The Utilization of Bee Products as a Holistic Approach to Managing Polycystic Ovarian Syndrome-Related Infertility
Abstract
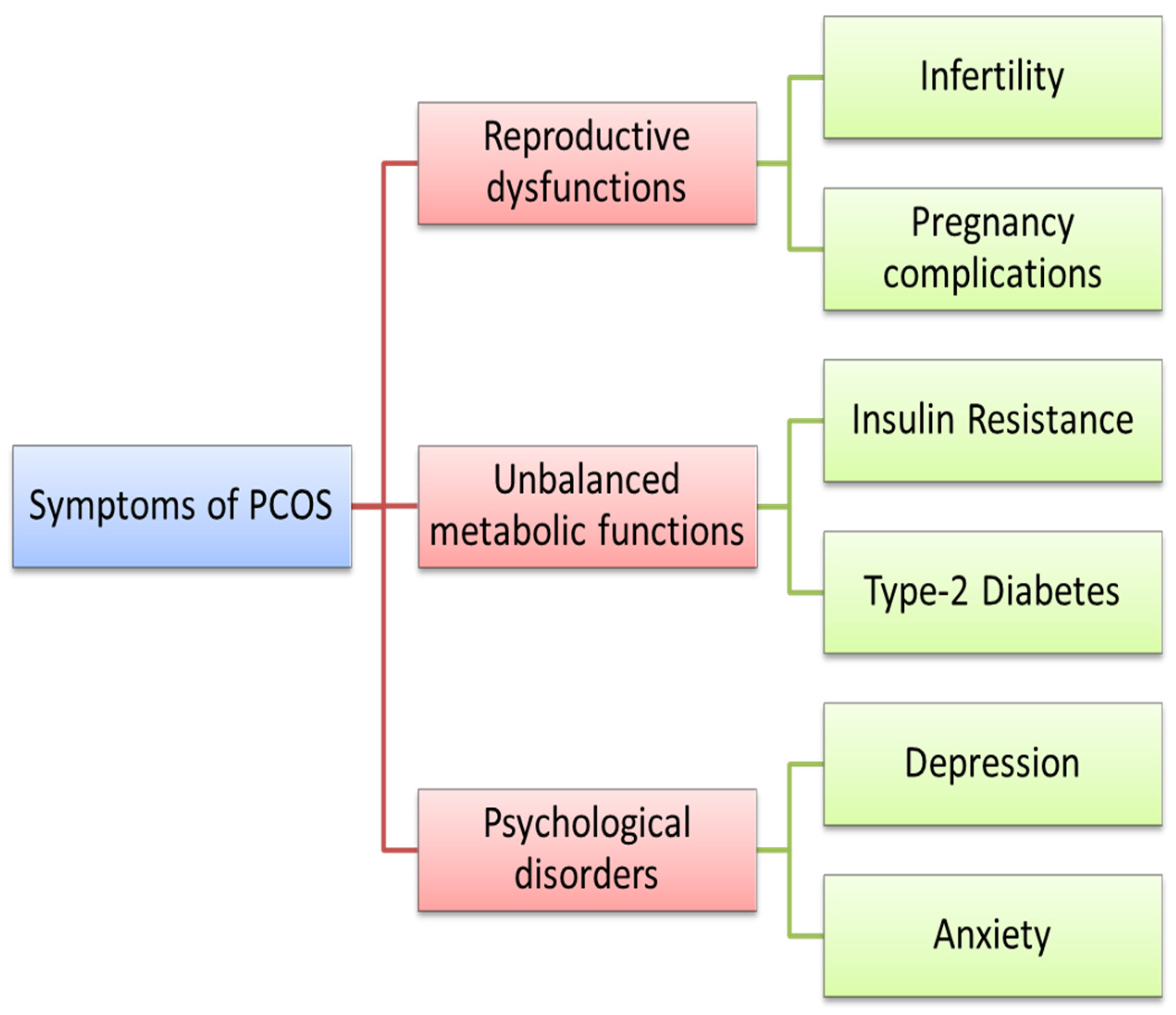
:1. Introduction
2. Study Design and Research
3. Honey
4. Honeybee Venom
5. Royal Jelly
6. Bee Pollen
7. Propolis
8. Potential Limitations
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J. Clin. Endocrinol. Metab. 2020, 106, e1071–e1083. [Google Scholar] [CrossRef] [PubMed]
- Poojari, P.; Padgaonkar, A.; Paramanya, A.; Ali, A. Compendium of polycystic ovarian syndrome and its relevance in glycation and diabetes. J. Exp. Clin. Med. 2022, 39, 256–268. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with High Levels of Antioxidants Can Provide Protection to Healthy Human Subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef]
- Salama, S.; Shou, Q.; El-Wahed, A.A.A.; Elias, N.; Xiao, J.; Swillam, A.; Umair, M.; Guo, Z.; Daglia, M.; Wang, K.; et al. Royal Jelly: Beneficial Properties and Synergistic Effects with Chemotherapeutic Drugs with Particular Emphasis in Anticancer Strategies. Nutrients 2022, 14, 4166. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Ugusman, A.; Zaid, S.S.M.; Mokhtar, M.H. Kelulut Honey Improves Folliculogenesis, Steroidogenic, and Aromatase Enzyme Profiles and Ovarian Histomorphology in Letrozole-Induced Polycystic Ovary Syndrome Rats. Nutrients 2022, 14, 4364. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Ugusman, A.; Mokhtar, M.H. Kelulut Honey Ameliorates Oestrus Cycle, Hormonal Profiles, and Oxidative Stress in Letrozole-Induced Polycystic Ovary Syndrome Rats. Antioxidants 2022, 11, 1879. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Ugusman, A.; Mokhtar, M.H. Kelulut Honey Regulates Sex Steroid Receptors in a Polycystic Ovary Syndrome Rat Model. Int. J. Mol. Sci. 2022, 23, 14757. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Ugusman, A.; Mokhtar, M.H. Effects of Kelulut Honey on Oestrus Cycle Regulation and Histomorphological Changes in Letrozole-Induced Polycystic Ovary Syndrome Rats: A Preliminary Study. Life 2022, 12, 890. [Google Scholar] [CrossRef]
