Low FODMAP Diet Relieves Visceral Hypersensitivity and Is Associated with Changes in Colonic Microcirculation in Water Avoidance Mice Model
Abstract
:1. Introduction
2. Materials and Methods
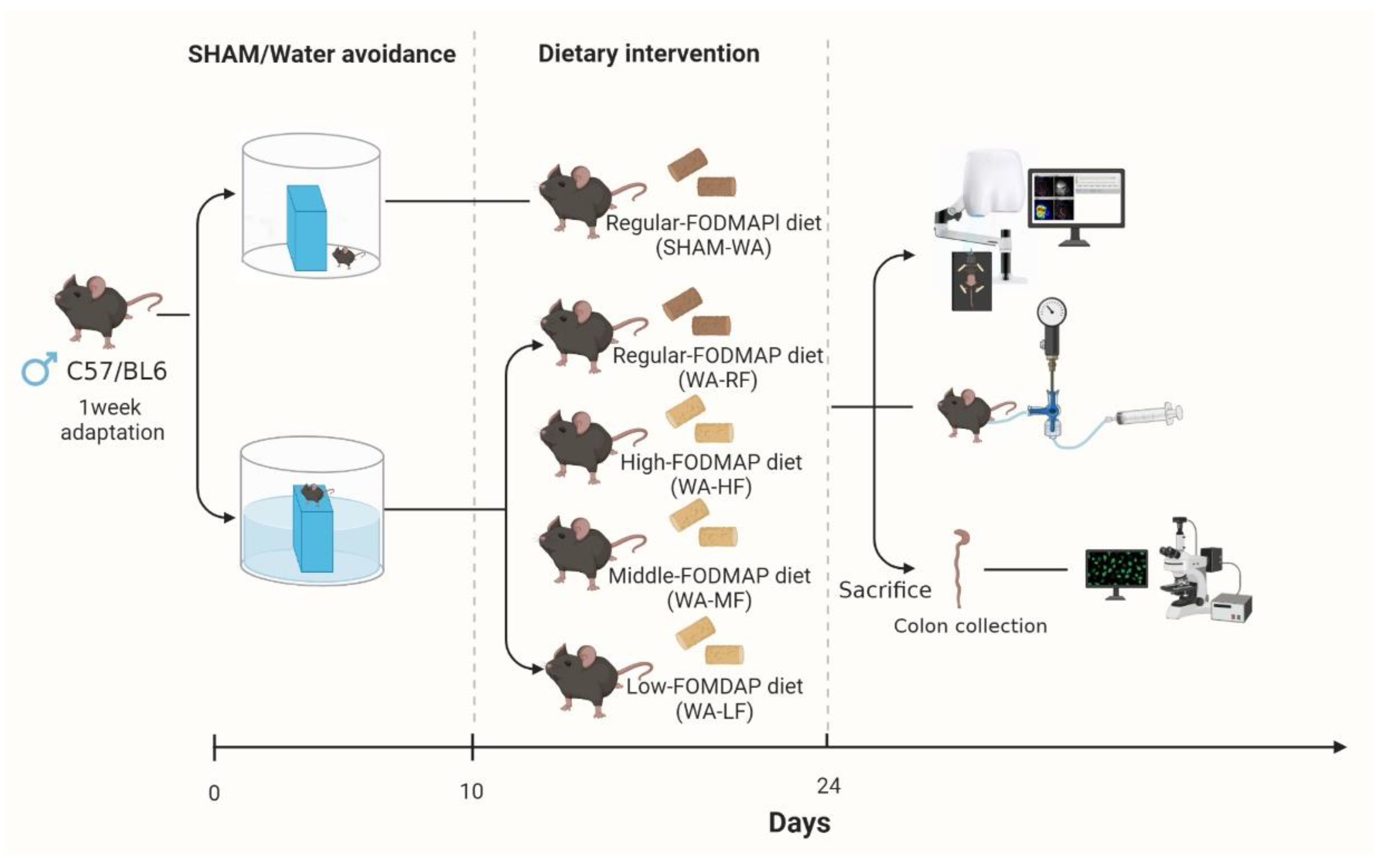
2.1. Animals and Water-Avoidance Procedures
2.1.1. Animals
2.1.2. Dietary Formula
2.1.3. WA Stress Protocol and Grouping
2.2. Visceral Hypersensitivity Measurement
2.3. Immunofluorescence Staining
2.4. Laser Speckle Contrast Imaging
2.5. Statistic Analyses
3. Results
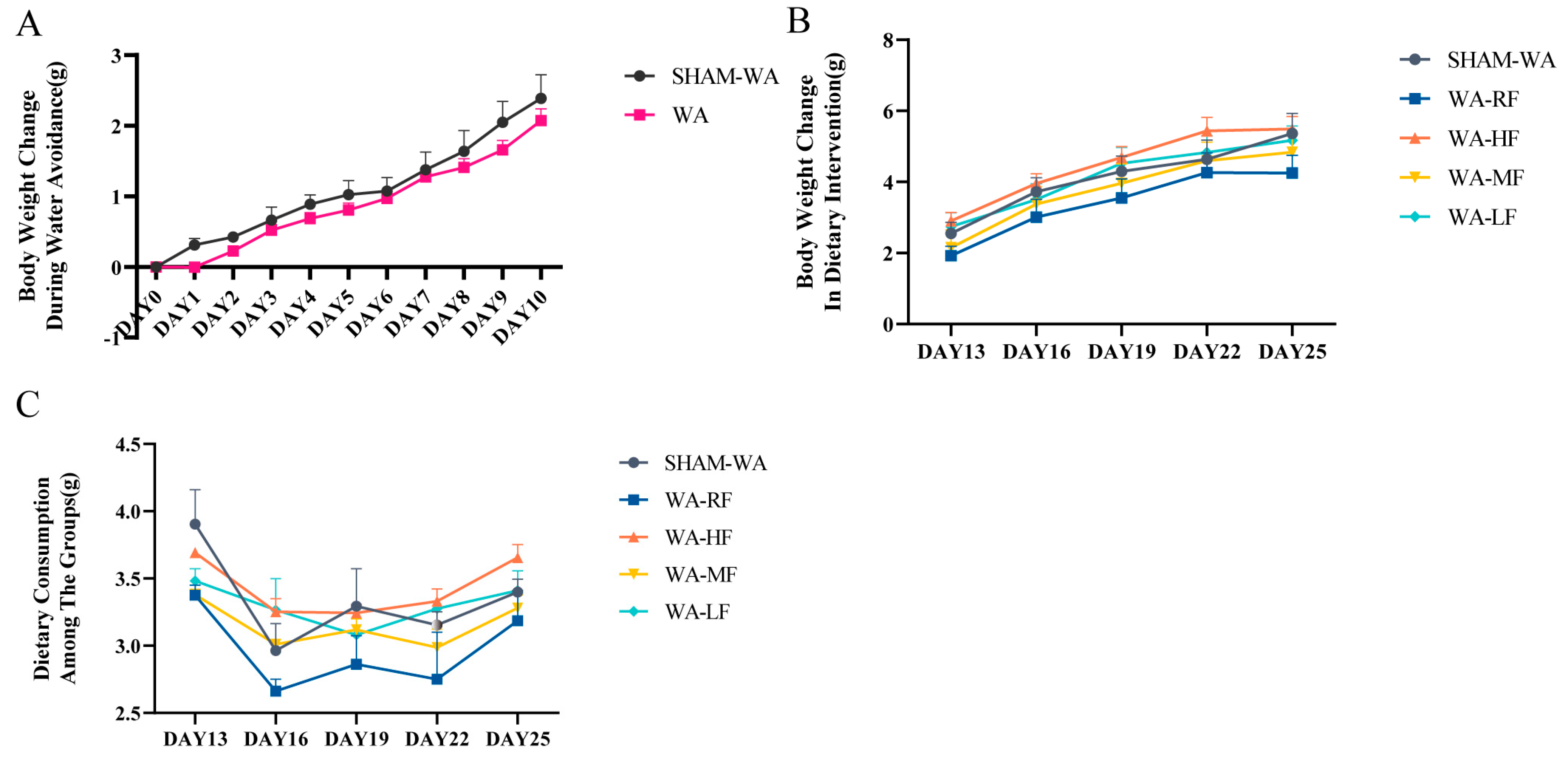
3.1. Body Weight Change and Dietary Consumption
3.2. Visceral Hypersensitivity
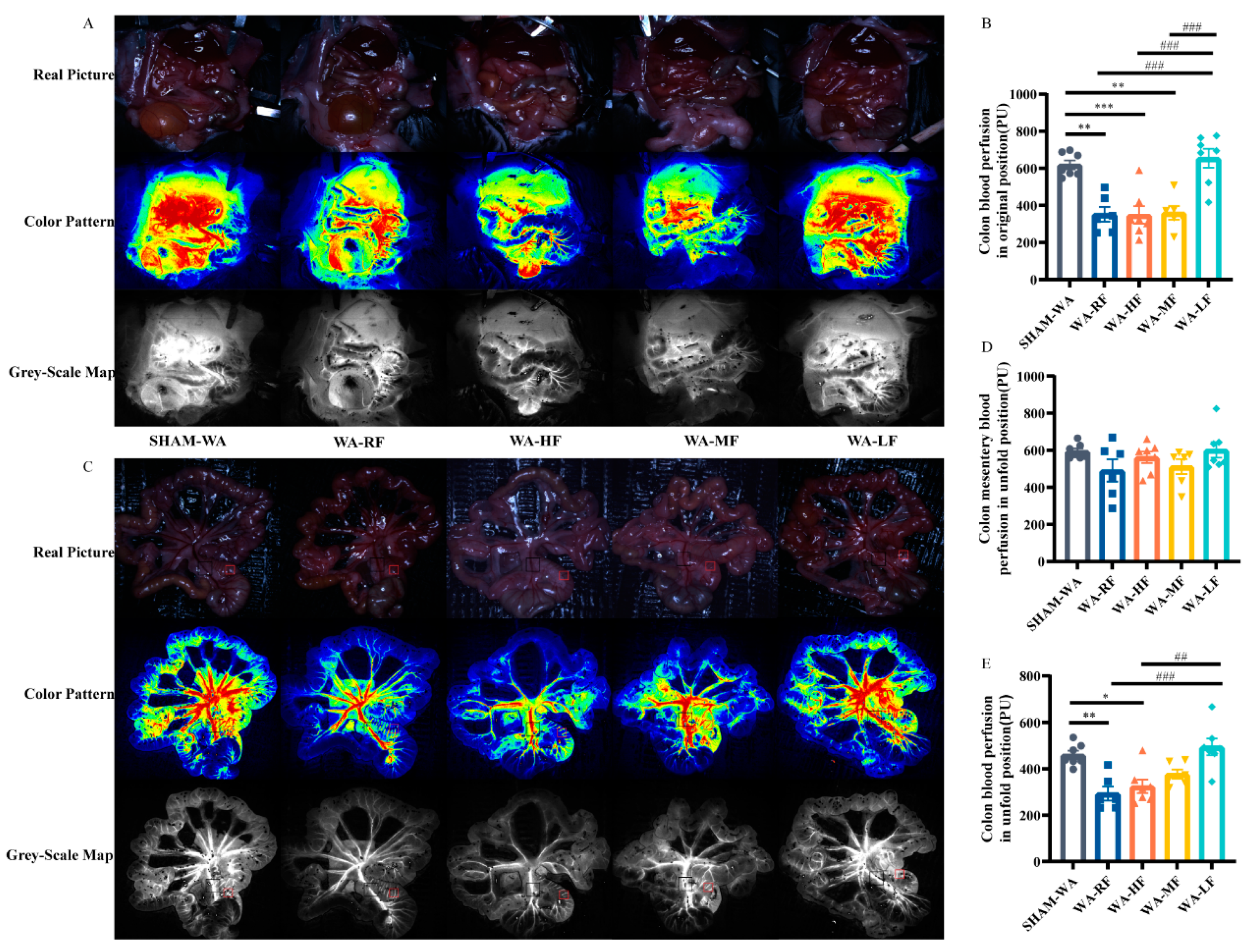
3.3. Colonic Microcirculation Blood Flow Decreased in the WA-RF, WA-HF, and WA-MF Groups, and Was Reversible in the WA-LF Group
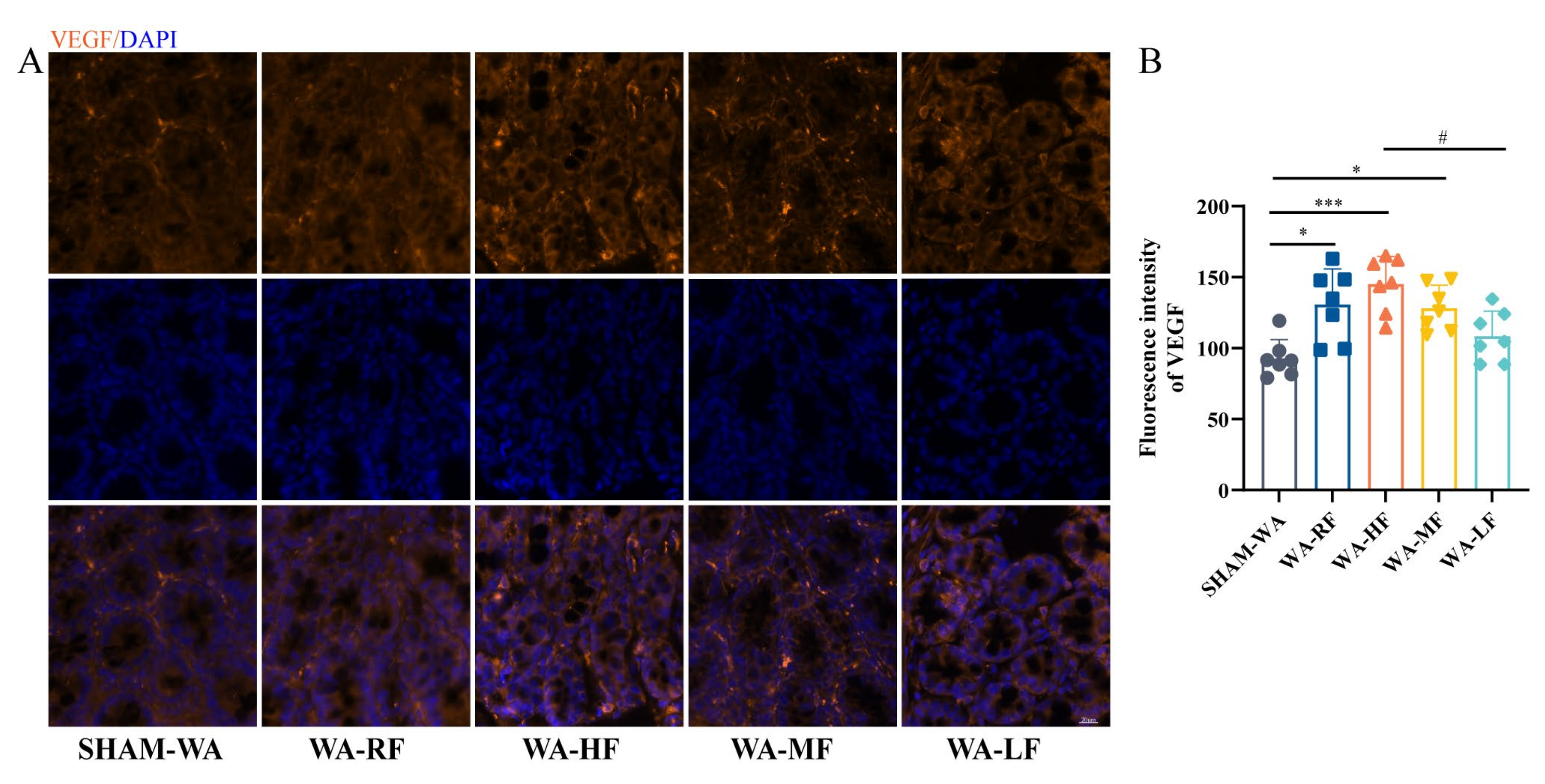
3.4. VEGF Fluorescence Intensity Increased in the WA-HF Group and Decreased in the WA-LF Group
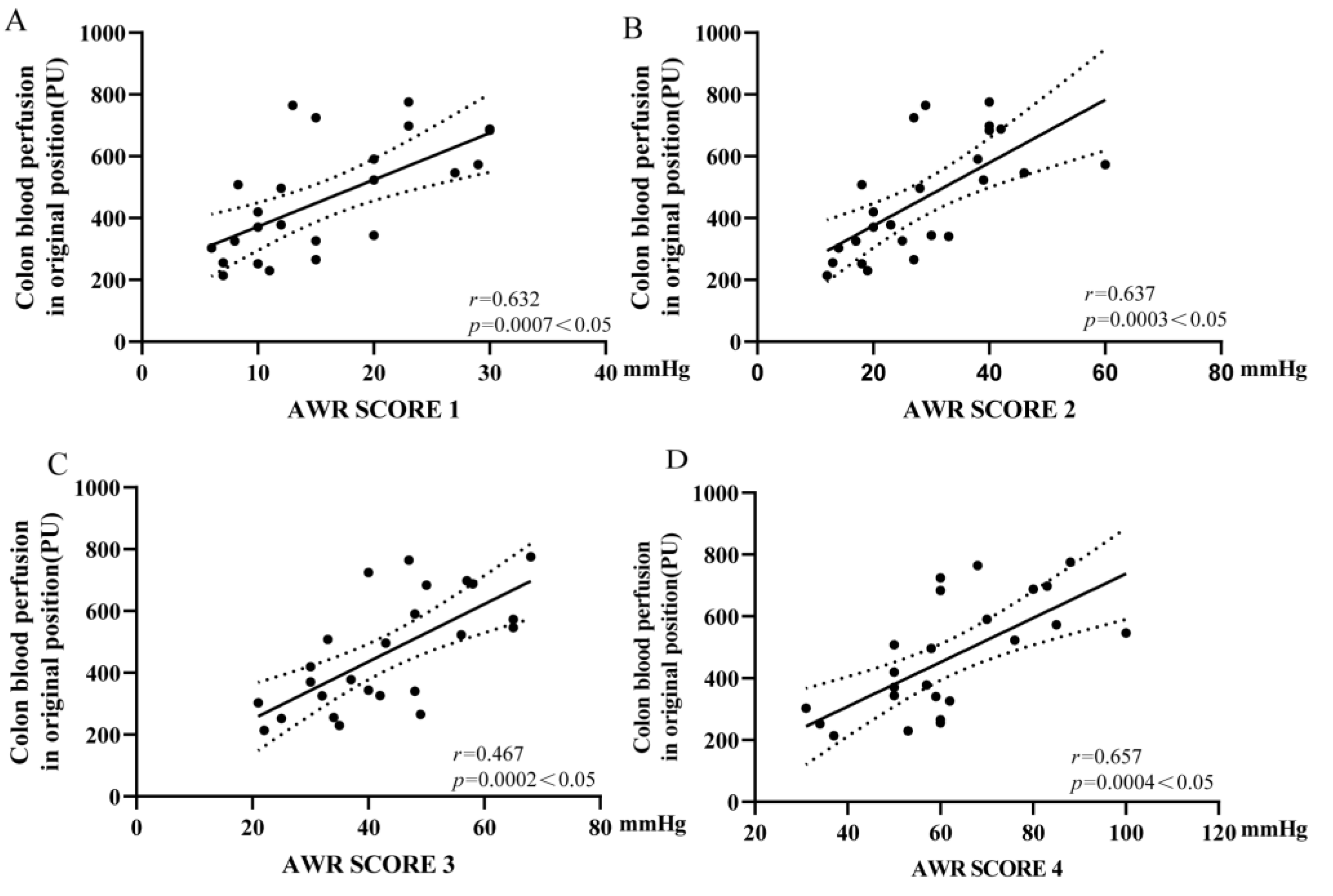
3.5. Colonic Microcirculation Blood Flow Was Positively Correlated with the Threshold of VH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical guideline: Management of irritable bowel syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273.e3. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Diagnosis and treatment of irritable bowel syndrome: A review. JAMA 2021, 325, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Jagielski, C.H.; Riehl, M.E. Behavioral strategies for irritable bowel syndrome: Brain-gut or gut-brain? Gastroenterol. Clin. 2021, 50, 581–593. [Google Scholar] [CrossRef]
- Grabauskas, G.; Wu, X.; Gao, J.; Li, J.-Y.; Turgeon, D.K.; Owyang, C. Prostaglandin E2, produced by mast cells in colon tissues from patients with irritable bowel syndrome, contributes to visceral hypersensitivity in mice. Gastroenterology 2020, 158, 2195–2207.e6. [Google Scholar] [CrossRef]
- Ghoshal, U.C. Postinfection irritable bowel syndrome. Gut Liver 2022, 16, 331–340. [Google Scholar] [CrossRef]
