Coeliac Disease Case–Control Study: Has the Time Come to Explore beyond Patients at Risk?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Analysis and Management
2.3. Statistical Analysis
2.4. Ethics and Approvals
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Wessels, M. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Mäki, M.; Kallonen, K.; Lähdeaho, M.L.; Visakorpi, J.K. Changing Pattern of Childhood Coeliac Disease in Finland. Acta Pediatr. Scand. 1988, 77, 408–412. [Google Scholar] [CrossRef]
- Popp, A.; Mäki, M. Changing pattern of childhood celiac disease epidemiology: Contributing factors. Front. Pediatr. 2019, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Rutz, R.; Ritzler, E.; Fierz, W.; Herzog, D. Prevalence of asymptomatic celiac disease in adolescents of eastern Switzerland. Swiss Med. Wkly. 2002, 132, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Pes, G.M.; Usai-Satta, P.; Salis, R.; Soro, S.; Colosso, B.M.Q.; Dore, M.P. Chronic autoimmune disorders are increased in coeliac disease. Medicine 2017, 96, e8562. [Google Scholar] [CrossRef]
- Neuhausen, S.L.; Steele, L.; Ryan, S.; Mousavi, M.; Pinto, M.; Osann, K.E.; Zone, J.J. Co-occurrence of celiac disease and other autoimmune diseases in celiacs and their first-degree relatives. J. Autoimmun. 2008, 31, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, L.; Wijmenga, C.; Murray, J.A.; Ludvigsson, J.F. Autoimmune Disease in First-Degree Relatives and Spouses of Individuals with Celiac Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 1271–1277. [Google Scholar] [CrossRef]
- Kahaly, G.; Frommer, L.; Schuppan, D. Celiac Disease and Glandular Autoimmunity. Nutrients 2018, 10, 814. [Google Scholar] [CrossRef]
- Stahl, M.G.; Geno Rasmussen, C.; Dong, F.; Waugh, K.; Norris, J.M.; Baxter, J.; ASK Study Group. Mass Screening for Celiac Disease: The Autoimmunity Screening for Kids Study. Am. J. Gastroenterol. 2021, 116, 180–187. [Google Scholar] [CrossRef]
- Kivelä, L.; Kaukinen, K.; Huhtala, H.; Lähdeaho, M.L.; Mäki, M.; Kurppa, K. At-Risk Screened Children with Celiac Disease are Comparable in Disease Severity and Dietary Adherence to Those Found because of Clinical Suspicion: A Large Cohort Study. J. Pediatr. 2017, 183, 115–121. [Google Scholar] [CrossRef]
