RETRACTED: A Mendelian Randomization Analysis Investigates Causal Associations between Inflammatory Bowel Diseases and Variable Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. MR Design
2.2. Selection of Genetic Instruments
2.3. GWAS Summary Statistics for IBD Cohorts
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the 36 Candidate Risk Factors
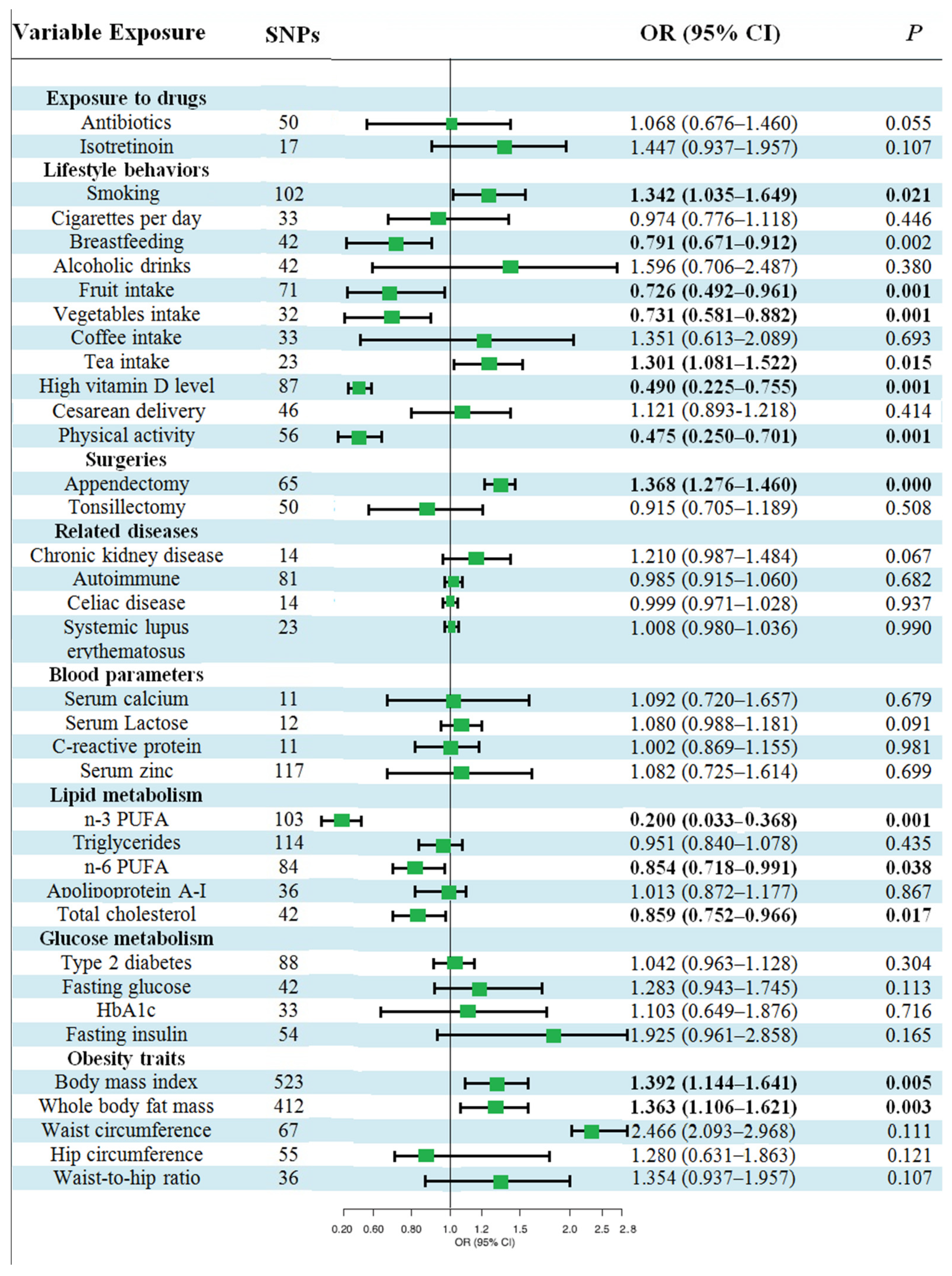
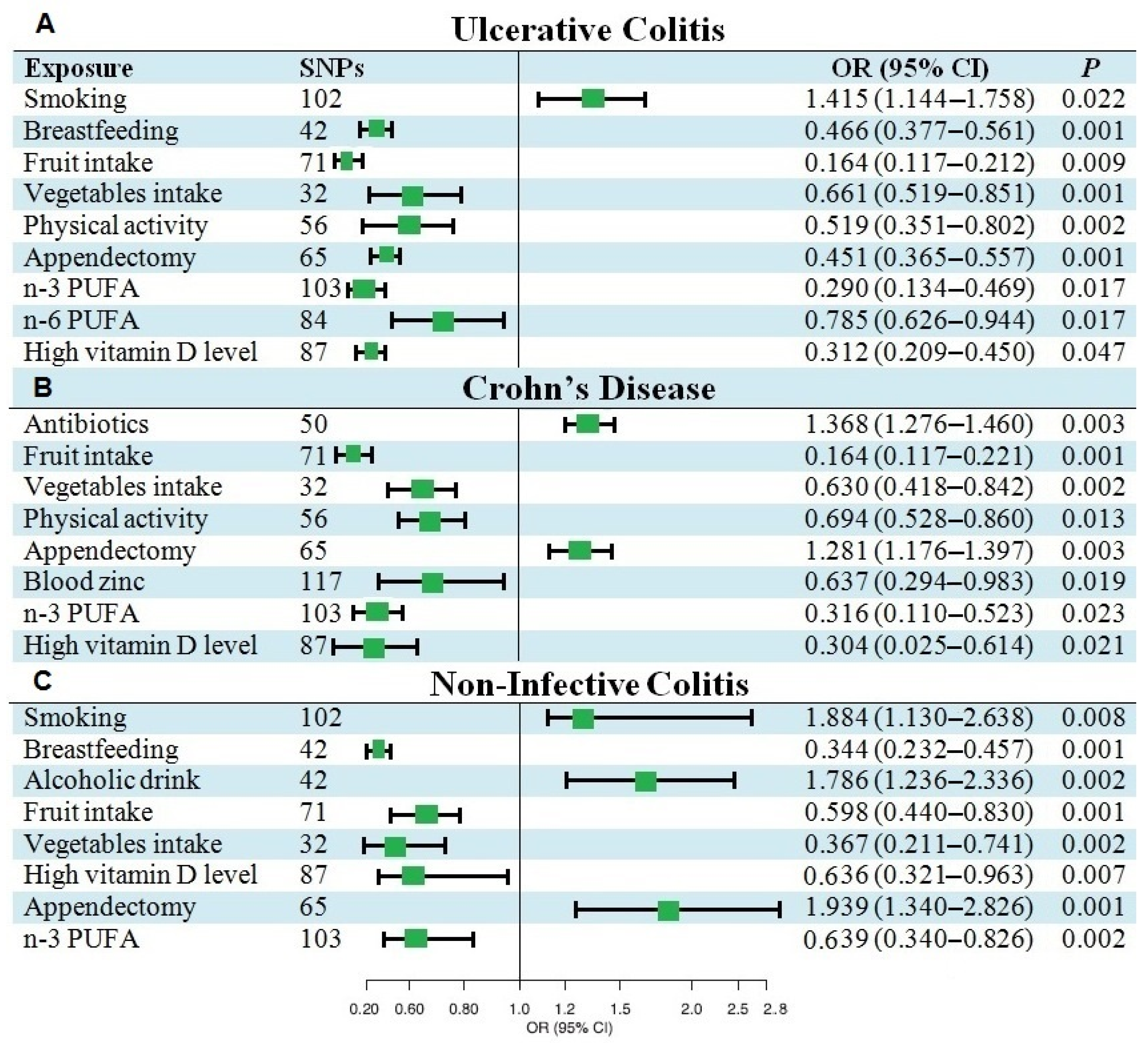
3.2. Causal Effects of Various Factors on UC
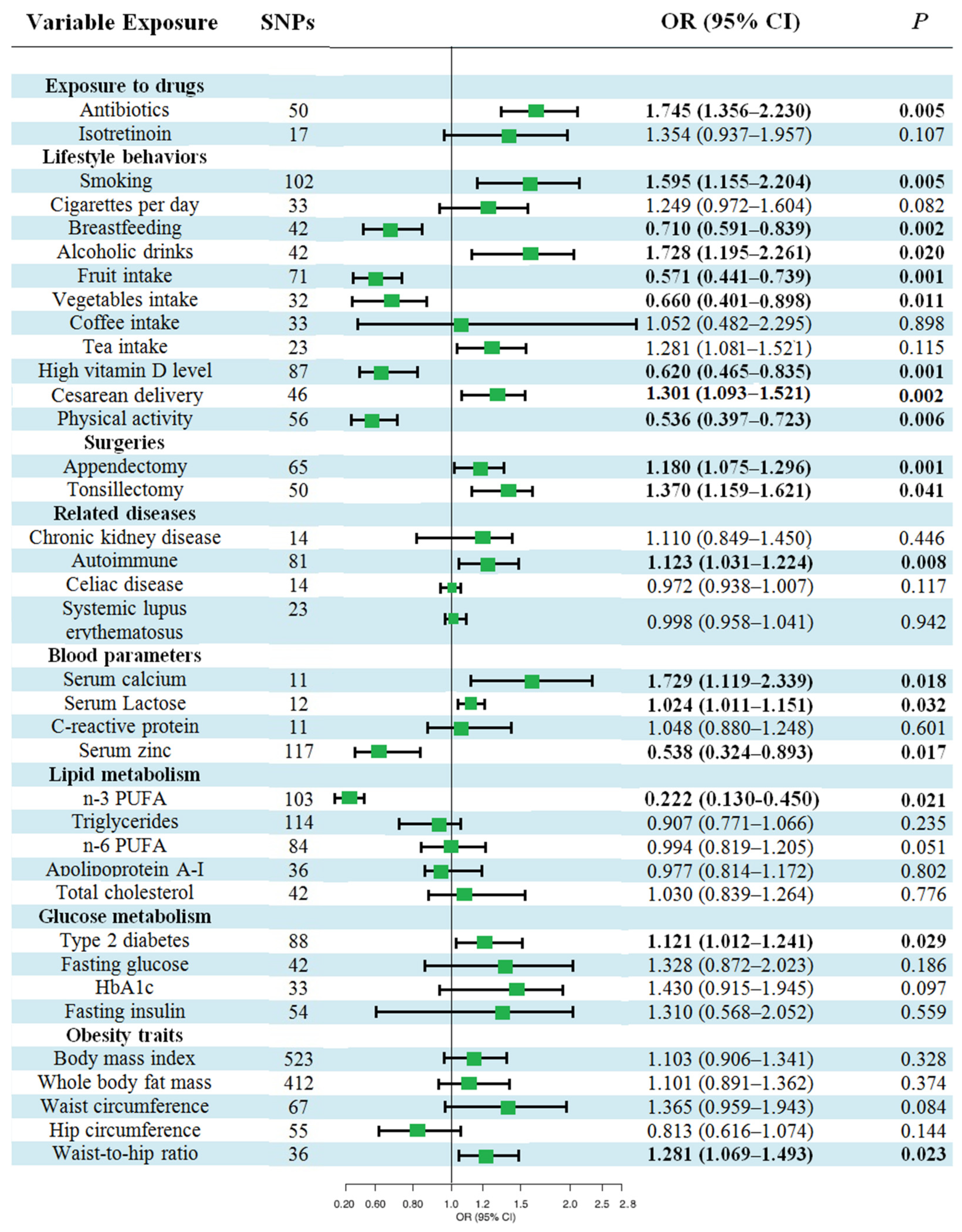
3.3. Causal Effects of Various Factors on CD
3.4. Causal Effects of Various Factors on NIC
3.5. Multivariable MR Analysis of IBD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shivashankar, R.; Lewis, J.D. The role of diet in inflammatory bowel disease. Curr. Gastroenterol. Rep. 2017, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Kaplan, G.G. Environmental risk factors for inflammatory bowel disease. Gastroenterol. Hepatol. 