- Sapmaz, T.; Sevgin, K.; Topkaraoglu, S.; Tekayev, M.; Gumuskaya, F.; Efendic, F.; Pence, M.E.; Aktas, S.; Hekimoglu, G.; Irkorucu, O. Propolis protects ovarian follicular reserve and maintains the ovary against polycystic ovary syndrome (PCOS) by attenuating degeneration of zona pellucida and fibrous tissue. Biochem. Biophys. Res. Commun. 2022, 636, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Naseri, L.; Khazaei, M.R.; Khazaei, M. Synergic effect of bee pollen and metformin on proliferation and apoptosis of granulosa cells: Rat model of polycystic ovary syndrome. J. Food Biochem. 2021, 46, e13635. [Google Scholar] [CrossRef] [PubMed]
- Naseri, L.; Khazaei, M.R.; Khazaei, M. Potential Therapeutic Effect of Bee Pollen and Metformin Combination on Testosterone and Estradiol Levels, Apoptotic Markers and Total Antioxidant Capacity in A Rat Model of Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2021, 15, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ab Hamid, N.; Abu Bakar, A.B.; Zain, A.A.M.; Hussain, N.H.N.; Othman, Z.A.; Zakaria, Z.; Mohamed, M. Composition of Royal Jelly (RJ) and Its Anti-Androgenic Effect on Reproductive Parameters in a Polycystic Ovarian Syndrome (PCOS) Animal Model. Antioxidants 2020, 9, 499. [Google Scholar] [CrossRef]
- Yasin, M.M.; Abdelfatah, E.; Hamada, H.; Yousef, A.; Shahin, M.; Mosaad, D. Effect of bee venom phonophoresis in obese polycystic ovarian women: A Single Blind Randomized Controlled Trial. J. Appl. Pharm. Sci. 2018, 8, 159–164. [Google Scholar] [CrossRef]
- Karimzadeh, L.; Nabiuni, M.; Kouchesfehani, H.M.; Adham, H.; Bagheri, A.; Sheikholeslami, A. Effect of bee venom on IL-6, COX-2 and VEGF levels in polycystic ovarian syndrome induced in Wistar rats by estradiol valerate. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 32. [Google Scholar] [CrossRef] [Green Version]
- Pouyanmanesh, F.; Nabiuni, M.; Nasri, S.; Nazari, Z.; Karimzadeh, L. The effect of honey bee venom on levels of lipids and anti-mullerian hormone in a rat with polycystic ovarian syndrome. Feyz J. Kashan Univ. Med. Sci. 2013, 17, 239–246. Available online: http://feyz.kaums.ac.ir/article-1-1954-en.html (accessed on 23 October 2022).