- Paul, S.P.; Sandhu, B.K.; Spray, C.H.; Basude, D.; Ramani, P. Evidence Supporting Serology-based Pathway for Diagnosing Celiac Disease in Asymptomatic Children from High-risk Groups. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Verdu, E.F.; Bai, J.C.; Lionetti, E. Coeliac disease. Lancet 2022, 399, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Laurikka, P.; Kivelä, L.; Kurppa, K.; Kaukinen, K. Review article: Systemic consequences of coeliac disease. Aliment. Pharmacol. Ther. 2022, 56 (Suppl. S1), S64–S72. [Google Scholar] [CrossRef]
- Solís, D.P.; Pascual, M.L.C.; Sangrador, C.O.; Burriel, J.I.G.; Visus, F.S.V.; Arocena, F.J.E.; Riechmann, E.R. Spanish National Registry of Paediatric Coeliac Disease: Changes in the Clinical Presentation in the 21st Century. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Walker-Smith, J.A. Revised criteria for diagnosis of coeliac disease Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch. Dis. Child. 1990, 65, 909–911. [Google Scholar]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; ESPGHAN Gastroenterology Committee. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- De Onis, M. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Bonamico, M.; Nenna, R. Implications of mass screening for childhood celiac disease. Ped. Health 2009, 3, 413–415. [Google Scholar] [CrossRef]
- Krzywicka, B.; Herman, K.; Kowalczyk-Zając, M.; Pytrus, T. Celiac Disease and Its Impact on the Oral Health Status—Review of the Literature. Adv. Clin. Exp. Med. 2014, 23, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Pellerin, G.; Mager, D. Prevalence of Metabolic Bone Disease in Children with Celiac Disease Is Independent of Symptoms at Diagnosis. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 589–593. [Google Scholar] [CrossRef]
- Björck, S.; Brundin, C.; Karlsson, M.; Agardh, D. Reduced Bone Mineral Density in Children with Screening-detected Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 526–532. [Google Scholar] [CrossRef]
- Verkasalo, M.A.; Raitakari, O.T.; Viikari, J.; Marniemi, J.; Savilahti, E. Undiagnosed silent coeliac disease: A risk for underachievement? Scand. J. Gastroenterol. 2005, 40, 1407–1412. [Google Scholar] [CrossRef]
- Nurminen, S.; Kivelä, L.; Taavela, J.; Huhtala, H.; Mäki, M.; Kaukinen, K.; Kurppa, K. Factors associated with growth disturbance at celiac disease diagnosis in children: A retrospective cohort study. BMC Gastroenterol. 2015, 15, 125. [Google Scholar] [CrossRef]
- Bingley, P.J.; Norcross, A.J.; Lock, R.J.; Ness, A.R.; Jones, R.W. Undiagnosed coeliac disease at age seven: Population based prospective birth cohort study. BMJ 2004, 328, 322–323. [Google Scholar] [CrossRef]