2010, 6, 339–346. [Google Scholar]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Burke, K.E.; Boumitri, C.; Ananthakrishnan, A.N. Modifiable environmental factors in inflammatory bowel disease. Curr. Gastroenterol. Rep. 2017, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.F. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Kahrstrom, C.T.; Pariente, N.; Weiss, U. Intestinal microbiota in health and disease. Nature 2016, 535, 47. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Klenske, E.; Bojarski, C.; Waldner, M.; Rath, T.; Neurath, M.F.; Atreya, R. Targeting mucosal healing in Crohn’s disease: What the clinician needs to know. Therap. Adv. Gastroenterol. 2019, 12, 1756284819856865. [Google Scholar] [CrossRef]
- Liu, J.Z.; Van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef] [PubMed]
- De Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.G.; et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Abegunde, A.T.; Muhammad, B.H.; Bhatti, O.; Ali, T. Environmental risk factors for inflammatory bowel diseases: Evidence based literature review. World J. Gastroenterol. 2016, 22, 6296. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Nguyen, D.D.; Sauk, J.; Yajnik, V.; Xavier, R.J. Genetic polymorphisms in metabolizing enzymes modifying the association between smoking and inflammatory bowel diseases. Inflamm. Bowel Dis. 2014, 20, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Fiocchi, C.; Zhu, X.; Ripke, S.; Kamboh, M.I.; Rebert, N.; Duerr, R.H.; Achkar, J.P. Gene-gene and gene-environment interactions in ulcerative colitis. Hum. Genet. 2014, 133, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Wedow, R.; Okbay, A.; Kong, E.; Maghzian, O.; Zacher, M.; Nguyen-Viet, T.A.; Bowers, P.; Sidorenko, J.; Karlsson Linnér, R.; et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 2018, 50, 1112–1121. [Google Scholar] [CrossRef]
- The FinnGen Consortium. FinnGen Documentation of R5 Release. Available online: https://finngen.gitbook.io/documentation (accessed on 12 July 2022).
