Delayed Macronutrients’ Target Achievement in Parenteral Nutrition Reduces the Risk of Hyperglycemia in Preterm Newborn: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approval, Ethics and Patient Consent
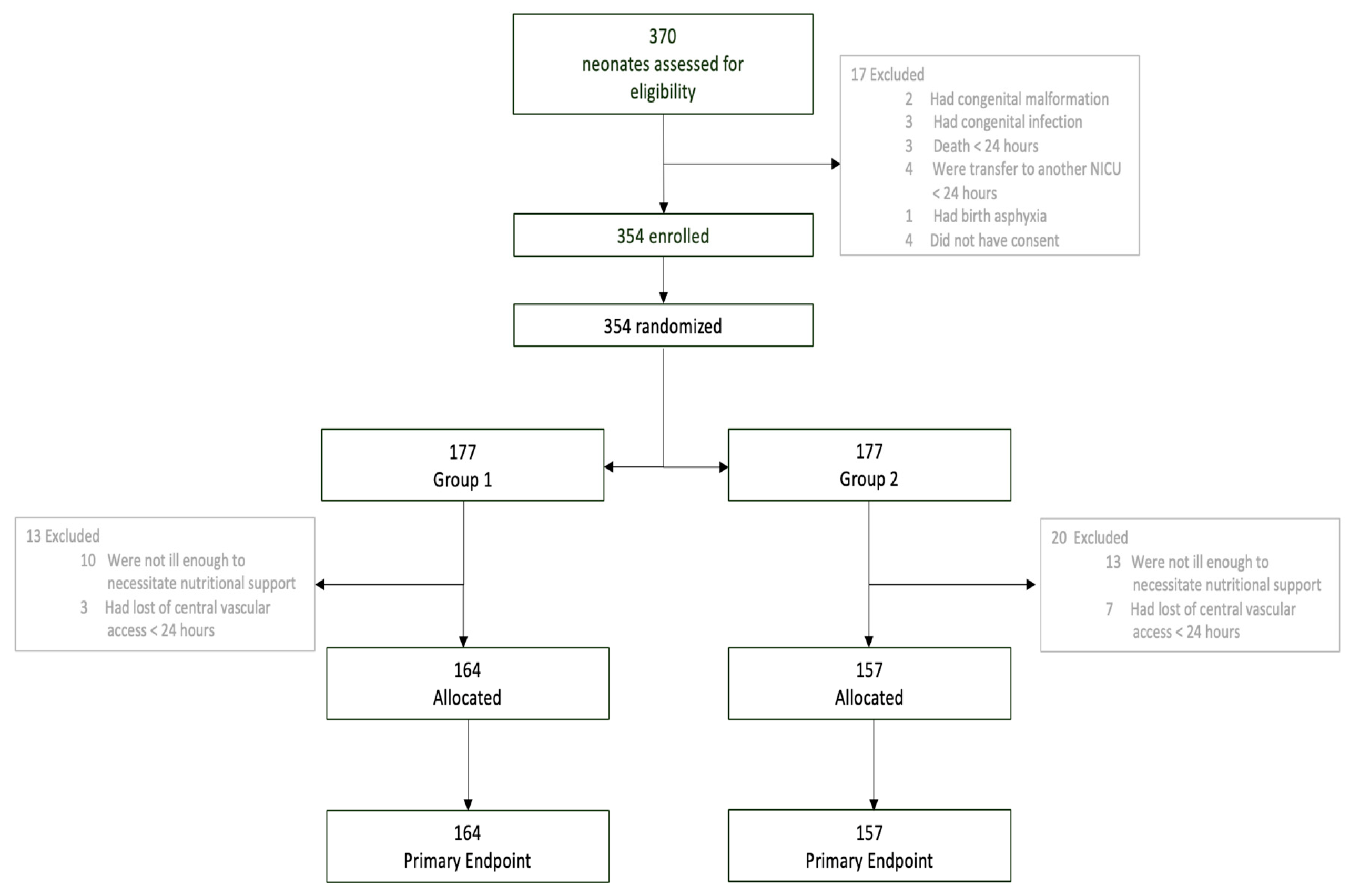
2.2. Population and Randomization
2.3. Outcome
2.4. Nutritional Protocol
2.5. Data Collection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Goudoever, J.B.; Carnielli, V.; Darmaun, D.; Sainz de Pipaon, M.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Amino Acids. Clin. Nutr. 2018, 37, 2315–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joosten, K.; Verbruggen, S. PN Administration in Critically Ill Children in Different Phases of the Stress Response. Nutrients 2022, 14, 1819. [Google Scholar] [CrossRef]
- Poindexter, B.B.; Langer, J.C.; Dusick, A.M.; Ehrenkranz, R.A. Early Provision of Parenteral Amino Acids in Extremely Low Birth Weight Infants: Relation to Growth and Neurodevelopmental Outcome. J. Pediatr. 2006, 148, 300–305.e1. [Google Scholar] [CrossRef] [PubMed]
- Moyses, H.E.; Johnson, M.J.; Leaf, A.A.; Cornelius, V.R. Early Parenteral Nutrition and Growth Outcomes in Preterm Infants: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2013, 97, 816–826. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, S.; Ichiba, H.; Tanaka, Y.; Harada, S.; Matsumura, H.; Kan, A.; Asada, Y.; Shintaku, H. Early and Intensive Nutritional Strategy Combining Parenteral and Enteral Feeding Promotes Neurodevelopment and Growth at 18 months of Corrected Age and 3 years of Age in Extremely Low Birth Weight Infants. Early Hum. Dev. 2016, 100, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R. 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). J. Pediatr. Gastroenterol. Nutr. 2005, 41, 4. [Google Scholar]
- Stensvold, H.J.; Strommen, K.; Lang, A.M.; Abrahamsen, T.G.; Steen, E.K.; Pripp, A.H.; Ronnestad, A.E. Early Enhanced Parenteral Nutrition, Hyperglycemia, and Death Among Extremely Low-Birth-Weight Infants. JAMA Pediatr. 2015, 169, 1003–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrin, G.; Coscia, A.; Boscarino, G.; Faccioli, F.; Di Chiara, M.; Greco, C.; Onestà, E.; Oliva, S.; Aloi, M.; Dito, L.; et al. Long-Term Effects on Growth of an Energy-Enhanced Parenteral Nutrition in Preterm Newborn: A Quasi-Experimental Study. PLoS ONE 2020, 15, e0235540. [Google Scholar] [CrossRef]