- Auricchio, R.; Stellato, P.; Bruzzese, D.; Cielo, D.; Chiurazzi, A.; Galatola, M.; Greco, L. Growth rate of coeliac children is compromised before the onset of the disease. Arch. Dis. Child. 2020, 105, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, L.; Popp, A.; Arvola, T.; Huhtala, H.; Kaukinen, K.; Kurppa, K. Long-term health and treatment outcomes in adult coeliac disease patients diagnosed by screening in childhood. United Eur. Gastroenterol. J. 2018, 6, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Van Koppen, E.J.; Schweizer, J.J.; Csizmadia, C.G.; Krom, Y.; Hylkema, H.B.; Van Geel, A.M.; Koopman, H.M.; Verloove-Vanhorick, S.P.; Mearin, M.L. Long-term health and quality-of-life consequences of mass screening for childhood celiac disease: A 10-year follow-up study. Pediatrics 2009, 123, e582–e588. [Google Scholar] [CrossRef]
- Kinos, S.; Kurppa, K.; Ukkola, A.; Collin, P.; Lähdeaho, M.L.; Huhtala, H.; Kekkonen, L.; Mäki, M.; Kaukinen, K. Burden of illness in screen-detected children with celiac disease and their families. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 412–416. [Google Scholar] [CrossRef]
- Oliveira, G.N.; Mohan, R.; Fagbemi, A. Review of celiac disease presentation in a pediatric tertiary centre. Arq. De Gastroenterol. 2018, 55, 86–93. [Google Scholar] [CrossRef]
- Al Sarkhy, A.; El Mouzan, M.I.; Saeed, E.; Alanazi, A.; Alghamdi, S.; Anil, S.; Assiri, A. Clinical characteristics of celiac disease and dietary adherence to gluten-free diet among Saudi children. J. Pediatr. Gastroenterol. Hepatol. Nutr. 2015, 18, 23–29. [Google Scholar] [CrossRef]
- Spijkerman, M.; Tan, I.L.; Kolkman, J.J.; Withoff, S.; Wijmenga, C.; Visschedijk, M.C.; Weersma, R.K. A large variety of clinical features and concomitant disorders in celiac disease—A cohort study in the Netherlands. Dig. Liver Dis. 2016, 48, 499–505. [Google Scholar] [CrossRef]
- Kho, A. Coeliac disease in children in Christchurch, New Zealand: Presentation and patterns from 2000–2010. World J. Clin. Pediatr. 2015, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Riznik, P.; De Leo, L.; Dolinsek, J.; Gyimesi, J.; Klemenak, M.; Koletzko, B.; Koletzko, S.; Korponay-Szabó, I.R.; Krencnik, T.; Not, T. Clinical Presentation in Children With Coeliac Disease in Central Europe. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Ravikumara, M.; Nootigattu, V.; Sandhu, B. Ninety Percent of Celiac Disease Is Being Missed. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 497–499. [Google Scholar] [CrossRef]