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.E.; Mägi, R.; Strawbridge, R.J.; Pers, T.H.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015, 518, 187–196. [Google Scholar] [CrossRef]
- Pattaro, C.; Teumer, A.; Gorski, M.; Chu, A.Y.; Li, M.; Mijatovic, V.; Garnaas, M.; Tin, A.; Sorice, R.; Li, Y.; et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat. Commun. 2016, 7, 10023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, Y.; Zhu, W.; Gong, J.; Gu, L.; Zhang, W.; Guo, Z.; Li, N.; Li, J. Cesarean delivery and risk of inflammatory bowel disease: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2014, 49, 834–844. [Google Scholar] [CrossRef]
- Trynka, G.; Hunt, K.A.; Bockett, N.A.; Romanos, J.; Mistry, V.; Szperl, A.; Bakker, S.F.; Bardella, M.T.; Bhaw-Rosun, L.; Castillejo, G.; et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat. Genet. 2011, 43, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Bentham, J.; Morris, D.L.; Cunninghame Graham, D.S.; Pinder, C.L.; Tombleson, P.; Behrens, T.W.; Martín, J.; Fairfax, B.P.; Knight, J.C.; Chen, L.; et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 2015, 47, 1457–1464. [Google Scholar] [CrossRef]
- O’Seaghdha, C.M.; Wu, H.; Yang, Q.; Kapur, K.; Guessous, I.; Zuber, A.M.; Köttgen, A.; Stoudmann, C.; Teumer, A.; Kutalik, Z.; et al. Meta-analysis of genome-wide association studies identifies six new Loci for serum calcium concentrations. PLoS Genet. 2013, 9, e1003796. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef]
- Ligthart, S.; Vaez, A.; Võsa, U.; Stathopoulou, M.G.; De Vries, P.S.; Prins, B.P.; Van der Most, P.J.; Tanaka, T.; Naderi, E.; Rose, L.M.; et al. Genome analyses of >200,000 individuals identify 58 loci for chronic inflammation and highlight pathways that link inflammation and complex disorders. Am. J. Hum. Genet. 2018, 103, 691–706. [Google Scholar] [CrossRef]
- Xue, A.; Wu, Y.; Zhu, Z.; Zhang, F.; Kemper, K.E.; Zheng, Z.; Yengo, L.; Lloyd-Jones, L.R.; Sidorenko, J.; Wu, Y. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 2018, 9, 2941. [Google Scholar] [CrossRef]
- Chen, J.; Spracklen, C.N.; Marenne, G.; Varshney, A.; Corbin, L.J.; Luan, J.A.; Willems, S.M.; Wu, Y.; Zhang, X.; Horikoshi, M.; et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 2021, 53, 840–860. [Google Scholar] [CrossRef]
- Ungaro, R.; Bernstein, C.N.; Gearry, R.; Hviid, A.; Kolho, K.L.; Kronman, M.P.; Shaw, S.; Van Kruiningen, H.; Colombel, J.F.; Atreja, A. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: A meta-analysis. Am. J. Gastroenterol. 2014, 109, 1728–1738. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jamal, M.M.; Nguyen, E.T.; Bechtold, M.L.; Nguyen, D.L. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 210–216. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Khalili, H.; Gower-Rousseau, C.; Olen, O.; Benchimol, E.I.; Lynge, E.; Nielsen, K.R.; Brassard, P.; Vutcovici, M.; Bitton, A.; et al. Sex-based differences in incidence of inflammatory bowel diseases—Pooled analysis of population-based studies from western countries. Gastroenterology 2018, 155, 1079–1089. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017, 152, 313–321. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Mahid, S.S.; Minor, K.S.; Soto, R.E.; Hornung, C.A.; Galandiuk, S. Smoking and inflammatory bowel disease: A meta-analysis. Mayo. Clin. Proc. 2006, 81, 1462–1471. [Google Scholar] [CrossRef]
- Calkins, B.M. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig. Dis. Sci. 1989, 34, 1841–1854. [Google Scholar] [CrossRef]
