1) | 119 (24.0) | 88 (17.7) | 57 (15.4) | 105 (21.4) | |
| 25–29.9 kg/m2, n (%) | 861 (46.5) | 221 (44.6) | 210 (42.3) | 183 (49.6) | 247 (50.3) | |
| ≥30 kg/m2, n (%) | 622 (33.6) | 155 (31.3) | 199 (40.0) | 129 (35.0) | 139 (28.3) | |
| Missing, n | 75 | 3 | 1 | 62 | 9 | |
| Frailty status, n (%) | <0.001 | |||||
| Robust | 642 (47.6) | 203 (41.8) | 270 (54.3) | 169 (46.3) | - | |
| Pre-frail | 507 (37.6) | 195 (40.1) | 134 (27.0) | 178 (48.8) | - | |
| Frail | 199 (14.8) | 88 (18.1) | 93 (18.7) | 18 (4.9) | - | |
| Missing, n | 579 | 12 | 1 | 66 | 500 | |
| Smoking status, n (%) | <0.001 | |||||
| Current | 115 (6.2) | 44 (8.8) | 40 (8.0) | 10 (2.8) | 21 (4.3) | |
| Past | 521 (28.2) | 186 (37.5) | 133 (26.7) | 60 (16.8) | 142 (28.7) | |
| Never | 1210 (65.5) | 266 (53.6) | 325 (65.3) | 288 (80.4) | 331 (67.0) | |
| Missing, n | 81 | 2 | 0 | 73 | 6 | |
| Health status, n (%) | ||||||
| Multimorbidity | 396 (21.9) | 99 (20.2) | 126 (25.7) | 58 (16.3) | 113 (23.5) | <0.001 |
| Missing, n | 115 | 8 | 8 | 80 | 19 | |
| Biomarkers | ||||||
| 25-OH-D3 (nM) | 56.51 ± 33.74 | 58.36 ± 32.16 | 62.97 ± 34.75 | 64.85 ± 37.00 | 41.23 ± 25.50 | <0.001 |
| 25-OH-D3: <25 nM, n (%) | 142 (8.8) | 40 (8.0) | 42 (8.5) | 36 (8.5) | 24 (11.9) | |
| 25-OH-D3: 25–49.9 nM, n (%) | 535 (33.1) | 188 (37.8) | 149 (30.1) | 128 (30.3) | 70 (34.7) | |
| 25-OH-D3: ≥50 nM, n (%) | 939 (57.5) | 269 (54.1) | 304 (61.4) | 258 (61.1) | 108 (53.5) | |
| Total Carotenoids (µM) | 2.96 ± 1.70 | 3.09 ± 1.63 | 3.09 ± 1.70 | 1.70 ± 0.93 | 3.77 ± 1.67 | <0.001 |
| α-Carotene (µM) | 0.12 ± 0.13 | 0.17 ± 0.14 | 0.12 ± 0.13 | 0.12 ± 0.12 | 0.09 ± 0.09 | <0.001 |
| β-Carotene (µM) | 0.41 ± 0.36 | 0.45 ± 0.37 | 0.43 ± 0.36 | 0.31 ± 0.28 | 0.45 ± 0.40 | < 0.001 |
| Lycopene (µM) | 1.64 ± 1.23 | 1.56 ± 1.09 | 1.91 ± 1.23 | 0.57 ± 0.41 | 2.39 ± 1.18 | < 0.001 |
| Lutein + Zeaxanthin (µM) | 0.29 ± 0.19 | 0.32 ± 0.22 | 0.24 ± 0.13 | 0.24 ± 0.17 | 0.35 ± 0.19 | < 0.001 |
| β-Cryptoxanthin (µM) | 0.49 ± 0.47 | 0.59 ± 0.54 | 0.40 ± 0.37 | 0.46 ± 0.36 | 0.50 ± 0.55 | < 0.001 |
| Retinol (µM) | 1.78 ± 0.65 | 2.04 ± 0.57 | 2.10 ± 0.67 | 1.17 ± 0.38 | 1.71 ± 0.48 | < 0.001 |
| α-Tocopherol (µM) | 36.81 ± 12.02 | 46.27 ± 9.72 | 43.61 ± 10.08 | 27.01 ± 6.42 | 28.97 ± 6.88 | < 0.001 |
| γ-Tocopherol (µM) | 1.00 ± 0.63 | 1.22 ± 0.64 | 1.24 ± 0.68 | 0.66 ± 0.37 | 0.81 ± 0.53 | < 0.001 |
| 3-Nitrotyrosine (pmol/mg) | 4.76 ± 3.67 | 7.01 ± 4.00 | 3.54 ± 2.49 | 3.67 ± 3.24 | - | < 0.001 |
References
- Population Division of the United Nations Department of Economic and Social Affairs. World Population Ageing 2020 Highlights: Living Arrangements of Older Persons (ST/ESA/SER.A/451. World Population Ageing 2020 Highlights. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Sep/un_pop_2020_pf_ageing_10_key_messages.pdf (accessed on 20 October 2022).