- Ivashkin, V.; Poluektov, Y.; Kogan, E.; Shifrin, O.; Sheptulin, A.; Kovaleva, A.; Kurbatova, A.; Krasnov, G.; Poluektova, E. Disruption of the pro-inflammatory, anti-inflammatory cytokines and tight junction proteins expression, associated with changes of the composition of the gut microbiota in patients with irritable bowel syndrome. PLoS ONE 2021, 16, e0252930. [Google Scholar] [CrossRef]
- Witt, S.T.; Bednarska, O.; Keita, Å.V.; Icenhour, A.; Jones, M.P.; Elsenbruch, S.; Söderholm, J.D.; Engström, M.; Mayer, E.A.; Walter, S. Interactions between gut permeability and brain structure and function in health and irritable bowel syndrome. Neuroimage Clin. 2019, 21, 101602. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, doubts, and hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, S.J.; Parker, F.C.; Muir, J.G.; Gibson, P.R. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: Randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–771. [Google Scholar] [CrossRef]
- Kamphuis, J.B.J.; Guiard, B.; Leveque, M.; Olier, M.; Jouanin, I.; Yvon, S.; Tondereau, V.; Rivière, P.; Guéraud, F.; Chevolleau, S.; et al. Lactose and fructo-oligosaccharides increase visceral sensitivity in mice via glycation processes, increasing mast cell density in colonic mucosa. Gastroenterology 2020, 158, 652–663.e6. [Google Scholar] [CrossRef]
- Campbell, A.K.; Matthews, S.B.; Vassel, N.; Cox, C.D.; Naseem, R.; Chaichi, J.; Holland, I.B.; Green, J.; Wann, K.T. Bacterial metabolic ‘toxins’: A new mechanism for lactose and food intolerance, and irritable bowel syndrome. Toxicology 2010, 278, 268–276. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Gillilland, M.; Wu, X.; Leelasinjaroen, P.; Zhang, G.; Zhou, H.; Ye, B.; Lu, Y.; Owyang, C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J. Clin. Investig. 2018, 128, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Grabauskas, G.; Zhou, S.