- Fivez, T.; Kerklaan, D.; Mesotten, D.; Verbruggen, S.; Wouters, P.J.; Vanhorebeek, I.; Debaveye, Y.; Vlasselaers, D.; Desmet, L.; Casaer, M.P.; et al. Early versus Late Parenteral Nutrition in Critically Ill Children. N. Engl. J. Med. 2016, 374, 1111–1122. [Google Scholar] [CrossRef] [Green Version]
- van Puffelen, E.; Jacobs, A.; Verdoorn, C.J.M.; Joosten, K.F.M.; van den Berghe, G.; Ista, E.; Verbruggen, S.C.A.T. International Survey of De-Implementation of Initiating Parenteral Nutrition Early in Paediatric Intensive Care Units. BMC Health Serv. Res. 2019, 19, 379. [Google Scholar] [CrossRef] [Green Version]
- van Puffelen, E.; Hulst, J.M.; Vanhorebeek, I.; Dulfer, K.; Van den Berghe, G.; Verbruggen, S.C.A.T.; Joosten, K.F.M. Outcomes of Delaying Parenteral Nutrition for 1 Week vs Initiation Within 24 Hours Among Undernourished Children in Pediatric Intensive Care: A Subanalysis of the PEPaNIC Randomized Clinical Trial. JAMA Netw. Open 2018, 1, e182668. [Google Scholar] [CrossRef]
- Galderisi, A.; Facchinetti, A.; Steil, G.M.; Ortiz-Rubio, P.; Cavallin, F.; Tamborlane, W.V.; Baraldi, E.; Cobelli, C.; Trevisanuto, D. Continuous Glucose Monitoring in Very Preterm Infants: A Randomized Controlled Trial. Pediatrics 2017, 140, e20171162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrin, G.; Berni Canani, R.; Passariello, A.; Messina, F.; Conti, M.G.; Caoci, S.; Smaldore, A.; Bertino, E.; De Curtis, M. Zinc Supplementation Reduces Morbidity and Mortality in Very-Low-Birth-Weight Preterm Neonates: A Hospital-Based Randomized, Placebo-Controlled Trial in an Industrialized Country. Am. J. Clin. Nutr. 2013, 98, 1468–1474. [Google Scholar] [CrossRef] [Green Version]
- Naeem, A.; Ahmed, I.; Silveyra, P. Bronchopulmonary Dysplasia: An Update on Experimental Therapeutics. Eur. Med. J. 2019, 4, 20–29. [Google Scholar] [CrossRef]
- Bertino, E.; Di Nicola, P.; Varalda, A.; Occhi, L.; Giuliani, F.; Coscia, A. Neonatal Growth Charts. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. S1), 67–69. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, F.; Cheikh Ismail, L.; Bertino, E.; Bhutta, Z.A.; Ohuma, E.O.; Rovelli, I.; Conde-Agudelo, A.; Villar, J.; Kennedy, S.H. Monitoring Postnatal Growth of Preterm Infants: Present and Future. Am. J. Clin. Nutr. 2016, 103, 635S–647S. [Google Scholar] [CrossRef] [Green Version]