- Karimzadeh, L.; Nabiuni, M.; Sheikholeslami, A.; Irian, S. Bee venom treatment reduced C-reactive protein and improved follicle quality in a rat model of estradiol valerate-induced polycystic ovarian syndrome. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Nabiuni, M.; Nasri, S.; Poyanmanesh, F.; Karimzadeh, L.; Nazari, Z. Honey Bee Venom Modulates Hyperglycemia in Response to Hyperandrogenism in Polycystic Ovarian Syndrome-Induced Wistar Rats, Chapters. In International Conference on Applied Life Sciences; Nejadkoorki, F., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of sugars and phenolic compounds in honey powders with the use of GC–MS, FTIR spectroscopy, and X-ray diffraction. Sci. Rep. 2020, 10, 16269. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M. Chemical Composition of Honey. In Bee Products-Chemical and Biological Properties; Springer: Cham, Switzerland, 2017; pp. 43–82. [Google Scholar]
- Moniruzzaman, M.; Rodríguez, I.; Ramil, M.; Cela, R.; Sulaiman, S.; Gan, S. Assessment of gas chromatography time-of-flight accurate mass spectrometry for identification of volatile and semi-volatile compounds in honey. Talanta 2014, 129, 505–515. [Google Scholar] [CrossRef]
- Suto, M.; Kawashima, H.; Nakamura, Y. Determination of Organic Acids in Honey by Liquid Chromatography with Tandem Mass Spectrometry. Food Anal. Methods 2020, 13, 2249–2257. [Google Scholar] [CrossRef]
- Jang, M.; Lee, M.J.; Lee, J.M.; Bae, C.-S.; Kim, S.; Ryu, J.H.; Cho, I.-H. Oriental Medicine Kyung-Ok-Ko Prevents and Alleviates Dehydroepiandrosterone-Induced Polycystic Ovarian Syndrome in Rats. PLoS ONE 2014, 9, e87623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadoret, V.; Jarrier-Gaillard, P.; Papillier, P.; Monniaux, D.; Guérif, F.; Dalbies-Tran, R. Leukemia inhibitory factor modulates the differentiation of granulosa cells during sheep in vitro preantral to antral follicle development and improves oocyte meiotic competence. Mol. Hum. Reprod. 2021, 27, gaab051. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Yang, H.; Lee, S.R.; Kwon, S.W.; Hong, E.-J.; Lee, H.W. Welsh Onion Root (Allium fistulosum) Restores Ovarian Functions from Letrozole Induced-Polycystic Ovary Syndrome. Nutrients 2018, 10, 1430. [Google Scholar] [CrossRef] [Green Version]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. Enhancement of BDNF Concentration and Restoration of the Hypothalamic-Pituitary-Adrenal Axis Accompany Reduced Depressive-Like Behaviour in Stressed Ovariectomised Rats Treated with Either Tualang Honey or Estrogen. Sci. World J. 2014, 2014, 310821. [Google Scholar] [CrossRef] [Green Version]
- Jakimiuk, A.J.; Weitsman, S.R.; Yen, H.-W.; Bogusiewicz, M.; Magoffin, D.A. Estrogen Receptor α and β Expression in Theca and Granulosa Cells from Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2002, 87, 5532–5538. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Sulaiman, S.A.; Othman, N.H. Oral Administration of Tualang and Manuka Honeys Modulates Breast Cancer Progression in Sprague-Dawley Rats Model. Evid.-Based Complement. Altern. Med. 2017, 2017, 5904361. [Google Scholar] [CrossRef] [Green Version]
- Nabiuni, M.; Parivar, K.; Zeynali, B.; Sheikholeslami, A.; Karimzadeh, L. 41. Anti-Inflammatory Effect of Honey Bee Venom on Wistar Rats Induced Poly Cystic Ovarian Syndrome by Estradiol Valerate. Toxicon 2012, 60, 115–116. [Google Scholar] [CrossRef]