- Whitburn, J.; Rao, S.R.; Paul, S.P.; Sandhu, B.K. Diagnosis of celiac disease is being missed in over 80% of children particularly in those from socioeconomically deprived backgrounds. Eur. J. Pediatr. 2021, 180, 1941–1946. [Google Scholar] [CrossRef]
- Kvamme, J.M.; Sørbye, S.; Florholmen, J.; Halstensen, T.S. Population-based screening for celiac disease reveals that the majority of patients are undiagnosed and improve on a gluten-free diet. Sci. Rep. 2022, 12, 12647. [Google Scholar] [CrossRef]
- Mårild, K.; Söderling, J.; Bozorg, S.R.; Everhov, Å.H.; Lebwohl, B.; Green, P.H.; Neovius, M.; Ludvigsson, J.F. Costs and Use of Health Care in Patients with Celiac Disease: A Population-Based Longitudinal Study. Am. J. Gastroenterol. 2020, 115, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, A.; Kurppa, K.; Collin, P.; Huhtala, H.; Forma, L.; Kekkonen, L.; Mäki, M.; Kaukinen, K. Use of health care services and pharmaceutical agents in coeliac disease: A prospective nationwide study. BMC Gastroenterol. 2012, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, V.; Kurppa, K.; Huhtala, H.; Mäki, M.; Kekkonen, L.; Kaukinen, K. Delayed celiac disease diagnosis predisposes to reduced quality of life and incremental use of health care services and medicines: A prospective nationwide study. United Eur. Gastroenterol. J. 2018, 6, 567–575. [Google Scholar] [CrossRef]
- Mattila, E.; Kurppa, K.; Ukkola, A.; Collin, P.; Huhtala, H.; Forma, L.; Lähdeaho, M.L.; Kekkonen, L.; Mäki, M.; Kaukinen, K. Burden of illness and use of health care services before and after celiac disease diagnosis in children. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Mearns, E.S.; Taylor, A.; Boulanger, T.; Craig, K.J.; Gerber, M.; Leffler, D.A.; Lebwohl, B. Systematic Literature Review of the Economic Burden of Celiac Disease. Pharmacoeconomics 2019, 37, 45–61. [Google Scholar] [CrossRef]
- Norström, F.; Lindholm, L.; Sandström, O.; Nordyke, K.; Ivarsson, A. Delay to celiac disease diagnosis and its implications for health-related quality of life. BMC Gastroenterol. 2011, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Card, T.R.; Kaukinen, K.; Bai, J.; Zingone, F.; Sanders, D.S.; Murray, J.A. Screening for celiac disease in the general population and in high-risk groups. United Eur. Gastroenterol. J. 2015, 3, 106–120. [Google Scholar] [CrossRef]