-Y.; Gao, J.; Zhang, Y.; Owyang, C. High FODMAP diet causes barrier loss via lipopolysaccharide-mediated mast cell activation. JCI Insight 2021, 6, e146529. [Google Scholar] [CrossRef]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut 2022, 71, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.J.; Caminero, A.; Jiménez Vargas, N.N.; Soltys, C.L.; Jaramillo Polanco, J.O.; Lopez Lopez, C.D.; Constante, M.; Lourenssen, S.R.; Verdu, E.F.; Muir, J.G.; et al. The impact of dietary fermentable carbohydrates on a postinflammatory model of irritable bowel syndrome. Neurogastroenterol. Motil. 2019, 31, e13675. [Google Scholar] [CrossRef] [PubMed]
- Slomski, A. The Low-FODMAP diet helps IBS symptoms, but questions remain. JAMA 2020, 323, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, J.B.J.; Reber, L.; Eutamène, H.; Theodorou, V. Increased fermentable carbohydrate intake alters colonic mucus barrier function through glycation processes and increased mast cell counts. FASEB J. 2022, 36, e22297. [Google Scholar] [CrossRef]
- Barbara, G.; Wang, B.; Stanghellini, V.; de Giorgio, R.; Cremon, C.; Di Nardo, G.; Trevisani, M.; Campi, B.; Geppetti, P.; Tonini, M.; et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 2007, 132, 26–37. [Google Scholar] [CrossRef]
- Wu, J.; Masuy, I.; Biesiekierski, J.R.; Fitzke, H.E.; Parikh, C.; Schofield, L.; Shaikh, H.; Bhagwanani, A.; Aziz, Q.; Taylor, S.A.; et al. Gut-brain axis dysfunction underlies FODMAP-induced symptom generation in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2022, 55, 670–682. [Google Scholar] [CrossRef]
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, pathophysiology, and clinical application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef]
- Thorburn, T.; Aali, M.; Lehmann, C. Immune response to systemic inflammation in the intestinal microcirculation. Front. Biosci. Landmark Ed. 2018, 23, 782–795. [Google Scholar]
- Matheson, P.J.; Wilson, M.A.; Garrison, R.N. Regulation of intestinal blood flow. J. Surg. Res. 2000, 93, 182–196. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.A.; Cook, S.F.; Sands, B.E.; Ajene, A.N.; Miller, D.P.; Walker, A.M. Occurrence of colon ischemia in relation to irritable bowel syndrome. Am. J. Gastroenterol. 2004, 99, 486–491. [Google Scholar] [CrossRef]
