Effects of Parental Dietary Restriction on Offspring Fitness in Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Strain and Husbandry
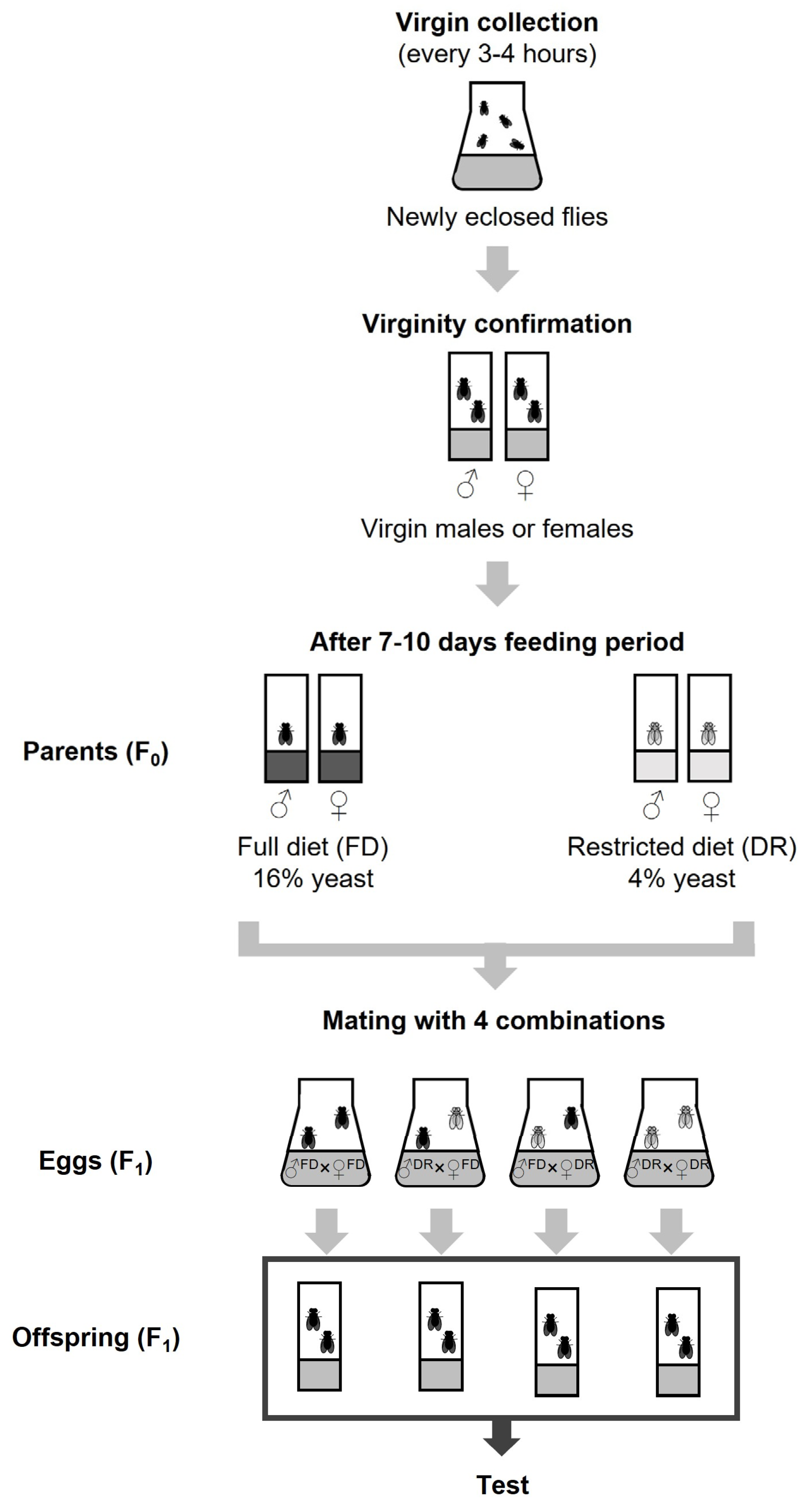
2.2. Fly Collection and Parental Breeding Design
2.3. Lifespan
2.4. Stress Resistance
2.5. Developmental Viability and Time
2.6. Fecundity
2.7. Feeding Rate
2.8. Body Weight
2.9. Statistical Analysis
3. Results
3.1. Dietary Restriction Increases the Lifespan and Reduces the Fecundity in Parent Flies
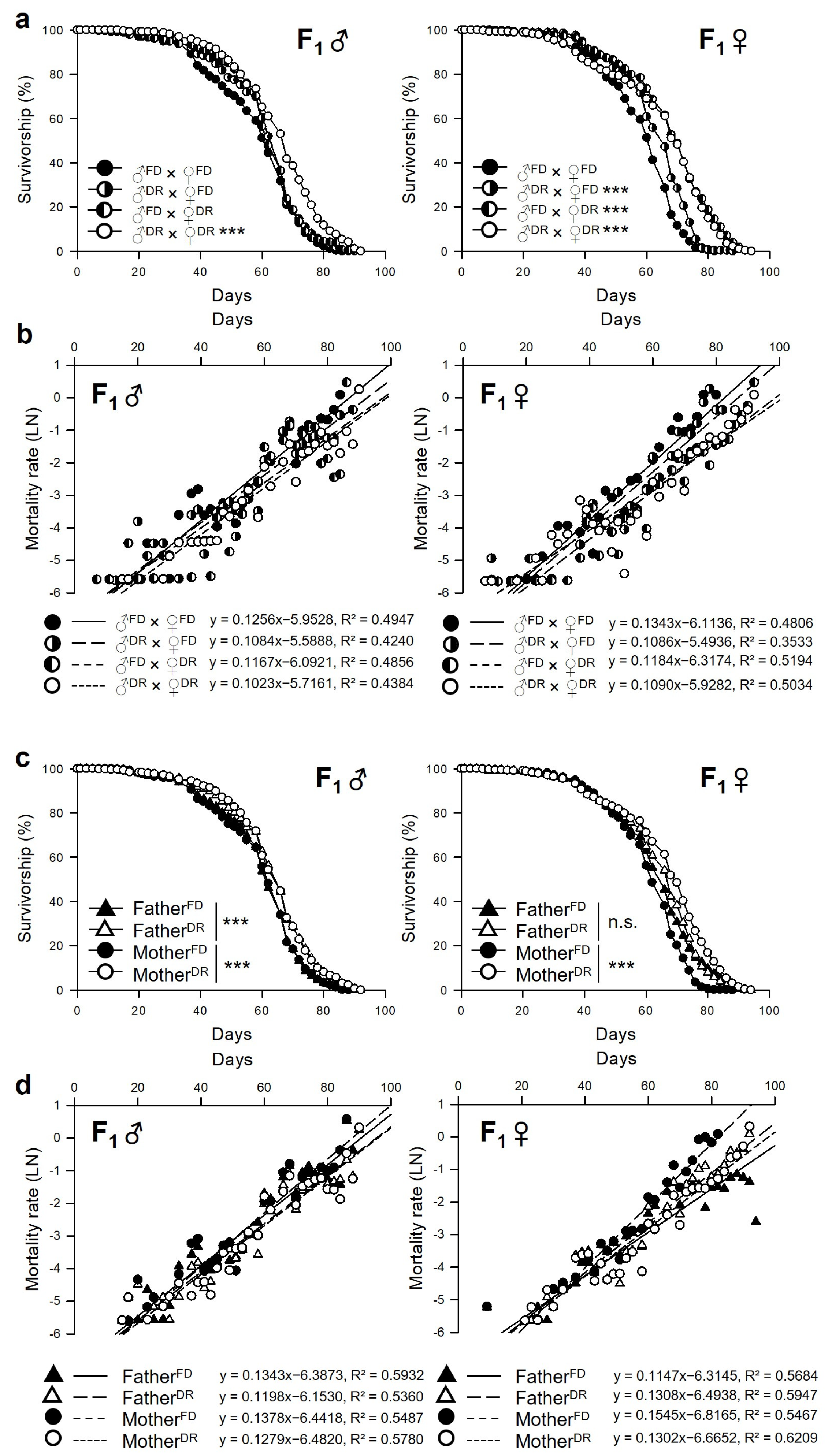
3.2. Parental Dietary Restriction Increases the Lifespan of the Offspring Flies
3.3. Parental (F0) Dietary Restriction Increases the Resistance to Various Stressors in Offspring (F1) Flies
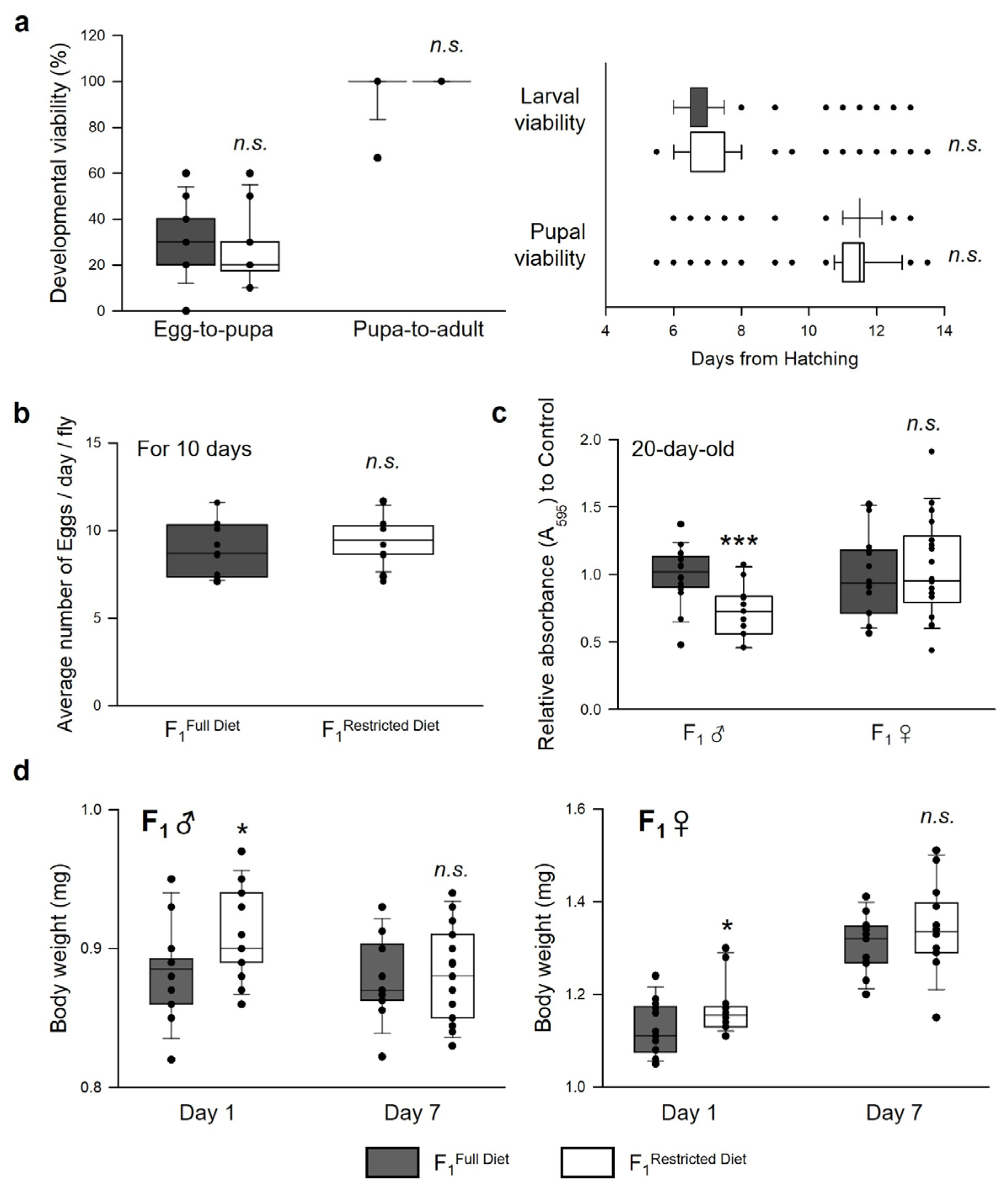
3.4. Parental Dietary Restriction Affects the Offspring’s Body Weight and Feeding Rate but Not the Development and Fecundity of the Offspring Flies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935, 10, 63–79. [Google Scholar] [CrossRef] [Green Version]
- Piper, M.D.; Bartke, A. Diet and aging. Cell Metab. 2008, 8, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.C.; Fretwell, S.D. The optimal balance between size and number of offspring. Am. Nat. 1974, 108, 499–506. [Google Scholar] [CrossRef]
- Stopher, K.V.; Pemberton, J.M.; Clutton-Brock, T.H.; Coulson, T. Individual differences, density dependence and offspring birth traits in a population of red deer. Proc. Biol. Sci. 2008, 275, 2137–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, C.N.; Barker, D.J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Int. J. Epidemiol. 1992, 42, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansing, A.I. A nongenic factor in the longevity of rotifers. Ann. N. Y. Acad. Sci. 1954, 57, 455–464. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Fox, C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998, 13, 403–407. [Google Scholar] [CrossRef]
