Macauba (Acrocomia aculeata) Pulp Oil Prevents Adipogenesis, Inflammation and Oxidative Stress in Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Chemical Characterization of Macauba Pulp Oil
2.3. Animals and Experimental Design
2.4. Biochemical Analysis
2.5. Homogenate Preparation and Oxidative Stress Levels
2.6. Total Antioxidant Capacity of Serum and Liver
2.7. PPAR-γ, PPAR-α, NF-κB, and TLR-4 Quantification
2.8. Determination of Gene Expression in Adipose Tissue and Liver by Reverse Transcriptase Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Histomorphometric Analysis of Adipose and Liver Tissues
2.10. Statistical Analyses
3. Results
3.1. Chemical Characterization of Macauba Pulp Oil
3.2. Effects of Macauba Pulp Oil on Biometric Measures, Food Intake, and Lipid Profile
3.3. Total Antioxidant Capacity and Oxidative Stress Marker Levels in Mice
3.4. Effects of Macauba Pulp Oil on NF-κB, TLR-4, and PPAR-(α, γ) Quantification
3.5. Effects of Macauba Pulp Oil on Gene Expression in Adipose and Hepatic Tissues
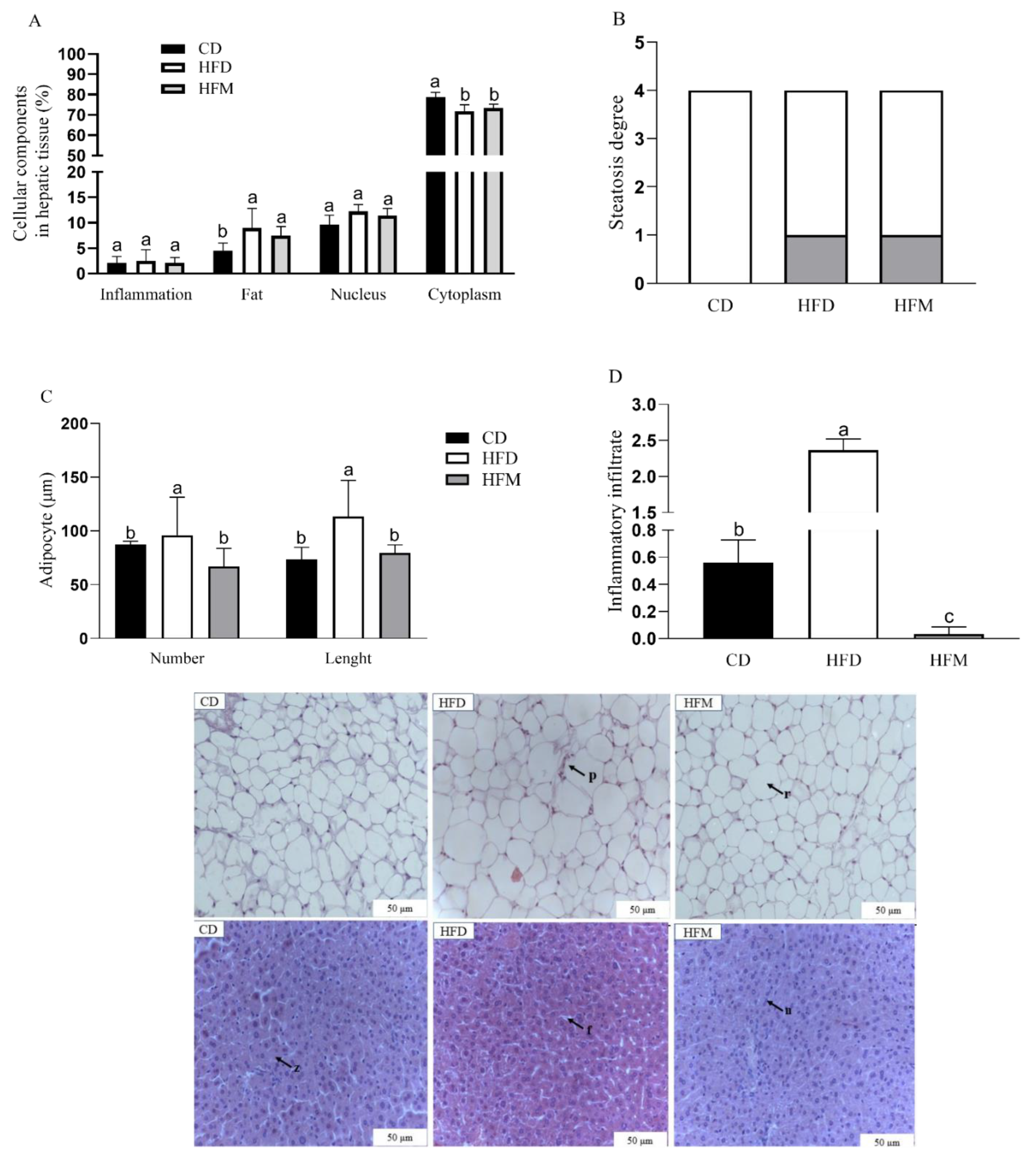
3.6. Effects of Macauba Pulp Oil on Histological Morphometrics of Liver and Adipose Tissues
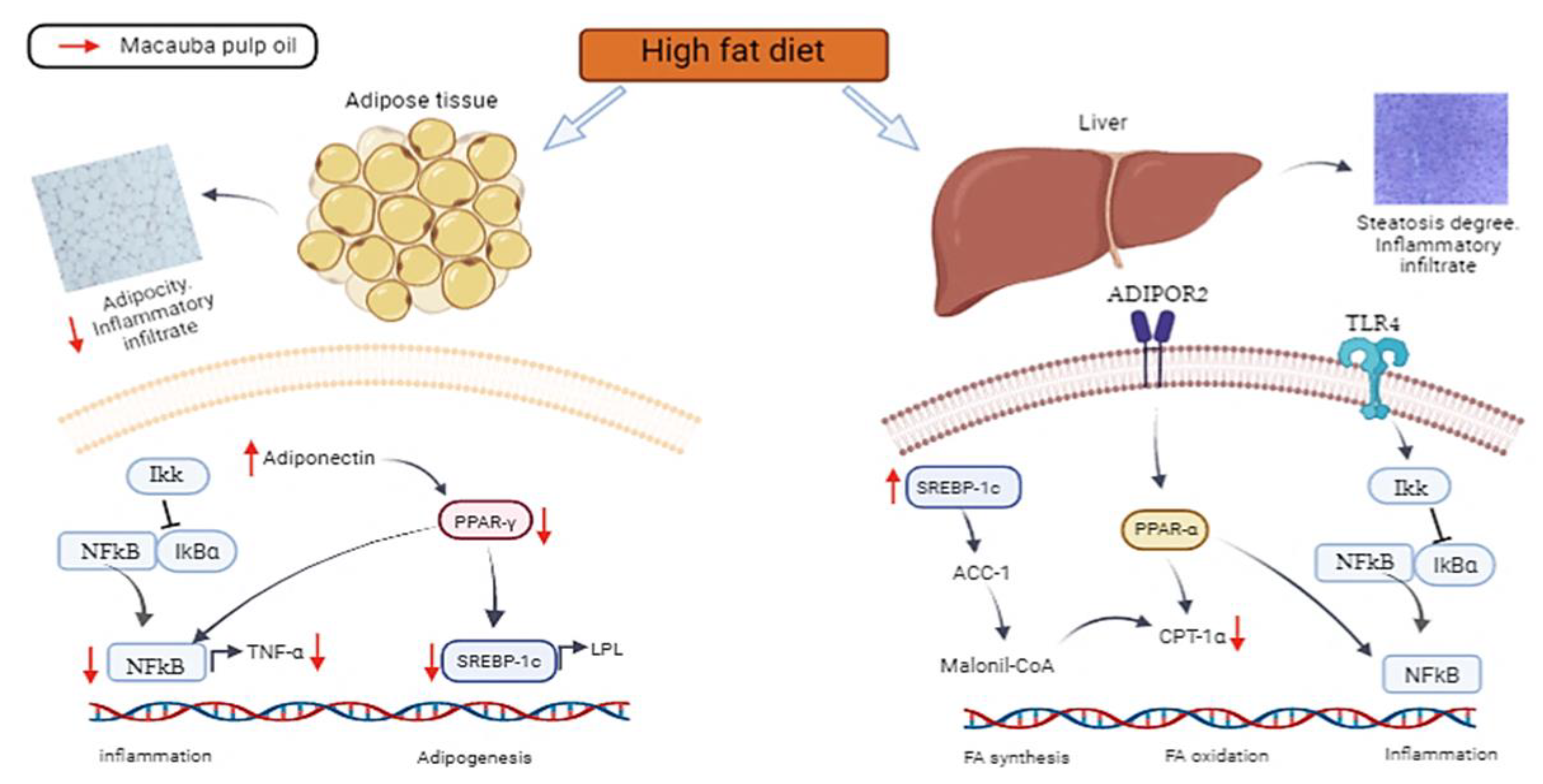
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, WHO. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 27 July 2020).