- Chen, B.-R.; Du, L.-J.; He, H.-Q.; Kim, J.J.; Zhao, Y.; Zhang, Y.-W.; Luo, L.; Dai, N. Fructo-oligosaccharide intensifies visceral hypersensitivity and intestinal inflammation in a stress-induced irritable bowel syndrome mouse model. World J. Gastroenterol. 2017, 23, 8321–8333. [Google Scholar] [CrossRef] [PubMed]
- Bradesi, S.; Schwetz, I.; Ennes, H.S.; Lamy, C.M.R.; Ohning, G.; Fanselow, M.; Pothoulakis, C.; McRoberts, J.A.; Mayer, E.A. Repeated exposure to water avoidance stress in rats: A new model for sustained visceral hyperalgesia. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G42–G53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Accarie, A.; Vanuytsel, T. Animal models for functional gastrointestinal disorders. Front. Psychiatry 2020, 11, 509681. [Google Scholar] [CrossRef] [PubMed]
- Tao, E.; Long, G.; Yang, T.; Chen, B.; Guo, R.; Ye, D.; Fang, M.; Jiang, M. Maternal separation induced visceral hypersensitivity evaluated via novel and small size distention balloon in post-weaning mice. Front. Neurosci. 2021, 15, 803957. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Y.; Xia, Q.; Yu, T.-T.; Zhu, J.-T.; Zhu, D. Transmissive-detected laser speckle contrast imaging for blood flow monitoring in thick tissue: From Monte Carlo simulation to experimental demonstration. Light Sci. Appl. 2021, 10, 241. [Google Scholar] [CrossRef]
- Behem, C.R.; Graessler, M.F.; Pinnschmidt, H.O.; Duprée, A.; Sandhu, H.K.; Debus, E.S.; Wipper, S.H.; Trepte, C.J.C. Volume based resuscitation and intestinal microcirculation after ischaemia/reperfusion injury: Results of an exploratory aortic clamping study in pigs. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Heeman, W.; Steenbergen, W.; van Dam, G.; Boerma, E.C. Clinical applications of laser speckle contrast imaging: A review. J. Biomed. Opt. 2019, 24, 080901. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.D. In vivo evaluation of microcirculation by coherent light scattering. Nature 1975, 254, 56–58. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the Theme: Cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C350–C360. [Google Scholar] [CrossRef] [Green Version]