- Kojima, G. Frailty as a Predictor of Future Falls Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Frailty as a predictor of fractures among community-dwelling older people: A systematic review and meta-analysis. Bone 2016, 90, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lupon, J.; Vidan, M.T.; Ferguson, C.; Gastelurrutia, P.; Newton, P.J.; Macdonald, P.S.; Bueno, H.; Bayes-Genis, A.; Woo, J.; et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwee, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Gerontopole Brussels Study, g. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.E1–1163.E17. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Manas, L.; Feart, C.; Mann, G.; Vina, J.; Chatterji, S.; Chodzko-Zajko, W.; Gonzalez-Colaco Harmand, M.; Bergman, H.; Carcaillon, L.; Nicholson, C.; et al. Searching for an operational definition of frailty: A Delphi method based consensus statement: The frailty operative definition-consensus conference project. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Gordon, A.L.; Masud, T.; Gladman, J.R. Now that we have a definition for physical frailty, what shape should frailty medicine take? Age Ageing 2014, 43, 8–9. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Mitnitski, A.; Collerton, J.; Martin-Ruiz, C.; Jagger, C.; von Zglinicki, T.; Rockwood, K.; Kirkwood, T.B. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015, 13, 161. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda, M.; Arauna, D.; Garcia, F.; Albala, C.; Palomo, I.; Fuentes, E. Frailty in Aging and the Search for the Optimal Biomarker: A Review. Biomedicines 2022, 10, 1426. [Google Scholar] [CrossRef]
- Kochlik, B.; Franz, K.; Henning, T.; Weber, D.; Wernitz, A.; Herpich, C.; Jannasch, F.; Aykac, V.; Muller-Werdan, U.; Schulze, M.B.; et al. Frailty is characterized by biomarker patterns reflecting inflammation or muscle catabolism in multi-morbid patients. J. Cachexia Sarcopenia Muscle 2022, 14, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Erusalimsky, J.D.; Grillari, J.; Grune, T.; Jansen-Duerr, P.; Lippi, G.; Sinclair, A.J.; Tegner, J.; Vina, J.; Durrance-Bagale, A.; Minambres, R.; et