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A complex interplay of multiple molecular determinants and pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Schmidt, H.; Lai, B.; Ge, K. Transcriptional and epigenomic regulation of adipogenesis. Mol. Cell. Biol. 2019, 39, e11. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Gougeon, R.; Thibault, L. A highly saturated fat-rich diet is more obesogenic than diets with lower saturated fat content. Nutr. Res. 2010, 30, 632–643. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Jorge, N. Characterization of the pulp and kernel oils from Syagrus oleracea, Syagrus romanzoffiana, and Acrocomia aculeata. J. Food Sci. 2011, 76, C1156–C1161. [Google Scholar] [CrossRef]
- Lieb, V.M.; Schex, R.; Esquivel, P.; Jiménez, V.M.; Schmarr, H.G.; Carle, R.; Steingass, C.B. Fatty acids and triacylglycerols in the mesocarp and kernel oils of maturing Costa Rican Acrocomia aculeata fruits. NFS J. 2019, 14–15, 6–13. [Google Scholar] [CrossRef]
- Díaz, A.C.; Fiñana, I.T.; Granados, J.M.M.; Méndez, M.V.R.; Dorado, G.; Sánchez, M.C.R.; Valverde, C.N.; Gómez, J.M.Q. Serum from postmenopausal women treated with a by-product of olive-oil extraction process stimulates osteoblastogenesis and inhibits adipogenesis in human mesenchymal stem-cells (MSC). Exp. Gerontol. 2017, 90, 71–78. [Google Scholar] [CrossRef]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef]
- Müller, L.; Theile, K.; Böhm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1578810728. [Google Scholar]
- Pinheiro-Sant’Ana, H.M.; Guinazi, M.; Da Silva, D.O.; Della Lucia, C.M.; De Lazzari, B.R.; Brandão, S.C.C. Method for simultaneous analysis of eight vitamin E isomers in various foods by high performance liquid chromatography and fluorescence detection. J. Chromatogr. A 2011, 1218, 8496–8502. [Google Scholar] [CrossRef]
- Fontelles, M.J. Metodologias da pesquisa: Diretrizes para o cálculo do tamanho da amostra. Rev. Para. Med. 2010, 24, 57–64. [Google Scholar]
- Schoemaker, M.H.; Kleemann, R.; Morrison, M.C.; Verheij, J.; Salic, K.; Van Tol, E.A.F.; Kooistra, T.; Weilinga, P.Y. A casein hydrolysate based formulation attenuates obesity and associated non-alcoholic fatty liver disease and atherosclerosis in LDLr-/- Leiden mice. PLoS ONE 2017, 12, e0180648. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Novelli Filho, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Kim, S.; Hong, J.; Jeon, R.; Kim, H.S. Adzuki bean ameliorates hepatic lipogenesis and proinflammatory mediator expression in mice fed a high-cholesterol and high-fat diet to induce nonalcoholic fatty liver disease. Nutr. Res. 2016, 36, 90–100. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Marklund, S. Pyrogallol autooxidation. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1985; pp. 243–247. [Google Scholar]