- Aihie Sayer, A.; Dunn, R.; Langley-Evans, S.; Cooper, C. Prenatal exposure to a maternal low protein diet shortens life span in rats. Gerontology 2001, 47, 9–14. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Wayman, A.; Ekizoglou, S.; Martin, M.S.; Hales, C.N.; Ozanne, S.E. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R368–R373. [Google Scholar] [CrossRef]
- Martin-Gronert, M.S.; Tarry-Adkins, J.L.; Cripps, R.L.; Chen, J.H.; Ozanne, S.E. Maternal protein restriction leads to early life alterations in the expression of key molecules involved in the aging process in rat offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R494–R500. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, O.H.; Olsen, H.L.; Frandsen, L.; Nielsen, P.E.; Nielsen, F.C.; Grunnet, N.; Quistorff, B. A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J. Biomed. Sci. 2010, 17, S38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ost, A.; Lempradl, A.; Casas, E.; Weigert, M.; Tiko, T.; Deniz, M.; Pantano, L.; Boenisch, U.; Itskov, P.M.; Stoeckius, M.; et al. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell 2014, 159, 1352–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buescher, J.L.; Musselman, L.P.; Wilson, C.A.; Lang, T.; Keleher, M.; Baranski, T.J.; Duncan, J.G. Evidence for transgenerational metabolic programming in Drosophila. Dis. Model Mech. 2013, 6, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Pollock, C.A.; Saad, S. Aberrant DNA Methylation Mediates the Transgenerational Risk of Metabolic and Chronic Disease Due to Maternal Obesity and Overnutrition. Genes 2021, 12, 1653. [Google Scholar] [CrossRef]
- Nowacka-Woszuk, J.; Madeja, Z.E.; Chmurzynska, A. Prenatal caloric restriction alters lipid metabolism but not hepatic Fasn gene expression and methylation profiles in rats. BMC Genet. 2017, 18, 78. [Google Scholar] [CrossRef] [Green Version]
- Nowacka-Woszuk, J.; Szczerbal, I.; Malinowska, A.M.; Chmurzynska, A. Transgenerational effects of prenatal restricted diet on gene expression and histone modifications in the rat. PLoS ONE 2018, 13, e0193464. [Google Scholar] [CrossRef] [Green Version]