- Heeman, W.; Dijkstra, K.; Hoff, C.; Koopal, S.; Pierie, J.-P.; Bouma, H.; Boerma, E.C. Application of laser speckle contrast imaging in laparoscopic surgery. Biomed. Opt. Express 2019, 10, 2010–2019. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Miao, P.; Yu, J.; Qiu, Y.; Zhu, Y.; Tong, S. Influences of Hypothermia on the cortical blood supply by laser speckle imaging. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Scholz, M.; Lomer, M.C.; Ralph, F.S.; Irving, P.M.; Lindsay, J.O.; Fava, F.; Tuohy, K.; Whelan, K. Gut microbiota associations with diet in irritable bowel syndrome and the effect of low FODMAP diet and probiotics. Clin. Nutr. 2021, 40, 1861–1870. [Google Scholar] [CrossRef]
- Hayase, E.; Hayase, T.; Jamal, M.A.; Miyama, T.; Chang, C.-C.; Ortega, M.R.; Ahmed, S.S.; Karmouch, J.L.; Sanchez, C.A.; Brown, A.N.; et al. Mucus-degrading bacteroides link carbapenems to aggravated graft-versus-host disease. Cell 2022, 185, 3705–3719.e14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Verne, G.N. New insights into visceral hypersensitivity--clinical implications in IBS. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Mertz, H.; Naliboff, B.; Munakata, J.; Niazi, N.; Mayer, E.A. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995, 109, 40–52. [Google Scholar] [CrossRef]
- Distrutti, E.; Salvioli, B.; Azpiroz, F.; Malagelada, J.R. Rectal function and bowel habit in irritable bowel syndrome. Am. J. Gastroenterol. 2004, 99, 131–137. [Google Scholar] [CrossRef]
- Bouin, M.; Plourde, V.; Boivin, M.; Riberdy, M.; Lupien, F.; Laganière, M.; Verrier, P.; Poitras, P. Rectal distention testing in patients with irritable bowel syndrome: Sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 2002, 122, 1771–1777. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Evangelista, S. Experimental models of irritable bowel syndrome and the role of the enteric neurotransmission. J. Clin. Med. 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Paine, P. Review article: Current and future treatment approaches for pain in IBS. Aliment. Pharmacol. Ther. 2021, 54 (Suppl. 1), S75–S88. [Google Scholar] [CrossRef] [PubMed]