- Yadav, P.; Ellinghaus, D.; Rémy, G.; Freitag-Wolf, S.; Cesaro, A.; Degenhardt, F.; Boucher, G.; Delacre, M.; Peyrin-Biroulet, L.; Pichavant, M.; et al. Genetic factors interact with tobacco smoke to modify risk for inflammatory bowel disease in humans and mice. Gastroenterology 2017, 153, 550–565. [Google Scholar] [CrossRef]
- Ng, S.C.; Tang, W.; Leong, R.W.; Chen, M.; Ko, Y.; Studd, C.; Niewiadomski, O.; Bell, S.; Kamm, M.A.; de Silva, H.J.; et al. Environmental risk factors in inflammatory bowel disease: A populationbased case-control study in Asia-Pacific. Gut 2015, 64, 1063–1071. [Google Scholar] [CrossRef]
- Reif, S.; Klein, I.; Arber, N.; Gilat, T. Lack of association between smoking and inflammatory bowel disease in Jewish patients in Israel. Gastroenterology 1995, 108, 1683–1687. [Google Scholar] [CrossRef]
- Reif, S.; Lavy, A.; Keter, D.; Fich, A.; Eliakim, R.; Halak, A.; Broide, E.; Niv, Y.; Ron, Y.; Patz, J.; et al. Lack of association between smoking and Crohn’s disease but the usual association with ulcerative colitis in Jewish patients in Israel: A multicenter study. Am. J. Gastroenterol. 2000, 95, 474–478. [Google Scholar] [CrossRef]
- Xu, L.; Lochhead, P.; Ko, Y.; Claggett, B.; Leong, R.W.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 46, 780–789. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Guo, H.; Jabir, M.S.; Liu, X.; Cui, W.; Li, D. Associations between Folate and Vitamin B12 Levels and Inflammatory Bowel Disease: A Meta-Analysis. Nutrients 2017, 9, 382. [Google Scholar] [CrossRef]
- Yazdanyar, S.; Kamstrup, P.R.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Penetrance of NOD2/CARD15 genetic variants in the general population. CMAJ 2010, 182, 661–665. [Google Scholar] [CrossRef]
- Ortizo, R.; Lee, S.Y.; Nguyen, E.T.; Jamal, M.M.; Bechtold, M.M.; Nguyen, D.L. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: A meta-analysis of casecontrolled and cohort studies. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1064–1070. [Google Scholar] [CrossRef]
- Jones, D.T.; Osterman, M.T.; Bewtra, M.; Lewis, J.D. Passive smoking and inflammatory bowel disease: A meta-analysis. Am. J. Gastroenterol. 2008, 103, 2382–2393. [Google Scholar] [CrossRef]
- Nie, J.Y.; Zhao, Q. Beverage consumption and risk of ulcerative colitis: Systematic review and meta-analysis of epidemiological studies. Medicine 2017, 49, e9070. [Google Scholar] [CrossRef]
- Oz, H.S.; Chen, T.; de Villiers, W.J. Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef]
- Brückner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lügering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohns Colitis 2012, 6, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Jackson, T.; Sands, B.E.; Frisch, M.; Andersson, R.E.; Korzenik, J. The risk of developing Crohn’s disease after an appendectomy: A meta-analysis. Am. J. Gastroenterol. 2008, 103, 2925–2931. [Google Scholar] [CrossRef] [PubMed]
- Sahami, S.; Kooij, I.A.; Meijer, S.L.; Van den Brink, G.R.; Buskens, C.J.; Te Velde, A.A. The link between the appendix and ulcerative colitis: Clinical relevance and potential immunological mechanisms. Am. J. Gastroenterol. 2016, 111, 163–169. [Google Scholar] [CrossRef]
- Deng, P.; Wu, J. Meta-analysis of the association between appendiceal orifice inflammation and appendectomy and ulcerative colitis. Rev. Esp. Enferm. Dig. 2016, 108, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Vlachonikolis, I.G. Appendectomy and the development of ulcerative colitis: Results of a meta-analysis of published case-control studies. Am. J. Gastroenterol. 2000, 95, 171–176. [Google Scholar] [CrossRef]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nys, K.; Agostinis, P.; Vermeire, S. Autophagy: A new target or an old strategy for the treatment of Crohn’s disease? Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 395–401. [Google Scholar] [CrossRef]