- Norström, F.; Myléus, A.; Nordyke, K.; Carlsson, A.; Högberg, L.; Sandström, O.; Lindholm, L. Is mass screening for coeliac disease a wise use of resources? A health economic evaluation. BMC Gastroenterol. 2021, 21, 159. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.; Thom, H.; Sheppard, A.L.; Keeney, E.; O’Donnell, R.; Jackson, J.; Whiting, P.F. Defining the optimum strategy for identifying adults and children with coeliac disease: Systematic review and economic modelling. Health Technol. Assess. 2022, 26, 1–310. [Google Scholar] [CrossRef]
- Auricchio, R.; Tosco, A.; Piccolo, E.; Galatola, M.; Izzo, V.; Maglio, M.; Greco, L. Potential Celiac Children: 9-Year Follow-Up on a Gluten-Containing Diet. Am. J. Gastroenterol. 2014, 109, 913–921. [Google Scholar] [CrossRef]
- Auricchio, R.; Mandile, R.; Del Vecchio, M.R.; Scapaticci, S.; Galatola, M.; Maglio, M.; Greco, L. Progression of Celiac Disease in Children with Antibodies against Tissue Transglutaminase and Normal Duodenal Architecture. Gastroenterology 2019, 157, 413–420. [Google Scholar] [CrossRef]
- Sakhuja, S.; Holtz, L.R. Progression of pediatric celiac disease from potential celiac disease to celiac disease: A retrospective cohort study. BMC Pediatr. 2021, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Castellaneta, S.; Francavilla, R.; Pulvirenti, A.; Catassi, G.N.; Catassi, C. Long-Term Outcome of Potential Celiac Disease in Genetically at-Risk Children: The Prospective CELIPREV Cohort Study. J. Clin. Med. 2019, 8, 186. [Google Scholar] [CrossRef]
- Sandström, O.; Norström, F.; Carlsson, A.; Högberg, L.; Van der Palz, M.; Stenhammar, L.; Myléus, A. Five-year follow-up of new cases after a coeliac disease mass screening. Arch. Dis. Child. 2022, 107, 596–600. [Google Scholar] [CrossRef]
- Iorfida, D.; Valitutti, F.; Vestri, A.; Di Rocco, A.; Cucchiara, S.; Lubrano, R.; Montuori, M. Dietary Compliance and Quality of Life in Celiac Disease: A Long-Term Follow-Up of Primary School Screening-Detected Patients. Front. Pediatr. 2021, 9, 787938. [Google Scholar] [CrossRef]
- Fabiani, E.; Taccari, L.M.; Rätsch, I.M.; Di Giuseppe, S.; Coppa, G.V.; Catassi, C. Compliance with gluten-free diet in adolescents with screening-detected celiac disease: A 5-year follow-up study. J. Pediatr. 2000, 136, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Germone, M.; Phu, T.; Slosky, C.; Pan, Z.; Jones, A.; Stahl, M.; Mehta, P.; Shull, M.; Ariefdjohan, M.; Liu, E. Anxiety and Depression in Pediatric Patients with Celiac Disease: A Large Cross-Sectional Study. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.E.; Morrison-Rees, S.; Thapar, N.; Benninga, M.A.; Borrelli, O.; Broekaert, I.; Williams, J.G. Systematic review and meta-analysis: The incidence and prevalence of paediatric coeliac disease across Europe. Aliment. Pharmacol. Ther. 2021, 54, 109–128. [Google Scholar] [CrossRef] [PubMed]