- Nowacka-Woszuk, J.; Grzemski, A.; Sliwinska, M.; Chmurzynska, A. Hepatic DNA methylation and expression profiles under prenatal restricted diet in three generations of female rat fetuses. PLoS ONE 2019, 14, e0215471. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, T.-L.; Piper, M.D.W.; Robker, R.L.; Dowling, D.K. Maternal and paternal sugar consumption interact to modify offspring life history and physiology. Funct. Ecol. 2022, 36, 1124–1136. [Google Scholar] [CrossRef]
- Mautz, B.S.; Lind, M.I.; Maklakov, A.A. Dietary restriction improves fitness of aging parents but reduces fitness of their offspring in Nematodes. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 843–848. [Google Scholar] [CrossRef] [Green Version]
- Ivimey-Cook, E.R.; Sales, K.; Carlsson, H.; Immler, S.; Chapman, T.; Maklakov, A.A. Transgenerational fitness effects of lifespan extension by dietary restriction in Caenorhabditis elegans. Proc. Biol. Sci. 2021, 288, 20210701. [Google Scholar] [CrossRef]
- Stanford, K.I.; Rasmussen, M.; Baer, L.A.; Lehnig, A.C.; Rowland, L.A.; White, J.D.; So, K.; De Sousa-Coelho, A.L.; Hirshman, M.F.; Patti, M.E.; et al. Paternal exercise improves glucose metabolism in adult offspring. Diabetes 2018, 67, 2530–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Seo, W.; Lee, S.-H.; Lee, H.-Y.; Min, K.-J. Strain-specific effects of parental age on offspring in Drosophila melanogaster. Entomol. Res. 2019, 49, 187–202. [Google Scholar] [CrossRef]
- Dimofski, P.; Meyre, D.; Dreumont, N.; Leininger-Muller, B. Consequences of paternal nutrition on offspring health and disease. Nutrients 2021, 13, 2818. [Google Scholar] [CrossRef]
- Zajitschek, F.; Zajitschek, S.R.; Canton, C.; Georgolopoulos, G.; Friberg, U.; Maklakov, A.A. Evolution under dietary restriction increases male reproductive performance without survival cost. Proc. Biol. Sci. 2016, 283, 20152726. [Google Scholar] [CrossRef]