- Santos-López, J.A.; Garcimartín, A.; López-Oliva, M.E.; Bautista-Ávila, M.; González-Muñoz, M.J.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Chia Oil–Enriched Restructured Pork Effects on Oxidative and Inflammatory Status of Aged Rats Fed High Cholesterol/High Fat Diets. J. Med. Food. 2017, 20, 526–534. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analyses of nitrate, nitrite and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DeltaCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cupertino, M.C.; Costa, K.L.C.; Santos, D.C.M.; Novaes, R.D.; Condessa, S.S.; Neves, A.C.; Oliveira, J.A.; Matta, S.L.P. Long-lasting morphofunctional remodelling of liver parenchyma and stroma after a single exposure to low and moderate doses of cadmium in rats. Int. J. Exp. Pathol. 2013, 94, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Turlin, B.; Mendler, M.H.; Moirand, R.; Guyader, G.; Guillygomarc’h, A.; Deugnier, Y. Histologic features of the liver in insulin resistance–associated Iron overload: A study of 139 patients. Am. J. Clin. Pathol. 2001, 116, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Torales, L.I.; García-Alonso, J.; Piriago-Castón, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Chen, Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Pal, P.K.; Chattopadhyay, A.; Bandyopadhyay, D. Oleic acid protects against cadmium induced cardiac and hepatic tissue injury in male Wistar rats: A mechanistic study. Life Sci. 2020, 244, 117324. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Naguibi, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Schnorr, C.E.; Morrone, M.S.; Simões-Pires, A.; Bittencourt, L.D.; Zeidán-Chuliá, F.; Moreira, J.C.F. Supplementation of adult rats with moderate amounts of β–carotene modulates the redox status in plasma without exerting pro-oxidant effects in the brain: A safer alternative to food fortification with vitamin A? Nutrients 2014, 6, 5572–5582. [Google Scholar] [CrossRef]
- Sarada, S.; Dipti, P.; Anju, B.; Pauline, T.; Kain, A.; Sairam, M.; Sharma, S.; Ilavazhagan, G.; Kumar, D.; Selvamurthy, W. Antioxidant effect of β-carotene on hypoxia induced oxidative stress in male albino rats. J. Ethnopharmacol. 2002, 79, 149–153. [Google Scholar] [CrossRef]
- Pan, J.H.; Kim, M.J.; Kim, J.H.; Cho, Y.J.; Shin, H.S.; Sung, J.S.; Park, T.S.; Yoon, H.G.; Park, S.; Kim, Y.J. Inhibition of the lipogenesis in liver and adipose tissue of diet-induced obese C57BL/6 mice by feeding oleic acid-rich sesame oil. Food Sci. Biotechnol. 2015, 24, 1115–1121. [Google Scholar] [CrossRef]
- Ribot, J.; Felipe, F.; Bonet, M.L.; Palou, A. Changes of adiposity in response to Vitamin A status correlate with changes of PPARγ2 expression. Obes. Res. 2001, 9, 500–509. [Google Scholar] [CrossRef]
- Arankumar, E.A.; Sushil, K.J. Adiponectin, a therapeutic target for obesity, diabetes and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Lobo, G.P.; Amengual, J.; Li, H.N.; Golczak, M.; Bonet, M.L.; Palczewski, K.; von Lintig, J. β-carotene decreases peroxisome proliferator gamma activity and reduces lipid storage capacity of adipocytes in a β-carotene oxygenase 1-dependent manner. J. Biol. Chen. 2010, 285, 27891–27899. [Google Scholar] [CrossRef]
- Amengual, J.; Gouranton, E.; Van Helden, Y.G.; Hessel, S.; Ribot, J.; Kramer, E.; Kiec-Wilk, B.; Razny, U.; Lietz, G.; Wyss, A.; et al. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE 2011, 6, e20644. [Google Scholar] [CrossRef]