| Cases (n = 468) Asymptomatic at Diagnosis | Controls (n = 468) Symptomatic at Diagnosis | p-Value | |
|---|---|---|---|
| Background information | |||
| Male sex | 199 (42.5%) | 199 (42.5%) | NS |
| Age (years) | 8.2 (5.3–11.4) | 7.8 (4.8–11.2) | NS |
| Delivery type (C section) | 87 (18.6) | 107 (22.9) | NS |
| Breastfeeding | 103 (76.3) | 87 (79.2) | NS |
| Rotavirus vaccination | 69 (14.7) | 73 (15.6) | NS |
| Age at gluten introduction (months) | 6 (6–8) | 6.5 (6–8) | NS |
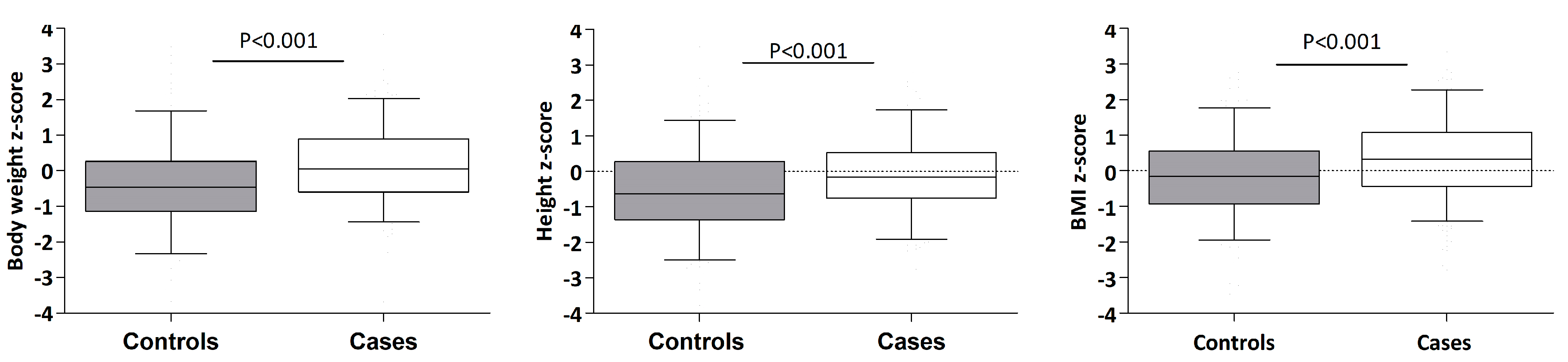
| Weight (z-score) | 0.16 (1.06) | −0.45 (1.19) | <0.001 |
| Height (z-score) | −0.12 (1.06) | −0.54 (1.22) | <0.001 |
| BMI (z-score) | 0.32 (1.15) | −0.19 (1.19) | <0.001 |
| Risk factors | |||
| Relatives with CD | <0.001 | ||
| No | 250 (54.1%) | 383 (82.7%) | |
| First degree | 161 (34.8%) | 29 (6.3%) | |
| Second degree | 42 (9.1%) | 47 (10.2%) | |
| First and second degree | 9 (1.9%) | 4 (0.9%) | |
| Diabetes | 62 (13.4%) | 4 (0.9%) | <0.001 |
| Thyroiditis | 18 (3.9%) | 5 (1.1%) | 0.006 |
| Down Syndrome | 10 (2.2%) | 0 (0.0%) | 0.001 |
| Laboratory parameters | |||
| IgA antiendomysium + | 325 (97.3) a | 328 (98.2) a | NS |
| TGA-IgA ≥ 10xUNL | 310 (66.2) | 353 (75.4) | 0.002 |
| HLA | (n = 409) | (n = 393) | NS |
| DQ2 | 345 (84.4%) | 338 (86.0%) | |
| DQ2/DQ8 | 40 (9.8%) | 34 (8.7%) | |
| DQ8 | 20 (4.9%) | 11 (2.8%) | |
| Others | 4 (1.0%) | 10 (2.5%) | |
| Marsh lesion grade | (n = 468) | (n = 371) | 0.017 b |
| 2 | 29 (6.2%) | 17 (4.6%) | |
| 3a | 162 (34.6%) | 96 (25.9%) | 0.002 c |
| 3b | 188 (40.2%) | 181 (48.8%) | |
| 3c | 89 (19.0%) | 77 (20.8%) |
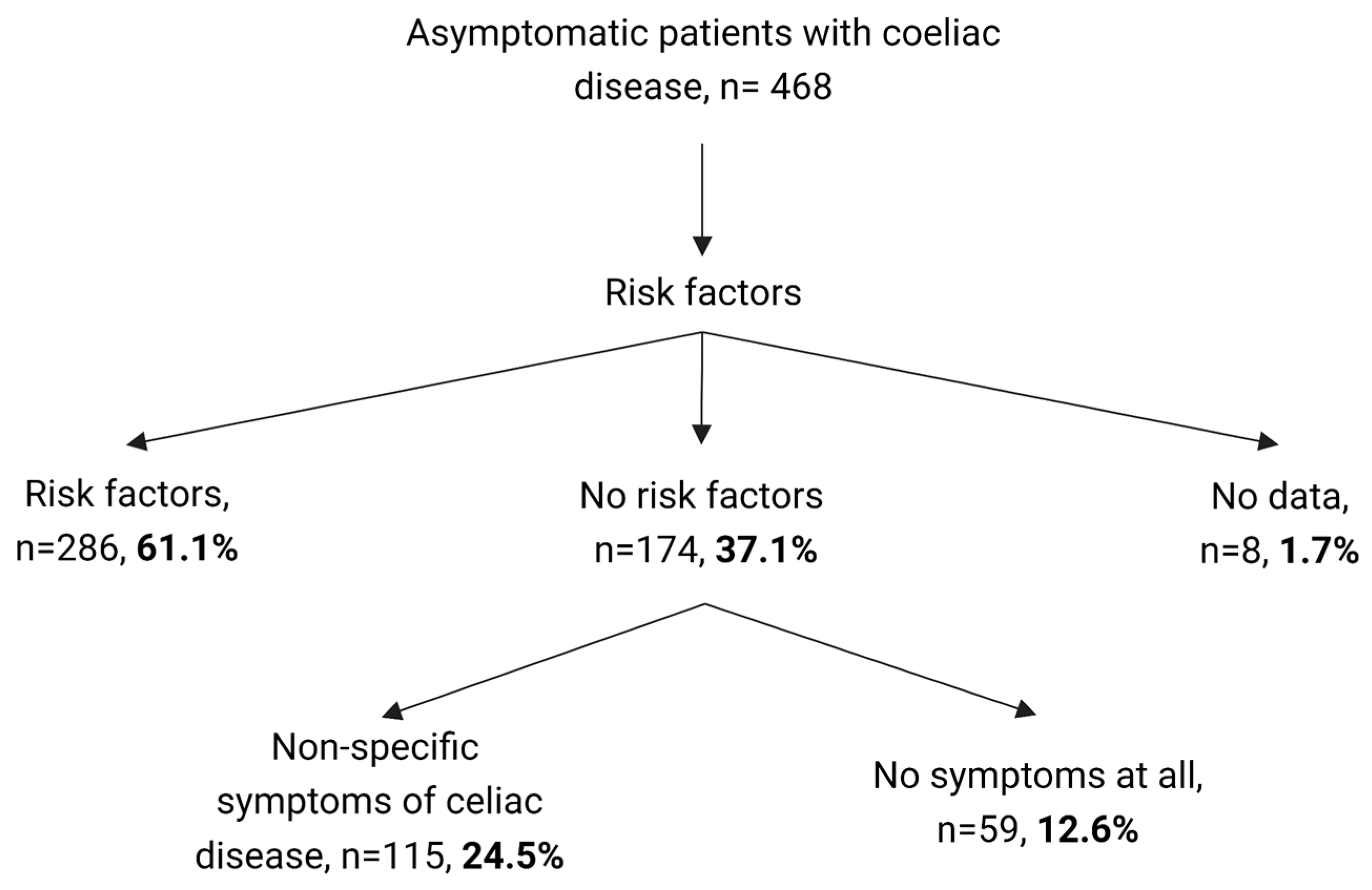
| CD Risk Factors a n = 286 | Non-Specific CD Symptoms b n = 115 | Other Reasons n = 59 | p-Value | |
|---|---|---|---|---|
| Background information | ||||
| Male sex (% from total) | 126 (44.1%) | 45 (39.1%) | 23 (39.0%) | 0.577 |
| Age (years) | 8.3 (5.3–11.4) | 8.1 (4.7–11.2) | 7.6 (4.4–11.5) | 0.603 |
| Age at gluten introduction (months) | 7 (6–8) | 6 (6–7) | 6 (6–7) | 0.022 |
| Weight (z-score) | 0.15 (0.97) | 0.14 (1.30) | 0.12 (0.88) | 0.973 |