al. In Search of ‘Omics’-Based Biomarkers to Predict Risk of Frailty and Its Consequences in Older Individuals: The FRAILOMIC Initiative. Gerontology 2016, 62, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kochlik, B.; Stuetz, W.; Peres, K.; Pilleron, S.; Feart, C.; Garcia Garcia, F.J.; Bandinelli, S.; Gomez-Cabrero, D.; Rodriguez-Manas, L.; Grune, T.; et al. Associations of fat-soluble micronutrients and redox biomarkers with frailty status in the FRAILOMIC initiative. J. Cachexia Sarcopenia Muscle 2019, 10, 1339–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Cabrero, D.; Walter, S.; Abugessaisa, I.; Minambres-Herraiz, R.; Palomares, L.B.; Butcher, L.; Erusalimsky, J.D.; Garcia-Garcia, F.J.; Carnicero, J.; Hardman, T.C.; et al. A robust machine learning framework to identify signatures for frailty: A nested case-control study in four aging European cohorts. Geroscience 2021, 43, 1317–1329. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Gutierrez Avila, G.; Alfaro-Acha, A.; Amor Andres, M.S.; De Los Angeles De La Torre Lanza, M.; Escribano Aparicio, M.V.; Humanes Aparicio, S.; Larrion Zugasti, J.L.; Gomez-Serranillo Reus, M.; Rodriguez-Artalejo, F.; et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo Study for Healthy Aging. J. Nutr. Health Aging 2011, 15, 852–856. [Google Scholar] [CrossRef]
- Rodriguez-Artalejo, F.; Graciani, A.; Guallar-Castillon, P.; Leon-Munoz, L.M.; Zuluaga, M.C.; Lopez-Garcia, E.; Gutierrez-Fisac, J.L.; Taboada, J.M.; Aguilera, M.T.; Regidor, E.; et al. Rationale and methods of the study on nutrition and cardiovascular risk in Spain (ENRICA). Rev. Esp. Cardiol. 2011, 64, 876–882. [Google Scholar] [CrossRef]
- Gomez-Cabello, A.; Vicente-Rodriguez, G.; Albers, U.; Mata, E.; Rodriguez-Marroyo, J.A.; Olivares, P.R.; Gusi, N.; Villa, G.; Aznar, S.; Gonzalez-Gross, M.; et al. Harmonization process and reliability assessment of anthropometric measurements in the elderly EXERNET multi-centre study. PLoS ONE 2012, 7, e41752. [Google Scholar] [CrossRef]