- Granados, N.; Amengual, J.; Ribot, J.; Palou, A.; Bonet, M.L. Distinct effects of oleic acid and its trans-isomer elaidic acid on the expression of myokines and adipokines in cell models. Br. J. Nutr. 2011, 105, 1226–1234. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA damage, and development of obesity-related diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Lubberts, E.; Alarcon-De-La-Lastra, C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef]
- Morari, J.; Torsoni, A.S.; Anhê, G.F.; Roman, E.A.; Cintra, D.E.; Ward, L.S.; Bordin, S.; Velloso, L.A. The role of proliferator-activated receptor gamma coactivator-1alpha in the fatty-acid-dependent transcriptional control of interleukin-10 in hepatic cells of rodents. Metabolism 2010, 59, 215–223. [Google Scholar] [CrossRef]
- Wang, Y.; Park, N.Y.; Jang, Y.; Ma, A.; Jiang, Q. Vitamin E γ-tocotrienol inhibits cytokine-stimulated NF-κB activation by induction of anti-inflammatory A20 via stress adaptive response due to modulation of sphingolipids. J. Immunol. 2015, 1195, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.-K.; Lee, S.-J.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Lee, H.; Kwon, Y.-G.; Chung, C.-K.; Kim, Y.-M. β-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-κB activation. Exp. Mol. Med. 2005, 37, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Moon, K.H.; Hardwick, J.P.; Gonzalez, F.J.; Song, B.J. Role of peroxisome proliferator-activated receptor-α in fasting-mediated oxidative stress. Free Radic. Biol. Med. 2010, 47, 767–778. [Google Scholar] [CrossRef]
- Jeon, T.L.; Osborne, T.F. SREBPs: Metabolic integrators in physiology and metabolism. Trends Endocrinol. Metab. 2012, 23, 65–72. [Google Scholar] [CrossRef]



| Ingredients (g/kg) | CD | HFD | HFM |
|---|---|---|---|
| Albumin * | 179.71 | 179.71 | 179.71 |
| Dextrinized starch | 155 | 155 | 155 |
| Sucrose | 100 | 100 | 100 |
| Soybean oil | 40 | 40 | - |
| Lard | 0 | 312 | 312 |
| Cellulose | 50 | 50 | 50 |
| Mineral mix | 35 | 35 | 35 |
| Vitamin mix | 10 | 10 | 10 |
| L-cystine | 1.8 | 1.8 | 1.8 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Corn starch | 425.99 | 113.99 | 113.99 |
| Macauba pulp oil | - | - | 40 |
| Carbohydrate (%) | 76.9 | 44.1 | 44.1 |
| Protein (%) | 18.9 | 18.9 | 18.9 |
| Lipids (%) | 4.20 | 37 | 37 |
| Caloric density (kcal g−1) | 3.85 | 5.41 | 5.41 |
| Genes | Forward | Reverse |
|---|---|---|
| SREBP-1c | CGC TAC CGT TCC TCT ATC AAT GAC | AGT TTC TGG TTG CTG TGC TGT AAG |
| ADIPOR2 | CAT GTT TGC CAC CCC TCA GTA | ATG CAA GGT AGG GAT TCC A |
| ACC-1 | TCA AGA CGG CTC AGG TCA TCA | AGG CGC CAA ACT TCA GCA TC |
| CPT-1α | GTA AGG CCA CTG ATG AAG GAA GA | ATT TGG GTC CGA GGT TGA CA |
| LPL | TCA ACC ACA GCA GCA AGA | CCG ATA CAA CCA GTC TAC TAC AA |
| Adiponectin | ATG AGT ACC AGA CTA ATG AGA C | GGC AGG ATT AAG AGG AAC A |
| TNF-α | TAT GGC TCA GGG TCC AAC TC | GCT CCA GTG AAT TCG GAA AG |
| SREBP-1c | GCC GAG ATG TGC GAA CTG | GGA AGT CAC TGT CTT GGT TGT T |
| β-actin | TTC GTT GCC GGT CCA CC | GCT TTG CAC ATG CCG GAG CC |