- Zajitschek, F.; Georgolopoulos, G.; Vourlou, A.; Ericsson, M.; Zajitschek, S.R.K.; Friberg, U.; Maklakov, A.A. Evolution Under dietary restriction decouples survival from fecundity in Drosophila melanogaster females. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1542–1548. [Google Scholar] [CrossRef]
- Lewontin, R.C. The effects of population density and composition on viability in Drosophila melanogaster. Evolution 1955, 9, 27–41. [Google Scholar] [CrossRef]
- Good, T.P.; Tatar, M. Age-specific mortality and reproduction respond to adult dietary restriction in Drosophila melanogaster. J. Insect Physiol. 2001, 47, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Tatar, M. Drosophila diet restriction in practice: Do flies consume fewer nutrients? Mech. Ageing Dev. 2006, 127, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, T.B. Evolution of ageing. Nature 1977, 270, 301–304. [Google Scholar] [CrossRef]
- Cypser, J.R.; Johnson, T.E. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B109–B114. [Google Scholar] [CrossRef] [Green Version]
- Kapahi, P.; Boulton, M.E.; Kirkwood, T.B.L. Positive correlation between mammalian life span and cellular resistance to stress. Free. Radic. Biol. Med. 1999, 26, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Anway, M.D.; Savenkova, M.I.; Gore, A.C.; Crews, D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE 2008, 3, e3745. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, Z.V.; van Straalen, N.M.; Ellers, J. Transgenerational effects of nutrition are different for sons and daughters. J. Evol. Biol. 2016, 29, 1317–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanungo, M.S. Genes and Aging; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar] [CrossRef]
- De Maio, A. Heat shock proteins: Facts, thoughts, and dreams. Shock 1999, 11, 1–12. [Google Scholar] [CrossRef]