- Cutolo, M.; Capellino, S.; Sulli, A.; Serioli, B.; Secchi, M.E.; Villaggio, B.; Straub, R.H. Estrogens and autoimmune diseases. Ann. N. Y. Acad. Sci. 2006, 1089, 538–547. [Google Scholar] [CrossRef]
- Upala, S.; Sanguankeo, A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: A systematic review and meta-analysis. Int. J. Obes. 2015, 39, 1197–1202. [Google Scholar] [CrossRef]
- Khan, M.F.; Wang, H. Environmental exposures and autoimmune diseases: Contribution of gut microbiome. Front. Immunol. 2020, 10, 3094. [Google Scholar] [CrossRef]
- Deng, Q.; Luo, Y.; Chang, C.; Wu, H.; Ding, Y.; Xiao, R. The emerging epigenetic role of CD8+ T cells in autoimmune diseases: A systematic review. Front. Immunol. 2019, 10, 856. [Google Scholar] [CrossRef]
- Xiong, H.F.; Wang, B.; Zhao, Z.H.; Hong, J.; Zhu, Y.; Zhou, X.; Xie, Y. Tonsillectomy and inflammatory bowel disease: A meta-analysis. Colorectal Dis. 2016, 18, 145–153. [Google Scholar] [CrossRef]
- M’Rabet, L.; Vos, A.P.; Boehm, G.; Garssen, J. Breast-feeding and its role in early development of the immune system in infants: Consequences for health later in life. J. Nutr. 2008, 138, 1782–1790. [Google Scholar] [CrossRef]
- Amre, D.K.; D’souza, S.; Morgan, K.; Seidman, G.; Lambrette, P.; Grimard, G.; Israel, D.; Mack, D.; Ghadirian, P.; Deslandres, C.; et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am. J. Gastroenterol. 2007, 102, 2016–2025. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; De Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.; Wang, W.; Zhang, D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 623–630. [Google Scholar] [CrossRef]
- Zeng, L.; Hu, S.; Chen, P.; Wei, W.; Tan, Y. Macronutrient intake and risk of Crohn’s disease: Systematic review and dose–response meta-analysis of epidemiological studies. Nutrients 2017, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, J.; Gao, Q.; Ma, M.; Lin, X.; Liu, J.; Li, J.; Zhao, Q. Carbohydrate and protein intake and risk of ulcerative colitis: Systematic review and dose-response meta-analysis of epidemiological studies. Clin. Nutr. 2017, 36, 1259–1265. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Li, F.; Zhang, D. Dietary fiber intake reduces risk of inflammatory bowel disease: Result from a meta-analysis. Nutr. Res. 2015, 35, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Meddings, J. The significance of the gut barrier in disease. Gut 2008, 57, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A.; Galiatsatos, P.; Xue, X. Systematic review and meta-analysis of lactose digestion, its impact on intolerance and nutritional effects of dairy food restriction in inflammatory bowel diseases. Nutr. J. 2016, 15, 67. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef]
- Chan, S.S.M.; Luben, R.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Lindgren, S.; Grip, O.; Bergmann, M.M.; Boeing, H.; Hallmans, G.; et al. Association between high dietary intake of the n-3 polyunsaturated fatty acid docosahexaenoic acid and reduced risk of Crohn’s disease. Aliment. Pharmacol. Ther. 2014, 39, 834–842. [Google Scholar] [CrossRef]
- De Silva, P.S.; Olsen, A.; Christensen, J.; Schmidt, E.B.; Overvaad, K.; Tjonneland, A.; Hart, A.R. An association between dietary arachidonic acid, measured in adipose tissue, and ulcerative colitis. Gastroenterology 2010, 139, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.; Davidson, L.A.; Ly, L.; Weeks, B.R.; Lupton, J.R.; McMurray, D.N. Immunomodulatory effects of (n-3) fatty acids: Putative link to inflammation and colon cancer. J. Nutr. 2007, 137, 200–204. [Google Scholar] [CrossRef]