- Tokuyama, O.; Nakamura, Y.; Musoh, A.; Honda, K.-I.; Ozaki, K.; Ishiko, O. Expression and distribution of cyclooxygenase-2 in human ovary during follicular development. Osaka City Med. J. 2003, 49, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Ali, A. Laparoscopic intraovarian injection of bee venom in the treatment of polycystic ovarian disease: A new modality. Obstet. Gynecol. 2000, 95, S15. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical Composition of Royal Jelly. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Xin, X.-X.; Chen, Y.; Chen, D.; Xiao, F.; Parnell, L.D.; Zhao, J.; Liu, L.; Ordovas, J.M.; Lai, C.-Q.; Shen, L.-R. Supplementation with Major Royal-Jelly Proteins Increases Lifespan, Feeding, and Fecundity in Drosophila. J. Agric. Food Chem. 2016, 64, 5803–5812. [Google Scholar] [CrossRef]
- Kunugi, H.; Ali, A.M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbari, E.; Khazaei, M.R.; Khazaei, M.; Nejati, V. Royal Jelly Promotes Ovarian Follicles Growth and Increases Steroid Hormones in Immature Rats. Int. J. Fertil. Steril. 2018, 11, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, F.; Ghanbari, E.; Khazaei, M. Improved hormonal and oxidative changes by Royal Jelly in the rat model of PCOS: An experimental study. Int. J. Reprod. Biomed. (IJRM) 2021, 19, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostić, A.; Milinčić, D.D.; Barać, M.B.; Shariati, M.A.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Kostić, A.; Milinčić, D.; Nedić, N.; Gašić, U.; Trifunović, B.; Vojt, D.; Tešić, Ž.L.; Pešić, M. Phytochemical Profile and Antioxidant Properties of Bee-Collected Artichoke (Cynara scolymus) Pollen. Antioxidants 2021, 10, 1091. [Google Scholar] [CrossRef]
- Kostić, A.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT 2019, 112, 108244. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid.-Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.K.; de Alencar, S.M.; Aguiar, C.L. Botanical Origin and Chemical Composition of Brazilian Propolis. J. Agric. Food Chem. 2002, 50, 2502–2506. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.; Gardana, C.; Pietta, A. Analytical methods for quality control of propolis. Fitoterapia 2002, 73, S7–S20. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.; Crane, E. Constituents of propolis. Apidologie 1987, 18, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, A.; Ahuja, V. Apitherapy—A sweet approach to dental diseases—Part I: Honey. J. Adv. Dent. Res. I 2010, 1, 81–86. [Google Scholar]
- Mitchell, A. Africanized killer bees: A case study. Crit. Care Nurse 2006, 26, 23–26. [Google Scholar] [CrossRef]
- Paola, F.; Pantalea, D.D.; Gianfranco, C.; Antonio, F.; Angelo, V.; Eustachio, N.; Elisabetta, D.L. Oral Allergy Syndrome in a Child Provoked by Royal Jelly. Case Rep. Med. 2014, 2014, 941248. [Google Scholar] [CrossRef] [Green Version]
- Harada, S.; Moriyama, T.; Tanaka, A. Two cases of royal jelly allergy provoked the symptoms at the time of their first intake. Arerugi = [Allergy] 2011, 60, 708–713. [Google Scholar]
- Cohen, S.H.; Yunginger, J.W.; Rosenberg, N.; Fink, J.N. Acute allergic reaction after composite pollen ingestion. J. Allergy Clin. Immunol. 1979, 64, 270–274. [Google Scholar] [CrossRef]
- Makris, M.; Koulouris, S.; Koti, I.; Aggelides, X.; Sideri, K.; Chliva, C.; Vassilatou, E.; Kalogeromitros, D. Temporal relationship of allergic rhinitis with asthma and other co-morbidities in a Mediterranean country: A retrospective study in a tertiary reference allergy clinic. Allergol. Immunopathol. 2010, 38, 246–253. [Google Scholar] [CrossRef]