- Pedrero-Chamizo, R.; Gomez-Cabello, A.; Delgado, S.; Rodriguez-Llarena, S.; Rodriguez-Marroyo, J.A.; Cabanillas, E.; Melendez, A.; Vicente-Rodriguez, G.; Aznar, S.; Villa, G.; et al. Physical fitness levels among independent non-institutionalized Spanish elderly: The elderly EXERNET multi-center study. Arch. Gerontol. Geriatr. 2012, 55, 406–416. [Google Scholar] [CrossRef]
- Pilia, G.; Chen, W.M.; Scuteri, A.; Orru, M.; Albai, G.; Dei, M.; Lai, S.; Usala, G.; Lai, M.; Loi, P.; et al. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006, 2, e132. [Google Scholar] [CrossRef]
- Henning, T.; Kochlik, B.; Kusch, P.; Strauss, M.; Juric, V.; Pignitter, M.; Marusch, F.; Grune, T.; Weber, D. Pre-Operative Assessment of Micronutrients, Amino Acids, Phospholipids and Oxidative Stress in Bariatric Surgery Candidates. Antioxidants 2022, 11, 774. [Google Scholar] [CrossRef]
- Stuetz, W.; Weber, D.; Dolle, M.E.; Jansen, E.; Grubeck-Loebenstein, B.; Fiegl, S.; Toussaint, O.; Bernhardt, J.; Gonos, E.S.; Franceschi, C.; et al. Plasma Carotenoids, Tocopherols, and Retinol in the Age-Stratified (35-74 Years) General Population: A Cross-Sectional Study in Six European Countries. Nutrients 2016, 8, 614. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.; Stuetz, W.; Bernhard, W.; Franz, A.; Raith, M.; Grune, T.; Breusing, N. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur. J. Clin. Nutr. 2014, 68, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfredi, G.; Midao, L.; Paul, C.; Cena, C.; Duarte, M.; Costa, E. Prevalence of frailty status among the European elderly population: Findings from the Survey of Health, Aging and Retirement in Europe. Geriatr. Gerontol. Int. 2019, 19, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Gagesch, M.; Chocano-Bedoya, P.O.; Abderhalden, L.A.; Freystaetter, G.; Sadlon, A.; Kanis, J.A.; Kressig, R.W.; Guyonnet, S.; DaSilva, J.A.P.; Felsenberg, D.; et al. Prevalence of Physical Frailty: Results from the DO-HEALTH Study. J. Frailty Aging 2022, 11, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Nicholl, B.I.; Jani, B.D.; Lee, D.; McQueenie, R.; Mair, F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018, 3, e323–e332. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Gomez-Diaz, R.; Gonzalez-Molina, A.; Vidal-Serrano, S.; Diez-Manglano, J.; Salgado, F.; Soto-Martin, M.; Ollero-Baturone, M.; on behalf of the Proteo Researchers. Oxidative Stress, Telomere Shortening, and Apoptosis Associated to Sarcopenia and Frailty in Patients with Multimorbidity. J. Clin. Med. 2020, 9, 2669. [Google Scholar] [CrossRef]