- Chan, S.S.; Luben, R.; Van Schaik, F.; Oldenburg, B.; Bueno-de-Mesquita, H.B.; Hallmans, G.; Karling, P.; Lindgren, S.; Grip, O.; Key, T.; et al. Carbohydrate intake in the etiology of Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2014, 20, 2013–2021. [Google Scholar] [CrossRef]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron Ruault, M.C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef]
- Sadeghian, M.; Saneei, P.; Siassi, F.; Esmaillzadeh, A. Vitamin D status in relation to Crohn’s disease: Meta-analysis of observational studies. Nutrition 2016, 32, 505–514. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association between inflammatory bowel disease and vitamin D deficiency: A systematic review and meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef]
- Sturniolo, G.C.; Di Leo, V.; Ferronato, A.; D’Odorico, A.; D’Inca, R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm. Bowel Dis. 2001, 7, 94–98. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Chan, A.T. Zinc intake and risk of Crohn’s disease and ulcerative colitis: A prospective cohort study. Int. J. Epidemiol. 2015, 44, 1995–2005. [Google Scholar] [CrossRef]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef]
- Wang, F.; Lin, X.; Zhao, Q.; Li, J. Fat intake and risk of ulcerative colitis: Systematic review and dose-response meta-analysis of epidemiological studies. J. Gastroenterol. Hepatol. 2017, 32, 19–27. [Google Scholar] [CrossRef]
- Fabisiak, N.; Fabisiak, A.; Watala, C.; Fichna, J. Fat-soluble vitamin deficiencies and inflammatory bowel disease: Systematic review and meta-analysis. J. Clin. Gastroenterol. 2017, 51, 878–889. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Schmitt, J.; Schwarz, K.; Baurecht, H.; Hotze, M.; Fölster-Holst, R.; Rodríguez, E.; Lee, Y.A.; Franke, A.; Degenhardt, F.; Lieb, W.; et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J. Allergy Clin. Immunol. 2016, 137, 130–136. [Google Scholar] [CrossRef]
- D’Haens, G. Systematic review: Second-generation vs. conventional corticosteroids for induction of remission in ulcerative colitis. Aliment Pharmacol. Ther. 2016, 44, 1018–1029. [Google Scholar] [CrossRef]
- Klement, E.; Cohen, R.V.; Boxman, J.; Joseph, A.; Reif, S. Breastfeeding and risk of inflammatory bowel disease: A systematic review with meta-analysis. Am. J. Clin. Nutr. 2004, 80, 1342–1352. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, K.Q.; Qin, X.R.; Wang, X.Y. Association between physical activity and inflammatory bowel disease risk: A meta-analysis. Dig. Liver Dis. 2016, 48, 1425–1431. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; von Bergen, M.; et al. Gut microbiota disturbance during antibiotics therapy: A multi-omic approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Etminan, M.; Bird, S.T.; Delaney, J.A.; Bressler, B.; Brophy, J.M. Isotretinoin and risk for inflammatory bowel disease: A nested casecontrol study and meta-analysis of published and unpublished data. JAMA Dermatol. 2013, 149, 216–220. [Google Scholar] [CrossRef]


| Exposure | SNPs | Ancestry | Unit | F | Source |
|---|---|---|---|---|---|
| Drugs | |||||
| Antibiotics | 50 | European | SD | 97.99 | PMID |
| Isotretinoin | 17 | European | SD | 72.22 | PMID |
| Lifestyle | |||||
| Smoking | 102 | European | SD | 39.98 | GSCAN |
| Cigarettes per day | 33 | European | SD | 87.24 | GSCAN |