- Villar, J.; Giuliani, F.; Bhutta, Z.A.; Bertino, E.; Ohuma, E.O.; Ismail, L.C.; Barros, F.C.; Altman, D.G.; Victora, C.; Noble, J.A.; et al. Postnatal Growth Standards for Preterm Infants: The Preterm Postnatal Follow-up Study of the INTERGROWTH-21 St Project. Lancet Glob. Health 2015, 3, e681–e691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tozzi, M.G.; Moscuzza, F.; Michelucci, A.; Lorenzoni, F.; Cosini, C.; Ciantelli, M.; Ghirri, P. ExtraUterine Growth Restriction (EUGR) in Preterm Infants: Growth Patterns, Nutrition, and Epigenetic Markers. A Pilot Study. Front. Pediatr. 2018, 6, 408. [Google Scholar] [CrossRef]
- Mehta, N.M.; Bechard, L.J.; Cahill, N.; Wang, M.; Day, A.; Duggan, C.P.; Heyland, D.K. Nutritional Practices and Their Relationship to Clinical Outcomes in Critically Ill Children—An International Multicenter Cohort Study. Crit. Care Med. 2012, 40, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- de Betue, C.T.; Verbruggen, S.C.; Schierbeek, H.; Chacko, S.K.; Bogers, A.J.; van Goudoever, J.B.; Joosten, K.F. Does a Reduced Glucose Intake Prevent Hyperglycemia in Children Early after Cardiac Surgery? A Randomized Controlled Crossover Study. Crit. Care 2012, 16, R176. [Google Scholar] [CrossRef] [Green Version]
- van Puffelen, E.; Vanhorebeek, I.; Joosten, K.F.M.; Wouters, P.J.; Van den Berghe, G.; Verbruggen, S.C.A.T. Early versus Late Parenteral Nutrition in Critically Ill, Term Neonates: A Preplanned Secondary Subgroup Analysis of the PEPaNIC Multicentre, Randomised Controlled Trial. Lancet Child Adolesc. Health 2018, 2, 505–515. [Google Scholar] [CrossRef]
- Boscarino, G.; Conti, M.G.; Gasparini, C.; Onestà, E.; Faccioli, F.; Dito, L.; Regoli, D.; Spalice, A.; Parisi, P.; Terrin, G. Neonatal Hyperglycemia Related to Parenteral Nutrition Affects Long-Term Neurodevelopment in Preterm Newborn: A Prospective Cohort Study. Nutrients 2021, 13, 1930. [Google Scholar] [CrossRef] [PubMed]
- Uthaya, S.; Longford, N.; Battersby, C.; Oughham, K.; Lanoue, J.; Modi, N. Early versus Later Initiation of Parenteral Nutrition for Very Preterm Infants: A Propensity Score-matched Observational Study. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 107, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ertl, T.; Gyarmati, J.; Gaál, V.; Szabó, I. Relationship between Hyperglycemia and Retinopathy of Prematurity in Very Low Birth Weight Infants. Neonatology 2006, 89, 56–59. [Google Scholar] [CrossRef]