- Dancso, B.; Spiro, Z.; Arslan, M.A.; Nguyen, M.T.; Papp, D.; Csermely, P.; Soti, C. The heat shock connection of metabolic stress and dietary restriction. Curr. Pharm. Biotechnol. 2010, 11, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norouzitallab, P.; Baruah, K.; Vandegehuchte, M.; Van Stappen, G.; Catania, F.; Vanden Bussche, J.; Vanhaecke, L.; Sorgeloos, P.; Bossier, P. Environmental heat stress induces epigenetic transgenerational inheritance of robustness in parthenogenetic Artemia model. FASEB J. 2014, 28, 3552–3563. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.; Partridge, L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008, 7, 200–203. [Google Scholar] [CrossRef] [Green Version]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, A.R.; Salvucci, I.D.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin. Sci. 2008, 114, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Strilbytska, O.; Velianyk, V.; Burdyliuk, N.; Yurkevych, I.S.; Vaiserman, A.; Storey, K.B.; Pospisilik, A.; Lushchak, O. Parental dietary protein-to-carbohydrate ratio affects offspring lifespan and metabolism in Drosophila. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 241, 110622. [Google Scholar] [CrossRef]
- Strilbytska, O.M.; Stefanyshyn, N.P.; Semaniuk, U.V.; Lushchak, O.V. Yeast concentration in the diet defines Drosophila metabolism of both parental and offspring generations. Ukr. Biochem. J. 2021, 93, 119–129. [Google Scholar] [CrossRef]
- Valtonen, T.M.; Kangassalo, K.; Polkki, M.; Rantala, M.J. Transgenerational effects of parental larval diet on offspring development time, adult body size and pathogen resistance in Drosophila melanogaster. PLoS ONE 2012, 7, e31611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matzkin, L.M.; Johnson, S.; Paight, C.; Markow, T.A. Preadult parental diet affects offspring development and metabolism in Drosophila melanogaster. PLoS ONE 2013, 8, e59530. [Google Scholar] [CrossRef] [PubMed]
- Roff, D.A. Evolution of Life Histories: Theory and Analysis; Springer Science & Business Media: Berlin, Germany, 1992. [Google Scholar]