| Breastfeeding | 42 | European | SD | 70.52 | GSCAN |
| Alcoholic drinks | 42 | European | SD | 68.99 | GSCAN |
| Fruit intake | 71 | European | SD | 98.13 | MRC-IEU |
| Vegetable intake | 32 | European | SD | 102 | MRC-IEU |
| Coffee intake | 33 | European | SD | 123.14 | MRC-IEU |
| Tea intake | 23 | European | SD | 111.19 | MRC-IEU |
| Vitamin D level | 87 | European | SD | 76.17 | MRC-IEU |
| Cesarean delivery | 46 | European | NA | 66.57 | PMID |
| Physical activity | 56 | European | SD | 43.6 | SSGAC |
| Surgeries | |||||
| Appendectomy | 65 | European | NA | 97.99 | FinnGen |
| Tonsillectomy | 50 | European | NA | 90.24 | FinnGen |
| Related diseases | |||||
| Type 2 diabetes | 88 | European | logOR | 72.02 | FinnGen |
| Chronic kidney disease | 14 | European | NA | 58.26 | PMID |
| Autoimmune | 81 | European | NA | 111.1 | FinnGen |
| Celiac disease | 14 | European | logOR | 323.75 | PMID |
| Systemic lupus erythematosus | 23 | European | logOR | 88.61 | PMID |
| Blood parameters | |||||
| Calcium | 11 | European | SD | 300.13 | PMID |
| Lactose | 12 | European | SD | 95.09 | PMID |
| C-reactive protein | 11 | European | NA | 160.09 | PMID |
| Zinc | 117 | European | SD | 79.24 | PMID |
| Lipid metabolism | |||||
| n-3 PUFA | 103 | European | SD | 82.52 | PMID |
| Triglycerides | 114 | European | SD | 114.05 | UK Biobank |
| n-6 PUFA | 84 | European | SD | 97.55 | PMID |
| Total cholesterol | 36 | European | SD | 114.03 | UK Biobank |
| Apolipoprotein A-I | 42 | European | SD | 125.4 | UK Biobank |
| Glucose metabolism | |||||
| HbA1c | 42 | European | SD | 118.71 | PMID |
| Fasting insulin | 33 | European | SD | 95.93 | PMID |
| Fasting glucose | 54 | European | SD | 60.13 | PMID |
| Obesity traits | |||||
| Whole-body fat mass | 412 | European | SD | 55.36 | GIANT |
| BMI | 523 | European | SD | 48.78 | GIANT |
| Waist-to-hip ratio | 36 | European | SD | 49.15 | Neale Lab |
| Waist circumference | 67 | European | SD | 57.45 | Neale Lab |
| Hip circumference | 55 | European | SD | 52.89 | GIANT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadh, M.J.; Pal, R.S.; Arias-Gonzáles, J.L.; Orosco Gavilán, J.C.; JC, D.; Mohany, M.; Al-Rejaie, S.S.; Bahrami, A.; Kadham, M.J.; Amin, A.H.; et al. RETRACTED: A Mendelian Randomization Analysis Investigates Causal Associations between Inflammatory Bowel Diseases and Variable Risk Factors. Nutrients 2023, 15, 1202. https://doi.org/10.3390/nu15051202
Saadh MJ, Pal RS, Arias-Gonzáles JL, Orosco Gavilán JC, JC D, Mohany M, Al-Rejaie SS, Bahrami A, Kadham MJ, Amin AH, et al. RETRACTED: A Mendelian Randomization Analysis Investigates Causal Associations between Inflammatory Bowel Diseases and Variable Risk Factors. Nutrients. 2023; 15(5):1202. https://doi.org/10.3390/nu15051202
Chicago/Turabian StyleSaadh, Mohamed J., Rashmi Saxena Pal, José Luis Arias-Gonzáles, Juan Carlos Orosco Gavilán, Darshan JC, Mohamed Mohany, Salim S. Al-Rejaie, Abolfazl Bahrami, Mustafa Jawad Kadham, Ali H. Amin, and et al. 2023. "RETRACTED: A Mendelian Randomization Analysis Investigates Causal Associations between Inflammatory Bowel Diseases and Variable Risk Factors" Nutrients 15, no. 5: 1202. https://doi.org/10.3390/nu15051202
APA StyleSaadh, M. J., Pal, R. S., Arias-Gonzáles, J. L., Orosco Gavilán, J. C., JC, D., Mohany, M., Al-Rejaie, S. S., Bahrami, A., Kadham, M. J., Amin, A. H., & Georgia, H. (2023). RETRACTED: A Mendelian Randomization Analysis Investigates Causal Associations between Inflammatory Bowel Diseases and Variable Risk Factors. Nutrients, 15(5), 1202. https://doi.org/10.3390/nu15051202