- Brugnerotto, P.; Seraglio, S.K.T.; Schulz, M.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Pyrrolizidine alkaloids and beehive products: A review. Food Chem. 2020, 342, 128384. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef] [PubMed]






| Bee Product | Studied Parameters | POCS Induced by | Study Model | Outcomes of the Study | Author | |
|---|---|---|---|---|---|---|
| 1 | Kelulut Honey 0.5, 1, and 2 g/kg/day 35 days | Oestrus Cycle Regulation Histomorphological Changes | letrozole 1 mg/kg/day of 21 days | Sprague–Dawley Rats (n = 24) | The increase in the corpus luteum and antral follicle improved the estrus cycle and reduced the effect of histomorphological changes in developing cystic follicles and ovaries in PCOS. | [7] |
| 2 | Kelulut Honey 1 g/kg/day 35 days | Oestrus Cycle, Hormonal Profile Oxidative Stress | letrozole 1 mg/kg/day of 21 days | Sprague–Dawley Rats (n = 42) | Co-administration of Kellut honey with metformin or clomiphene contributed positively to the hormonal profile, oxidative stress, and estrus cycle in PCOS rats. Additionally, it had no adverse effects on insulin, blood sugar, and rate of body weight gain in PCOS mice. | [8] |
| 3 | Kelulut Honey 1 g/kg/day 35 days | Sex Steroid Receptors | letrozole 1 mg/kg/day of 21 days | Sprague–Dawley Rats (n = 42) | This study shows that in PCOS, sex steroid receptors are expressed abnormally and that Kelulut honey treatment can normalize the expression of these receptors. | [9] |
| 4 | Kelulut Honey 1 g/kg/day 35 days | Folliculogenesis, Steroidogenic, and Aromatase Enzyme Profile, Ovarian Histomorphology | letrozole 1 mg/kg/day of 21 days | Sprague–Dawley Rats (n = 42) | It was found that Kellut honey positively affected Cyp17a1 and Cyp19a1 expression, folliculogenesis, and ovarian histomorphology in PCOS rats, and was more effective when applied together with clomiphene. | [10] |
| 5 | Propolis 50 mg/kg and 150 mg/kg | ovarian follicular reserve zona pellucida fibrous tissue | letrozole 1 mg/kg/day of 21 days | Wistar Rat (n = 24) | The application of 50 mg/kg propolis had a positive effect on the number of cystic and primary follicles. In the lower concentration groups, however, it did not completely restore PCOS-associated hormones and p53 expression. | [11] |
| 6 | Bee pollen 50, 100, and 200 mg/kg | proliferation and apoptosis of granulosa cells | 2 mg of estradiol valerate 60 days | Wistar Rats (n = 54) | The number of preantral and antral follicles increased in the PCOS group in which bee pollen and metformin were administered together. The number of cystic follicles decreased. TNF-α, NO levels, and Ki67 values were decreased in other groups. Apoptosis increased in bee pollen-applied groups. There was a positive change in PCOS symptoms in both bee pollen and metformin groups. | [12] |
| 7 | Bee pollen 50, 100, and 200 mg/kg | Testosterone and Estradiol Levels Apoptotic Markers Total Antioxidant Capacity | 2 mg of estradiol valerate 21 days | Wistar Rats (n = 54) | Estradiol and testosterone levels and Bcl-2 expression increased in the PCOS group. In the application group, these values decreased. Total antioxidant capacity and expression of Bax, Cas-3, and Sirt1 were decreased, but these values increased in the bee pollen and metformin group. | [13] |