- Vijendravarma, R.K.; Narasimha, S.; Kawecki, T.J. Effects of parental larval diet on egg size and offspring traits in Drosophila. Biol. Lett. 2010, 6, 238–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roff, D.A. Life History Evolution; Oxford University Press: Oxford, UK, 2001; pp. 1–7. [Google Scholar]
- Giraudo, S.Q.; Della-Fera, M.A.; Proctor, L.; Wickwire, K.; Ambati, S.; Baile, C.A. Maternal high fat feeding and gestational dietary restriction: Effects on offspring body weight, food intake and hypothalamic gene expression over three generations in mice. Pharmacol. Biochem. Behav. 2010, 97, 121–129. [Google Scholar] [CrossRef]
- Lagisz, M.; Blair, H.; Kenyon, P.; Uller, T.; Raubenheimer, D.; Nakagawa, S. Transgenerational effects of caloric restriction on appetite: A meta-analysis. Obes. Rev. 2014, 15, 294–309. [Google Scholar] [CrossRef]
- Prasad, N.G.; Shakarad, M.; Rajamani, M.; Joshi, A. Interaction between the effects of maternal and larval levels of nutrition on pre-adult survival in Drosophila melanogaster. Evol. Ecol. Res. 2003, 5, 903–911. [Google Scholar]
- Wright, T.M.; Fone, K.C.; Langley-Evans, S.C.; Voigt, J.P. Exposure to maternal consumption of cafeteria diet during the lactation period programmes feeding behaviour in the rat. Int. J. Dev. Neurosci. 2011, 29, 785–793. [Google Scholar] [CrossRef]
- Silva-Filha, E.; Costa, T.; Farias, B.; Santos, P.; Santana, M.; Macedo, F.; Santos, R.; Santana-Filho, V. Transgenerational effects on the feeding behavior of the offspring. FASEB J. 2015, 29, LB243. [Google Scholar] [CrossRef]
- Chandegra, B.; Tang, J.L.Y.; Chi, H.; Alic, N. Sexually dimorphic effects of dietary sugar on lifespan, feeding and starvation resistance in Drosophila. Aging 2017, 9, 2521–2528. [Google Scholar] [CrossRef] [Green Version]
- Magwere, T.; Chapman, T.; Partridge, L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, W.; Piper, M.D.W.; Partridge, L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005, 3, e223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lushchak, O.; Strilbytska, O.; Storey, K.B. Gender-specific effects of pro-longevity interventions in Drosophila. Mech. Ageing Dev. 2023, 209, 111754. [Google Scholar] [CrossRef]