- Mu, L.; Jiang, L.; Chen, J.; Xiao, M.; Wang, W.; Liu, P.; Wu, J. Serum Inflammatory Factors and Oxidative Stress Factors Are Associated with Increased Risk of Frailty and Cognitive Frailty in Patients with Cerebral Small Vessel Disease. Front. Neurol. 2021, 12, 786277. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodriguez-Manas, L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic. Biol. Med. 2020, 149, 72–77. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Sahni, S.; Dufour, A.B.; Fielding, R.A.; Newman, A.B.; Kiel, D.P.; Hannan, M.T.; Jacques, P.F. Total carotenoid intake is associated with reduced loss of grip strength and gait speed over time in adults: The Framingham Offspring Study. Am. J. Clin. Nutr. 2021, 113, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Blaum, C.; Guralnik, J.M.; Moncrief, D.T.; Ricks, M.O.; Fried, L.P. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin. Exp. Res. 2003, 15, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Hass, U.; Herpich, C.; Kochlik, B.; Weber, D.; Grune, T.; Norman, K. Dietary Inflammatory Index and Cross-Sectional Associations with Inflammation, Muscle Mass and Function in Healthy Old Adults. J. Nutr. Health Aging 2022, 26, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Leon-Munoz, L.M.; Garcia-Esquinas, E.; Lopez-Garcia, E.; Banegas, J.R.; Rodriguez-Artalejo, F. Major dietary patterns and risk of frailty in older adults: A prospective cohort study. BMC Med. 2015, 13, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talegawkar, S.A.; Bandinelli, S.; Bandeen-Roche, K.; Chen, P.; Milaneschi, Y.; Tanaka, T.; Semba, R.D.; Guralnik, J.M.; Ferrucci, L. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J. Nutr. 2012, 142, 2161–2166. [Google Scholar] [CrossRef] [Green Version]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Dietary quality is related to frailty in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Struijk, E.A.; Fung, T.T.; Sotos-Prieto, M.; Rodriguez-Artalejo, F.; Willett, W.C.; Hu, F.B.; Lopez-Garcia, E. Red meat consumption and risk of frailty in older women. J. Cachexia Sarcopenia Muscle 2022, 13, 210–219. [Google Scholar] [CrossRef]
- Struijk, E.A.; Fung, T.T.; Rodriguez-Artalejo, F.; Bischoff-Ferrari, H.A.; Hu, F.B.; Willett, W.C.; Lopez-Garcia, E. Protein intake and risk of frailty among older women in the Nurses’ Health Study. J. Cachexia Sarcopenia Muscle 2022, 13, 1752–1761. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Dawson-Hughes, B.; Harris, S.S.; Ceglia, L. Alkaline diets favor lean tissue mass in older adults. Am. J. Clin. Nutr. 2008, 87, 662–665. [Google Scholar] [CrossRef] [Green Version]
- Pilleron, S.; Weber, D.; Peres, K.; Colpo, M.; Gomez-Cabrero, D.; Stuetz, W.; Dartigues, J.F.; Ferrucci, L.; Bandinelli, S.; Garcia-Garcia, F.J.; et al. Patterns of circulating fat-soluble vitamins and carotenoids and risk of frailty in four European cohorts of older adults. Eur. J. Nutr. 2019, 58, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.C.; Emmert, S.; Boeckmann, L. Sunlight, Vitamin D, and Xeroderma Pigmentosum. Adv. Exp. Med. Biol. 2020, 1268, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Perez, D.; Sanchez-Flores, M.; Proietti, S.; Bonassi, S.; Costa, S.; Teixeira, J.P.; Fernandez-Tajes, J.; Pasaro, E.; Valdiglesias, V.; Laffon, B. Low Vitamin D Levels and Frailty Status in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Kochlik, B.; Stuetz, W.; Dolle, M.E.T.; Jansen, E.; Grubeck-Loebenstein, B.; Debacq-Chainiaux, F.; Bernhardt, J.; Gonos, E.S.; Capri, M.; et al. Medication Intake Is Associated with Lower Plasma Carotenoids and Higher Fat-Soluble Vitamins in the Cross-Sectional MARK-AGE Study in Older Individuals. J. Clin. Med. 2020, 9, 2072. [Google Scholar] [CrossRef]
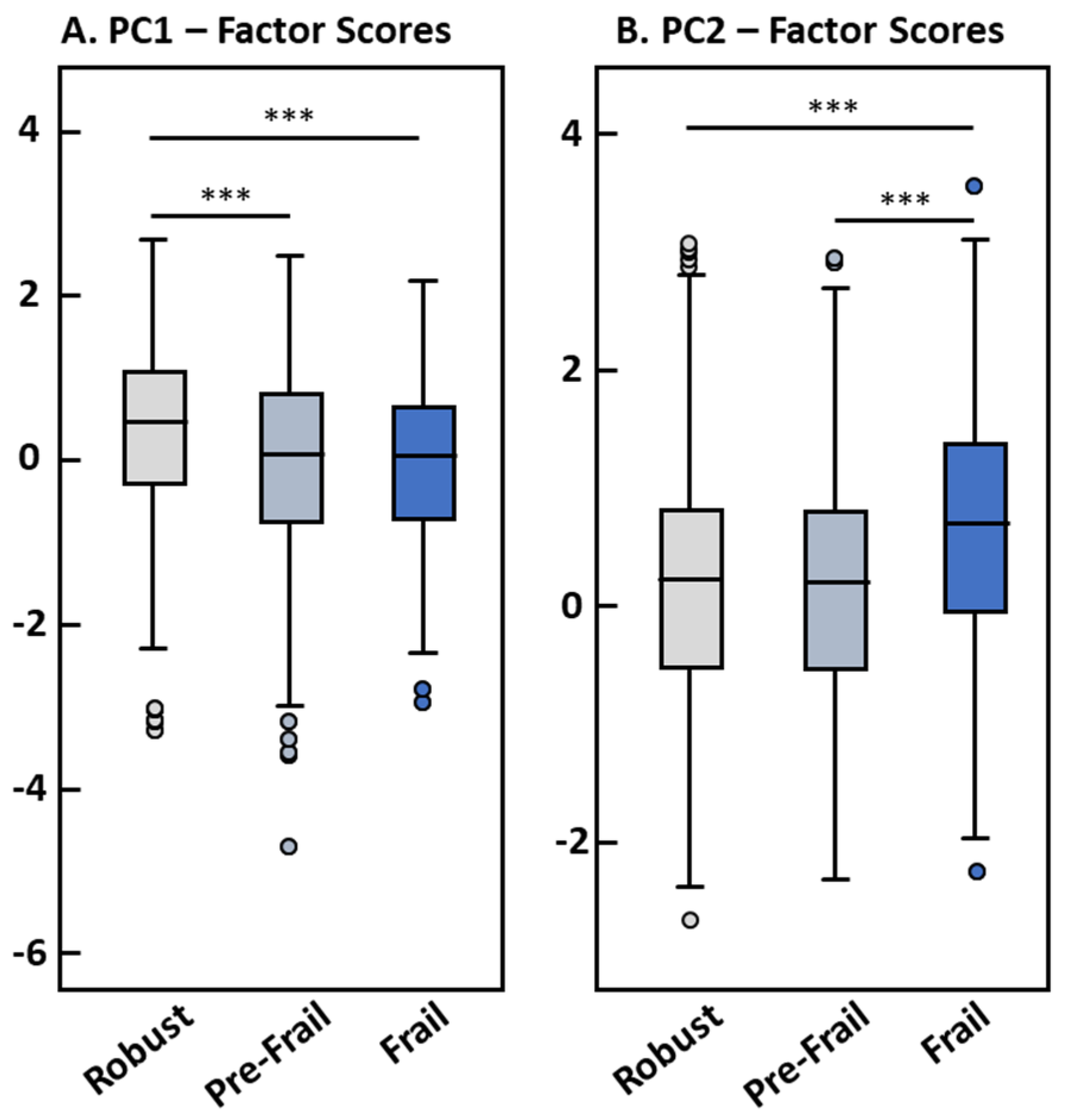
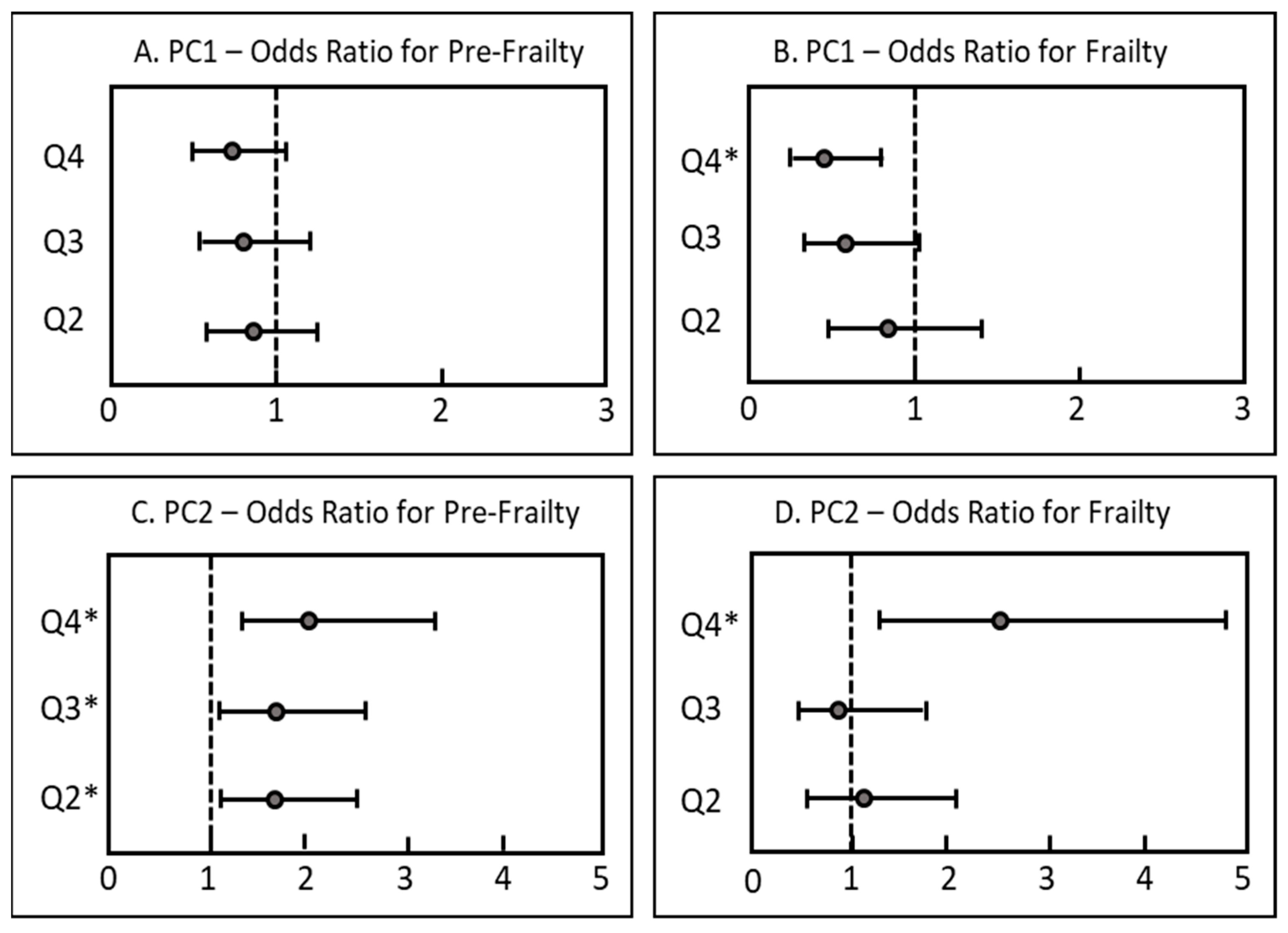
| Total | Robust | Pre-Frail | Frail | p-Value | |
|---|---|---|---|---|---|
| N (%) | 1348 | 642 (47.6) | 507 (37.6) | 199 (14.8) | - |