| Height (z-score) | −0.16 (1.02) | −0.07 (1.21) | −0.16 (0.89) | 0.716 |
| BMI (z-score) | 0.35 (1.03) | 0.24 (1.41) | 0.29 (1.15) | 0.686 |
| Laboratory parameters | n (% from total) | |||
| IgA antiendomysium + | 196 (96.5%) | 91 (98.9%) | 30 (96.7%) | 0.511 |
| TGA-IgA ≥ 10xUNL | 192 (67.1%) | 74 (64.3%) | 40 (67.8%) | 0.550 |
| HLA | NS | |||
| DQ2 | 205 (82.3%) | 85 (84.2%) | 48 (92.3%) | |
| DQ2/DQ8 | 29 (11.6%) | 8 (7.9%) | 3 (5.8%) | |
| DQ8 | 12 (4.8%) | 7 (6.9%) | 1 (1.9%) | |
| Others | 3 (1.2%) | 1 (1.0%) | 0 (0.0%) | |
| Marsh lesion grade | NS | |||
| 2 | 13 (4.5%) | 11 (9.6%) | 5 (8.5%) | |
| 3a | 108 (37.8%) | 31 (27.0%) | 19 (32.2%) | |
| 3b | 104 (36.4%) | 50 (43.5%) | 31 (52.5%) | |
| 3c | 61 (21.3%) | 23 (20.0%) | 4 (6.8%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillejo, G.; Ochoa-Sangrador, C.; Pérez-Solís, D.; Cilleruelo, M.L.; Donat, E.; García-Burriel, J.I.; Sánchez-Valverde, F.; Garcia-Calatayud, S.; Eizaguirre, F.J.; Martinez-Ojinaga, E.; et al. Coeliac Disease Case–Control Study: Has the Time Come to Explore beyond Patients at Risk? Nutrients 2023, 15, 1267. https://doi.org/10.3390/nu15051267
Castillejo G, Ochoa-Sangrador C, Pérez-Solís D, Cilleruelo ML, Donat E, García-Burriel JI, Sánchez-Valverde F, Garcia-Calatayud S, Eizaguirre FJ, Martinez-Ojinaga E, et al. Coeliac Disease Case–Control Study: Has the Time Come to Explore beyond Patients at Risk? Nutrients. 2023; 15(5):1267. https://doi.org/10.3390/nu15051267
Chicago/Turabian StyleCastillejo, Gemma, Carlos Ochoa-Sangrador, David Pérez-Solís, Maria Luz Cilleruelo, Ester Donat, Jose Ignacio García-Burriel, Félix Sánchez-Valverde, Salvador Garcia-Calatayud, Francisco Javier Eizaguirre, Eva Martinez-Ojinaga, and et al. 2023. "Coeliac Disease Case–Control Study: Has the Time Come to Explore beyond Patients at Risk?" Nutrients 15, no. 5: 1267. https://doi.org/10.3390/nu15051267
APA StyleCastillejo, G., Ochoa-Sangrador, C., Pérez-Solís, D., Cilleruelo, M. L., Donat, E., García-Burriel, J. I., Sánchez-Valverde, F., Garcia-Calatayud, S., Eizaguirre, F. J., Martinez-Ojinaga, E., Barros, P., Leis, R., Salazar, J. C., Barrio, J., Peña-Quintana, L., Luque, V., Polanco, I., Ribes, C., & Roman, E., on behalf of the Coeliac Disease Working Group of the Spanish Paediatric Gastroenterology, Hepatology and Nutrition Society (SEGHNP). (2023). Coeliac Disease Case–Control Study: Has the Time Come to Explore beyond Patients at Risk? Nutrients, 15(5), 1267. https://doi.org/10.3390/nu15051267