- Camus, M.F.; Piper, M.D.; Reuter, M. Sex-specific transcriptomic responses to changes in the nutritional environment. Elife 2019, 8, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, S.D.; Libert, S.; Skorupa, D. Flies and their golden apples: The effect of dietary restriction on Drosophila aging and age-dependent gene expression. Ageing Res. Rev. 2005, 4, 451–480. [Google Scholar] [CrossRef]
- Kirkpatrick, M.; Lande, R. The Evolution of Maternal Characters. Evolution 1989, 43, 485–503. [Google Scholar] [CrossRef]
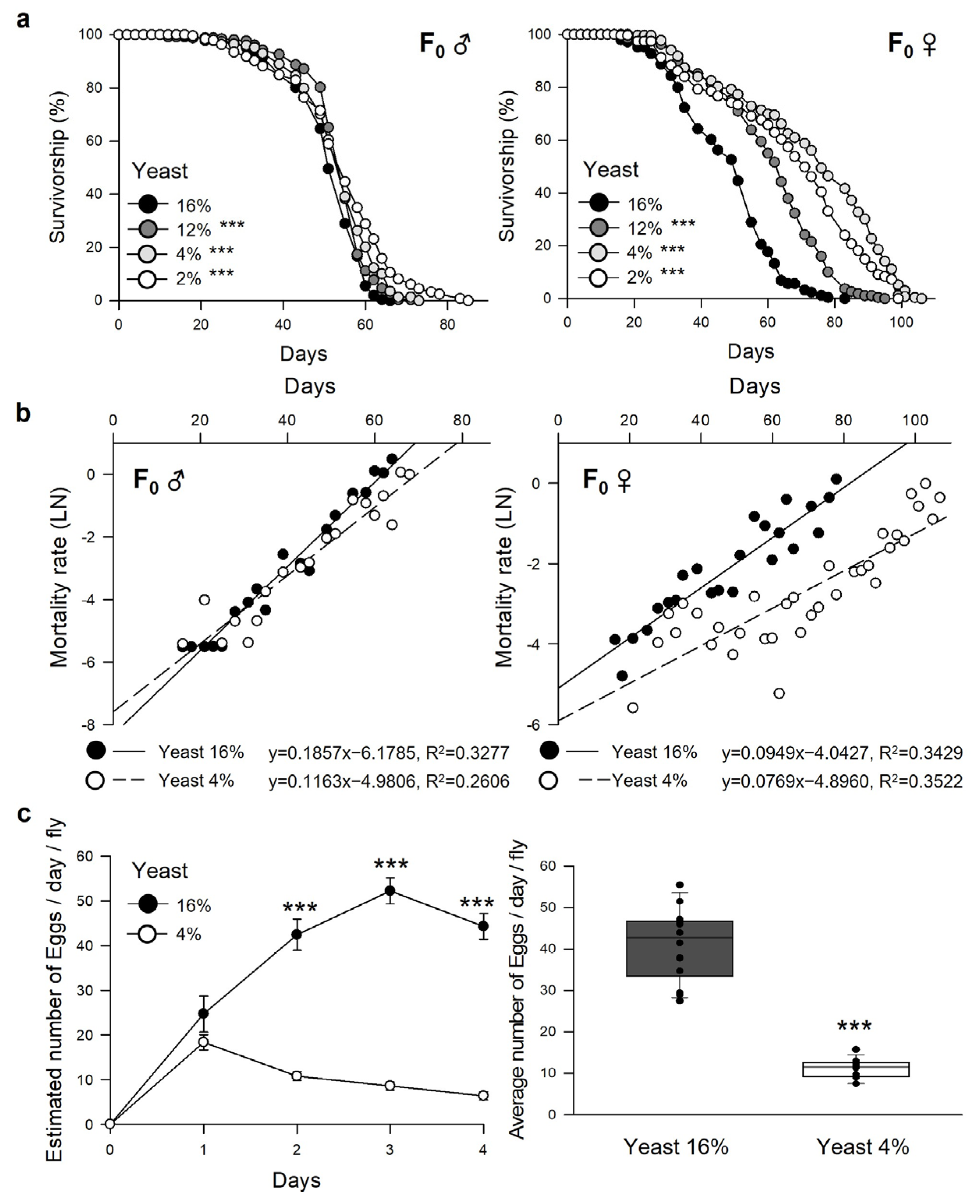

| Sex | Yeast (%) | n | Mean-Lifespan | Change | Median-Lifespan | Change | Max. Lifespan (75% Failures) | Change | χ2 | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 16 | 252 | 50.89 | ± | 0.57 | 51 | 58 | |||||
| 12 | 258 | 53.64 | ± | 0.53 | 5.4% | 55 | 7.8% | 58 | 0.0% | 13.5037 | 0.0002 | |
| 4 | 228 | 53.21 | ± | 0.66 | 4.6% | 55 | 7.8% | 60 | 3.4% | 19.0097 | <0.0001 | |
| 2 | 238 | 53.05 | ± | 0.78 | 4.2% | 55 | 7.8% | 62 | 6.9% | 28.8639 | <0.0001 | |
| Female | 16 | 249 | 47.66 | ± | 0.91 | 51 | 58 | |||||
| 12 | 269 | 60.41 | ± | 1.01 | 26.8% | 64 | 25.5% | 71 | 22.4% | 105.4658 | <0.0001 | |
| 4 | 269 | 72.36 | ± | 1.39 | 51.8% | 76 | 49.0% | 91 | 56.9% | 225.8365 | <0.0001 | |
| 2 | 275 | 66.73 | ± | 1.36 | 40.0% | 71 | 39.2% | 83 | 43.1% | 175.0049 | <0.0001 | |
| Sex | Group | n | Mean-Lifespan | Change | Median-Lifespan | Change | Max. Lifespan (75% Failures) | Change | χ2 | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | ♂FD × ♀FD | 268 | 55.87 | ± | 0.89 | 60 | 66 | |||||
| ♂DR × ♀FD | 274 | 58.23 | ± | 0.87 | 4.2% | 62 | 3.3% | 66 | 0.0% | 3.6747 | 0.0552 | |
| ♂FD × ♀DR | 266 | 58.80 | ± | 0.86 | 5.3% | 60 | 0.0% | 66 | 0.0% | 3.6341 | 0.0566 | |
| ♂DR × ♀DR | 264 | 63.42 | ± | 0.88 | 13.5% | 66 | 10.0% | 74 | 12.1% | 43.3299 | <0.0001 | |