- Perna, E.; Aguilera-Lizarraga, J.; Florens, M.V.; Jain, P.; Theofanous, S.A.; Hanning, N.; De Man, J.G.; Berg, M.; De Winter, B.; Alpizar, Y.A.; et al. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut 2021, 70, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolstanova, G.; Khomenko, T.; Deng, X.; Chen, L.; Tarnawski, A.; Ahluwalia, A.; Szabo, S.; Sandor, Z. Neutralizing anti-vascular endothelial growth factor (VEGF) antibody reduces severity of experimental ulcerative colitis in rats: Direct evidence for the pathogenic role of VEGF. J. Pharmacol. Exp. Ther. 2009, 328, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, E.K.; Çetinkaya, H.; Savaş, B.; Ensari, A.; Torgutalp, M.; Efe, C. Vascular endothelial growth factor, endostatin levels and clinical features among patients with ulcerative colitis and irritable bowel syndrome and among healthy controls: A cross-sectional analytical study. Sao Paulo Med. J. 2018, 136, 543–550. [Google Scholar] [CrossRef]
- Sofi, F.; Whittaker, A.; Gori, A.M.; Cesari, F.; Surrenti, E.; Abbate, R.; Gensini, G.F.; Benedettelli, S.; Casini, A. Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: A double-blinded randomised dietary intervention trial. Br. J. Nutr. 2014, 111, 1992–1999. [Google Scholar] [CrossRef] [Green Version]
- Christina Grape, R.N.; Theorell, T.; Wikström, B.-M.; Ekman, R. Choir singing and fibrinogen. VEGF, cholecystokinin and motilin in IBS patients. Med. Hypotheses 2009, 72, 223–225. [Google Scholar] [CrossRef]
- Joussen, A.M.; Poulaki, V.; Qin, W.; Kirchhof, B.; Mitsiades, N.; Wiegand, S.J.; Rudge, J.; Yancopoulos, G.D.; Adamis, A.P. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am. J. Pathol. 2002, 160, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Falkenhain, K.; Njiru, B.N.; Murtaza-Ali, M.; Ruiz-Uribe, N.E.; Haft-Javaherian, M.; Catchers, S.; Nishimura, N.; Schaffer, C.B.; Bracko, O. VEGF signalling causes stalls in brain capillaries and reduces cerebral blood flow in Alzheimer’s mice. Brain 2022, 145, 1449–1463. [Google Scholar] [CrossRef]