| GAPDH | AGG TTG TCT CCT GTC ACT TC | CTG TTG CTG TAG CCA TAT TC |
| Components | |
|---|---|
| Palmitic (C16:0) | 22.84% |
| Palmitoleic (C16:1) | 5.93% |
| Stearic (C18:0) | 1.23% |
| Oleic (C18:1n9c) | 49.32% |
| Linoleic (C18:2n6c) | 19.63% |
| Linolenic (C18:3n6c) | 1.05% |
| Oleic acid (mg/g) | 199.00 |
| Tocopherol (μg/g) | 40.80 |
| Total carotenoids (μg/g) | 207.52 |
| β-carotene (μg/g) | 163.63 |
| α-carotene (μg/g) | 21.03 |
| Lutein (μg/g) | 8.75 |
| Lycopene (μg/g) | 14.11 |
| CD | HFD | HFM | |
|---|---|---|---|
| Weight gain (g) | 4.01 ± 1.74 a | 4.14 ± 2.23 a | 3.66 ± 2.23 a |
| BMI (g/cm2) | 0.34 ± 0.02 a | 0.33 ± 0.01 a | 0.33 ± 0.02 a |
| Adiposity (%) | 0.71 ± 0.24 b | 2.43 ± 1.28 a | 2.26 ± 1.25 a |
| Food consumption (g/day) | 4.07 ± 0.16 a | 2.53 ± 0.41 b | 2.63 ± 0.42 b |
| Food efficiency (%) | 1.69 ± 0.60 b | 2.67 ± 1.46 a | 2.42 ± 1.55 a |
| Hepatosomatic index (%) | 3.61 ± 0.29 a | 3.72 ± 0.28 a | 3.48 ± 0.29 a |
| Oleic acid intake (mg/day) | - | - | 0.52 ± 0.08 |
| Carotenoid intake (µg/day) | - | - | 21.96 ± 4.11 |
| Tocopherol intake (mg/day) | 0.46 ± 0.01 a | 0.29 ± 0.03 b | 0.31 ± 0.03 b |
| Total cholesterol (mg dL−1) | 151.48 ± 13.79 b | 166.49 ± 15.51 a | 179.91 ± 6.87 a |
| Total triglycerides (mg dL−1) | 79.91 ± 4.71 a | 84.83 ± 5.63 a | 83.06 ± 5.09 a |
| HDL-c (mg dL−1) | 38.13 ± 4.29 a | 37.35 ± 5.79 a | 43.07 ± 5.64 a |
| LDL-c (mg dL−1) | 12.80 ± 2.11 b | 20.64 ± 5.25 a | 20.80 ± 5.20 a |
| Glucose (mg dL−1) | 160.67 ± 44.23 a | 182.58 ± 30.09 a | 197.31 ± 36.68 a |
| AST (mg dL−1) | 88.14 ± 21.88 a | 71.66 ± 21.30 a | 73.39 ± 19.04 a |
| ALT (mg dL−1) | 18.74 ± 9.88 a | 15.71 ± 5.63 a | 18.84 ± 9.26 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sant’ Ana, C.T.; Agrizzi Verediano, T.; Grancieri, M.; Toledo, R.C.L.; Tako, E.; Costa, N.M.B.; Martino, H.S.D.; de Barros, F.A.R. Macauba (Acrocomia aculeata) Pulp Oil Prevents Adipogenesis, Inflammation and Oxidative Stress in Mice Fed a High-Fat Diet. Nutrients 2023, 15, 1252. https://doi.org/10.3390/nu15051252
Sant’ Ana CT, Agrizzi Verediano T, Grancieri M, Toledo RCL, Tako E, Costa NMB, Martino HSD, de Barros FAR. Macauba (Acrocomia aculeata) Pulp Oil Prevents Adipogenesis, Inflammation and Oxidative Stress in Mice Fed a High-Fat Diet. Nutrients. 2023; 15(5):1252. https://doi.org/10.3390/nu15051252
Chicago/Turabian StyleSant’ Ana, Cíntia Tomaz, Thaísa Agrizzi Verediano, Mariana Grancieri, Renata Celi Lopes Toledo, Elad Tako, Neuza Maria Brunoro Costa, Hércia Stampini Duarte Martino, and Frederico Augusto Ribeiro de Barros. 2023. "Macauba (Acrocomia aculeata) Pulp Oil Prevents Adipogenesis, Inflammation and Oxidative Stress in Mice Fed a High-Fat Diet" Nutrients 15, no. 5: 1252. https://doi.org/10.3390/nu15051252
APA StyleSant’ Ana, C. T., Agrizzi Verediano, T., Grancieri, M., Toledo, R. C. L., Tako, E., Costa, N. M. B., Martino, H. S. D., & de Barros, F. A. R. (2023). Macauba (Acrocomia aculeata) Pulp Oil Prevents Adipogenesis, Inflammation and Oxidative Stress in Mice Fed a High-Fat Diet. Nutrients, 15(5), 1252. https://doi.org/10.3390/nu15051252