| Female | ♂FD × ♀FD | 267 | 56.04 | ± | 0.78 | 60 | 66 | |||||
| ♂DR × ♀FD | 278 | 60.50 | ± | 0.74 | 8.0% | 62 | 3.3% | 70 | 6.1% | 24.138 | <0.0001 | |
| ♂FD × ♀DR | 283 | 65.49 | ± | 0.92 | 16.9% | 68 | 13.3% | 76 | 15.2% | 109.4231 | <0.0001 | |
| ♂DR × ♀DR | 280 | 63.20 | ± | 1.03 | 12.8% | 68 | 13.3% | 76 | 15.2% | 92.7941 | <0.0001 | |
| Sex | Group | n | Mean-Lifespan | Change | Median-Lifespan | Change | Max. Lifespan (75% Failures) | Change | χ2 | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | FatherFD | 534 | 57.33 | ± | 0.62 | 60 | 66 | |||||
| FatherDR | 538 | 60.78 | ± | 0.63 | 6.0% | 62 | 3.3% | 70 | 6.1% | 24.2517 | <0.0001 | |
| MotherFD | 542 | 57.06 | ± | 0.62 | 60 | 66 | ||||||
| MotherDR | 530 | 61.10 | ± | 0.62 | 7.1% | 62 | 3.3% | 70 | 6.1% | 25.3409 | <0.0001 | |
| Female | FatherFD | 550 | 60.90 | ± | 0.64 | 62 | 70 | |||||
| FatherDR | 558 | 61.85 | ± | 0.64 | 1.6% | 66 | 6.5% | 72 | 2.9% | 0.7762 | 0.3783 | |
| MotherFD | 545 | 58.31 | ± | 0.55 | 60 | 66 | ||||||
| MotherDR | 563 | 64.35 | ± | 0.69 | 10.4% | 68 | 13.3% | 76 | 15.2% | 137.008 | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Lee, B.; Lee, E.-J.; Min, K.-J. Effects of Parental Dietary Restriction on Offspring Fitness in Drosophila melanogaster. Nutrients 2023, 15, 1273. https://doi.org/10.3390/nu15051273
Lee H-Y, Lee B, Lee E-J, Min K-J. Effects of Parental Dietary Restriction on Offspring Fitness in Drosophila melanogaster. Nutrients. 2023; 15(5):1273. https://doi.org/10.3390/nu15051273
Chicago/Turabian StyleLee, Hye-Yeon, Bora Lee, Eun-Ji Lee, and Kyung-Jin Min. 2023. "Effects of Parental Dietary Restriction on Offspring Fitness in Drosophila melanogaster" Nutrients 15, no. 5: 1273. https://doi.org/10.3390/nu15051273
APA StyleLee, H.-Y., Lee, B., Lee, E.-J., & Min, K.-J. (2023). Effects of Parental Dietary Restriction on Offspring Fitness in Drosophila melanogaster. Nutrients, 15(5), 1273. https://doi.org/10.3390/nu15051273