- Martínez-Martínez, L.-A.; Mora, T.; Vargas, A.; Fuentes-Iniestra, M.; Martínez-Lavín, M. Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: A review of case-control studies. J. Clin. Rheumatol. 2014, 20, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Katherine Jurek, M.; Seavey, H.; Guidry, M.; Slomka, E.; Hunter, S.D. The effects of slow deep breathing on microvascular and autonomic function and symptoms in adults with irritable bowel syndrome: A pilot study. Neurogastroenterol. Motil. 2022, 34, e14275. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Manabe, N.; Hata, J.; Kusunoki, H.; Ishii, M.; Sato, M.; Kamada, T.; Shiotani, A.; Haruma, K. Characterization of autonomic dysfunction in patients with irritable bowel syndrome using fingertip blood flow. Neurogastroenterol. Motil. 2008, 20, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.V.; Kamm, M.A. Laser doppler flowmetry as a measure of extrinsic colonic innervation in functional bowel disease. Gut 2000, 46, 212–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boley, S.J.; Agrawal, G.P.; Warren, A.R.; Veith, F.J.; Levowitz, B.S.; Treiber, W.; Dougherty, J.; Schwartz, S.S.; Gliedman, M.L. Pathophysiologic effects of bowel distention on intestinal blood flow. Am. J. Surg. 1969, 117, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y. Hypothesis: Mechanism of irritable bowel syndrome in inflammatory bowel disease. Med. Hypotheses 2019, 132, 109324. [Google Scholar] [CrossRef] [PubMed]


| Raw Material | Regular FODMAP (2.1%) | High FODMAP (10%) | Medium FODMAP (5%) | Low FODMAP (0%) | ||||
|---|---|---|---|---|---|---|---|---|
| Weight (g) | Energy (Kcal) | Weight (g) | Energy (Kcal) | Weight (g) | Energy (Kcal) | Weight (g) | Energy (Kcal) | |
| Casein | 200 | 800 | 200 | 800 | 200 | 800 | 200 | 800 |
| Cystine | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 |
| Soybean oil | 70 | 630 | 70 | 630 | 70 | 630 | 70 | 630 |
| Lard | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sucrose | 100 | 400 | 100 | 400 | 100 | 400 | 100 | 400 |