| Females, % (n) | 62.0 (852) | 55.8 (358) | 65.9 (334) | 71.4 (142) | <0.001 |
| Age, years | 75.64 ± 5.95 | 73.65 ± 5.01 a | 76.24 ± 5.73 b | 80.55 ± 6.13 c | <0.001 |
| BMI, kg/m2 | 28.82 ± 4.61 | 28.21 ± 4.23 a | 29.28 ± 4.55 b | 29.55 ± 5.59 b | <0.001 |
| Current smoker, % (n) | 7.0 (93) | 8.9 (56) | 5.4 (27) | 5.0 (10) | 0.039 |
| Multimorbidity, % (n) | 21.1 (275) | 17.2 (107) | 21.6 (106) | 32.0 (62) | <0.001 |
| Biomarker | Total | Robust | Pre-Frail | Frail | p-Value |
|---|---|---|---|---|---|
| Total Carotenoids (µM) | 2.24 (2.16–2.32) | 2.58 (2.46–2.71) a | 2.00 (1.88–2.21) b | 1.91 (1.75–2.08) c | <0.001 |
| adjusted | 2.57 (2.44–2.70) a | 2.17 (2.04–2.30) b | 1.96 (1.75–2.20) b | <0.001 | |
| α-Carotene (µM) | 0.09 (0.09–0.10) | 0.10 (0.10–0.11) a | 0.09 (0.08–0.09) b | 0.09 (0.07–0.09) b | 0.001 |
| adjusted | 0.10 (0.09–0.11) | 0.09 (0.08–0.10) | 0.09 (0.08–0.10) | 0.042 | |
| β-Carotene (µM) | 0.29 (0.27–0.30) | 0.33 (0.31–0.36) a | 0.26 (0.24–0.28) b | 0.23 (0.19–26) b | <0.001 |
| adjusted | 0.32 (0.30–0.35) a | 0.27 (0.25–0.29 b | 0.24 (0.20–0.28) b | 0.001 | |
| Lycopene (µM) | 0.99 (0.94–1.05) | 1.18 (1.10–1.27) a | 0.82 (0.75–0.91) b | 0.89 (0.78–1.00) b | <0.001 |
| adjusted | 1.17 (1.08–1.25) a | 0.99 (0.90–1.08) b | 0.94 (0.79–1.11) a | 0.006 | |
| Lutein + Zeaxanthin (µM) | 0.22 (0.21–0.23) | 0.24 (0.23–0.26) a | 0.21 (0.20–0.22) b | 0.18 (0.16–0.20) b | <0.001 |
| adjusted | 0.24 (0.23–0.25) a | 0.21 (0.20–0.23) a,b | 0.18 (0.16–0.20) b | <0.001 | |
| β-Cryptoxanthin (µM) | 0.33 (0.32–0.35) | 0.37 (0.35–0.40) a | 0.33 (0.31–0.36) a | 0.23 (0.20–0.27) b | <0.001 |
| adjusted | 0.39 (0.36–0.42) a | 0.30 (0.27–0.33) b | 0.25 (0.21–0.30) b | <0.001 | |
| Retinol (µM) | 1.69 (1.65–1.73) | 1.71 (1.66–1.77) | 1.63 (1.57–1.69) | 1.69 (1.65–1.73) | 0.007 |
| adjusted | 1.75 (1.70–1.80) | 1.73 (1.68–1.79) | 1.66 (1.56–1.77) | 0.407 | |
| α-Tocopherol (µM) | 38.0 (37.3–38.7) | 38.6 (37.7–39.6) a | 36.15 (35.0–37.2) b | 40.9 (39.4–42.5) a | <0.001 |
| adjusted | 39.2 (38.4–40.0) | 37.9 (37.1–38.8) | 37.9 (36.2–39.6) | 0.097 | |
| γ-Tocopherol (µM) | 0.92 (0.89–0.95) | 0.95 (0.91–1.00) a | 0.84 (0.80–0.89) b | 1.01 (0.95–1.08) a | <0.001 |