- Slidsborg, C.; Jensen, L.B.; Rasmussen, S.C.; Fledelius, H.C.; Greisen, G.; Cour, M. de la. Early Postnatal Hyperglycaemia Is a Risk Factor for Treatment-Demanding Retinopathy of Prematurity. Br. J. Ophthalmol. 2018, 102, 14–18. [Google Scholar] [CrossRef]
- Lei, C.; Duan, J.; Ge, G.; Zhang, M. Association between Neonatal Hyperglycemia and Retinopathy of Prematurity: A Meta-Analysis. Eur. J. Pediatr. 2021, 180, 3433–3442. [Google Scholar] [CrossRef]
- Villeneuve, A.; Arsenault, V.; Lacroix, J.; Tucci, M. Neonatal Red Blood Cell Transfusion. Vox Sang. 2021, 116, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Poggi, C.; Bresci, C.; Corsini, I.; Frosini, S.; Pratesi, S. Early Fresh-Frozen Plasma Transfusion Decreases the Risk of Retinopathy of Prematurity: Plasma and Risk of ROP. Transfusion 2014, 54, 1002–1007. [Google Scholar] [CrossRef]
- Milanesi, B.G.; Lima, P.A.; Villela, L.D.; Martins, A.S.; Gomes-Junior, S.C.S.; Moreira, M.E.L.; Méio, M.D.B.B. Assessment of Early Nutritional Intake in Preterm Infants with Bronchopulmonary Dysplasia: A Cohort Study. Eur. J. Pediatr. 2021, 180, 1423–1430. [Google Scholar] [CrossRef]
- Temming, P.; Tröger, B.; Thonnissen, S.; Holterhus, P.; Schultz, C.; Härtel, C. The Effect of Hyperglycemia on Neonatal Immune Responses In-Vitro. J. Matern. Fetal Neonatal Med. 2012, 25, 94–98. [Google Scholar] [CrossRef]
- Zingg, W.; Tomaske, M.; Martin, M. Risk of Parenteral Nutrition in Neonates—An Overview. Nutrients 2012, 4, 1490–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.C.; Cairns, P.; Halliday, H.L.; Reid, M.; McClure, G.; Dodge, J.A. Randomised Controlled Trial of an Aggressive Nutritional Regimen in Sick Very Low Birthweight Infants. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 77, F4–F11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, C.; McGowan, P.; Herwitker, S.; Hart, A.E.; Turner, M.A. Postnatal Head Growth in Preterm Infants: A Randomized Controlled Parenteral Nutrition Study. Pediatrics 2014, 133, e120–e128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senterre, T. Practice of Enteral Nutrition in Very Low Birth Weight and Extremely Low Birth Weight Infants. In World Review of Nutrition and Dietetics; Koletzko, B., Poindexter, B., Uauy, R., Eds.; S. Karger AG: Basel, Switzerland, 2014; Volume 110, pp. 201–214. [Google Scholar] [CrossRef]