| Maltodextrin | 132 | 528 | 132 | 528 | 132 | 528 | 132 | 528 |
| Corn starch | 376 | 1504 | 297 | 1188 | 347 | 1388 | 397 | 1588 |
| Cellulose | 50 | 0 | 50 | 0 | 50 | 0 | 50 | 0 |
| Multimineral ain | 35 | 0 | 35 | 0 | 35 | 0 | 35 | 0 |
| Multidimensional | 10 | 40 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline | 2.5 | 0 | 2.5 | 0 | 2.5 | 0 | 2.5 | 0 |
| Fructose | 7.35 | 29.4 | 35 | 140 | 17.5 | 70 | 0 | 0 |
| Fructo-Oligosaccharide | 6.3 | 25.2 | 30 | 120 | 15 | 60 | 0 | 0 |
| Galacto-oligosaccharide | 7.35 | 29.4 | 35 | 140 | 17.5 | 70 | 0 | 0 |
| Sorbitol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 999.5 | 3998 | 999.5 | 3998 | 999.5 | 3998 | 999.5 | 3998 |
| Weight ratio | Energy ratio | Weight ratio | Energy ratio | Weight ratio | Energy ratio | Weight ratio | Energy ratio | |
| Protein | 0.203101551 | 0.203101551 | 0.203101551 | 0.203101551 | 0.203101551 | 0.203101551 | 0.203101551 | 0.203101551 |
| Fat | 0.070035018 | 0.157578789 | 0.070035018 | 0.157578789 | 0.070035018 | 0.157578789 | 0.070035018 | 0.157578789 |
| Carbohydrate | 0.63931966 | 0.63931966 | 0.63931966 | 0.63931966 | 0.63931966 | 0.63931966 | 0.63931966 | 0.63931966 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Yan, C.; Wu, Y.; Tao, E.; Guo, R.; Zhu, Z.; Chen, X.; Fang, M.; Jiang, M. Low FODMAP Diet Relieves Visceral Hypersensitivity and Is Associated with Changes in Colonic Microcirculation in Water Avoidance Mice Model. Nutrients 2023, 15, 1155. https://doi.org/10.3390/nu15051155
Hu C, Yan C, Wu Y, Tao E, Guo R, Zhu Z, Chen X, Fang M, Jiang M. Low FODMAP Diet Relieves Visceral Hypersensitivity and Is Associated with Changes in Colonic Microcirculation in Water Avoidance Mice Model. Nutrients. 2023; 15(5):1155. https://doi.org/10.3390/nu15051155
Chicago/Turabian StyleHu, Chenmin, Chenxi Yan, Yuhao Wu, Enfu Tao, Rui Guo, Zhenya Zhu, Xiaolong Chen, Marong Fang, and Mizu Jiang. 2023. "Low FODMAP Diet Relieves Visceral Hypersensitivity and Is Associated with Changes in Colonic Microcirculation in Water Avoidance Mice Model" Nutrients 15, no. 5: 1155. https://doi.org/10.3390/nu15051155