| 8 | Royal jelly 100, 200 and 400 mg/kg | Anti-Androgenic Effect | Testosterone 10 mg/kg | Sprague Dawley Rats (n = 40) | The T + 200RJ group had higher FSH (Follicule-Stimulating Hormone) levels and lower LH (Luteinizing hormone), testosterone, estradiol levels, lower malondialdehyde, lower glutathione peroxidase activity and higher total antioxidant capacity levels compared to the T group. It also showed recovery in various stages of ovarian follicular development. | [14] |
| 9 | Bee venom phonophoresis 1 μg/ml | Case Study | Obese PCOS Women (n = 46) | After 7 and 14 weeks of administration, the progesterone value increased but the LH and LH/FSH ratio decreased in the administration group. Dietary bee venom phonophoresis has a positive effect on the treatment of obese women with PCOS. | [15] | |
| 10 | Bee venom | IL-6, COX-2 and VEGF levels | 2 mg of estradiol valerate 60 days | Wistar Rats (n = 24) | The theca layer thickness, IL-6 levels, and the number and diameter of cysts were decreased in the HBV group. It showed increased expression of COX-2 and VEGF in the PCOS group. Irregular immunostaining occurred in HBV-treated rats. | [16] |
| 11 | Bee venom | Levels of lipids and anti-mullerian hormone | 2 mg of estradiol valerate 60 days | Wistar Rats (n = 63) | There was a decrease in the corpus luteum diameter in the PCOS group and an increase in the HBV group. Anti-mullerian hormone increased in the PCOS group and decreased in the honeybee venom group. Blood triglycerides and LDL(low-density-lipoprotein) cholestrol levels increased in the PCOS group and decreased in the HBV group. | [17] |
| 12 | Bee venom 0.5 mg/kg 14 days | C-reactive protein follicle quality | 2 mg of estradiol valerate 60 days | Wistar Rats | Honeybee venom reduced the theca layer thickness, number of cysts, and C-reactive protein levels, and showed the presence of the corpus luteum in the PCOS group. It was thought that honey bee venom would have a positive effect by inhibiting the level of C-reactive protein. | [18] |
| 13 | Bee venom 0.5 mg/kg 14 days | Hyperglycemia Hyperandrogenism | Estradiol Valerate (2 mg/100 grB.W) | Wistar Rats | Ovarian weight, testosterone, and estradiol levels increased in the experimental group. These values decreased in the group treated with honeybee venom. The blood glucose levels, the thickness of the theca layer, the number, and the diameter of cysts were reduced in the honeybee venom group compared to the PCOS group. In addition, the corpus luteum was detected in the group in which honey bee venom was administered. | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Paramanya, A.; Poojari, P.; Arslan-Acaroz, D.; Acaroz, U.; Kostić, A.Ž. The Utilization of Bee Products as a Holistic Approach to Managing Polycystic Ovarian Syndrome-Related Infertility. Nutrients 2023, 15, 1165. https://doi.org/10.3390/nu15051165
Ali A, Paramanya A, Poojari P, Arslan-Acaroz D, Acaroz U, Kostić AŽ. The Utilization of Bee Products as a Holistic Approach to Managing Polycystic Ovarian Syndrome-Related Infertility. Nutrients. 2023; 15(5):1165. https://doi.org/10.3390/nu15051165
Chicago/Turabian StyleAli, Ahmad, Additiya Paramanya, Payal Poojari, Damla Arslan-Acaroz, Ulas Acaroz, and Aleksandar Ž. Kostić. 2023. "The Utilization of Bee Products as a Holistic Approach to Managing Polycystic Ovarian Syndrome-Related Infertility" Nutrients 15, no. 5: 1165. https://doi.org/10.3390/nu15051165
APA StyleAli, A., Paramanya, A., Poojari, P., Arslan-Acaroz, D., Acaroz, U., & Kostić, A. Ž. (2023). The Utilization of Bee Products as a Holistic Approach to Managing Polycystic Ovarian Syndrome-Related Infertility. Nutrients, 15(5), 1165. https://doi.org/10.3390/nu15051165