| adjusted | 0.95 (0.91–0.99) | 0.93 (0.88–0.97) | 0.99 (0.90–1.09) | 0.475 | |
| 25-OH-D3 (nM) | 54.0 (52.5–55.6) | 57.7 (55.5–60.0) a | 52.9 (50.5–55.4)b | 45.9 (42.0–50.2) c | <0.001 |
| adjusted | 55.0 (52.5–57.7) | 51.8 (49.1–54.7) | 49.8 (44.9–55.3) | 0.125 | |
| 3-Nitrotyrosine (pmol/mg) | 3.62 (3.45–3.79) | 3.49 (3.27–3.72) | 3.59 (3.31–3.88) | 4.19 (3.68–4.77) | 0.008 |
| adjusted | 3.61 (3.37–3.86) | 3.49 (3.22–3.78) | 3.88 (3.33–4.52) | 0.466 |
| PC1 Biomarkers | PC1 Factor Loadings | PC2 Biomarkers | PC2 Factor Loadings |
|---|---|---|---|
| β-Carotene | 0.732 | Retinol | 0.601 |
| α-Tocopherol | 0.693 | γ-Tocopherol | 0.572 |
| α-Carotene | 0.648 | α-Tocopherol | 0.506 |
| Lutein + zeaxanthin | 0.598 | Lycopene | 0.161 |
| Lycopene | 0.583 | α-Carotene | −0.473 |
| β-Cryptoxanthin | 0.523 | β-Carotene | −0.458 |
| γ-Tocopherol | 0.507 | β-Cryptoxanthin | −0.437 |
| Retinol | 0.500 | Lutein + zeaxanthin | −0.273 |
| Variance explained [%] | 36.4 | Variance explained [%] | 20.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henning, T.; Kochlik, B.; Ara, I.; González-Gross, M.; Fiorillo, E.; Marongiu, M.; Cucca, F.; Rodriguez-Artalejo, F.; Carnicero Carreño, J.A.; Rodriguez-Mañas, L.; et al. Patterns of Dietary Blood Markers Are Related to Frailty Status in the FRAILOMIC Validation Phase. Nutrients 2023, 15, 1142. https://doi.org/10.3390/nu15051142
Henning T, Kochlik B, Ara I, González-Gross M, Fiorillo E, Marongiu M, Cucca F, Rodriguez-Artalejo F, Carnicero Carreño JA, Rodriguez-Mañas L, et al. Patterns of Dietary Blood Markers Are Related to Frailty Status in the FRAILOMIC Validation Phase. Nutrients. 2023; 15(5):1142. https://doi.org/10.3390/nu15051142
Chicago/Turabian StyleHenning, Thorsten, Bastian Kochlik, Ignacio Ara, Marcela González-Gross, Edoardo Fiorillo, Michele Marongiu, Francesco Cucca, Fernando Rodriguez-Artalejo, Jose Antonio Carnicero Carreño, Leocadio Rodriguez-Mañas, and et al. 2023. "Patterns of Dietary Blood Markers Are Related to Frailty Status in the FRAILOMIC Validation Phase" Nutrients 15, no. 5: 1142. https://doi.org/10.3390/nu15051142