| Birth Weight < 1000 g | Birth Weight ≥ 1000 g | |||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 1 | Group 2 | |
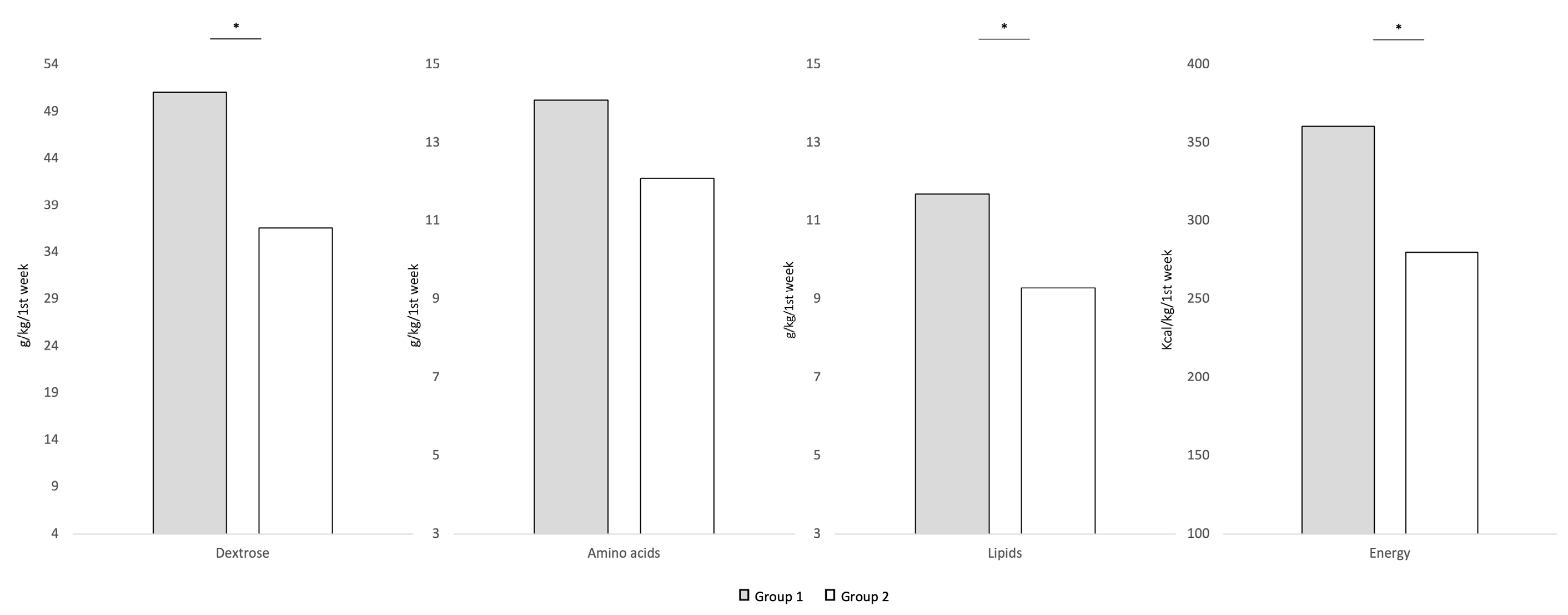
| Energy (kcal/kg/day) | ||||
| Starting dose (min–max) | 45–58 | 45–58 | 40–45 | 40–45 |
| Target dose (min–max) | 100–110 | 100–110 | 90–100 | 90–100 |
| Time of target achievement, days | 4–5 | 10–12 | 4–5 | 10–12 |
| Amino acids (g/kg/day) | ||||
| Starting dose (min–max) | 2.0–2.5 | 2.0–2.5 | 3.2–3.5 | 3.2–3.5 |
| Target dose (min–max) | 3.5–4.0 | 3.5–4.0 | 3.5–4.0 | 3.5–4.0 |
| Time of target achievement, days | 3–4 | 5–7 | 3–4 | 5–7 |
| Dextrose (g/kg/day) | ||||
| Starting dose (min–max) | 6.5–7.0 | 6.5–7.0 | 6.5–7 | 6.5–7.0 |
| Target dose (min–max) | 13.0–14.0 | 13.0–14.0 | 13.0–14.0 | 13.0–14.0 |
| Time of target achievement, days | 5–7 | 10–12 | 5–7 | 10–12 |
| Lipids (g/kg/day) | ||||
| Starting dose (min–max) | 1.5–2.0 | 1.5–2.0 | 1.0–1.5 | 1.0–1.5 |
| Target dose (min–max) | 3.5–4.0 | 3.5–4.0 | 3.0–3.5 | 3.0–3.5 |
| Time of target achievement, days | 3–5 | 5–7 | 3–5 | 5–7 |
| Group 1 n = 164 | Group 2 n = 157 | p | |
|---|---|---|---|
| Pre-natal characteristics | |||
| Antenatal corticosteroids 1, No. (%) | 112 (69.6) | 105 (70.0) | 0.516 |
| IUGR 2, No. (%) | 24 (14.9) | 28 (18.9) | 0.215 |
| Pregnancy-induced hypertension, No. (%) | 38 (23.6) | 30 (19.9) | 0.254 |
| Hypothyroidism, No. (%) | 20 (12.4) | 21 (14.1) | 0.395 |
| Twins, No. (%) | 51 (31.1) | 53 (34.6) | 0.291 |
| Gestational Diabetes, No. (%) | 16 (9.9) | 21 (14.5) | 0.149 |
| Mother’s age ≥ 35 years old, No. (%) | 65 (43.9) | 69 (45.4) | 0.444 |
| Cesarean section, No. (%) | 142 (86.6) | 132 (86.3) | 0.533 |
| Perinatal characteristics | |||
| Gestational age, weeks | 29.4 (29.0 to 29.8) | 29.6 (29.2 to 30.0) | 0.572 |
| Birth weight, g | 1225.7 (1170.4 to 1280.9) | 1301.9 (1241.4 to 1362.3) | 0.067 |
| Male sex, No. (%) | 88 (53.7) | 80 (51.6) | 0.400 |
| SGA 3, No. (%) | 37 (23) | 25 (16.9) | 0.116 |
| ELBW 4, No. (%) | 43 (26.2) | 30 (19.1) | 0.083 |
| 5-min Apgar score | 7.8 (7.6 to 8.0) | 7.9 (7.6 to 8.1) | 0.483 |
| pH at birth | 7.3 (7.2 to 7.3) | 7.3 (7.2 to 7.3) | 0.851 |
| Base excess on cord blood, mmol/L | −5.2 (−5.7 to −4.6) | −5.9 (−6.8 to −5.2) | 0.107 |
| CRIB II score 5 | 6.4 (5.8 to 7.0) | 5.7 (4.9 a 6.5) | 0.176 |
| Length of hospital stay, days | 63.8 (57.8 to 69.8) | 58.3 (53.4 to 63.3) | 0.167 |
| Group 1 | Group 2 | p | |
|---|---|---|---|
| n = 164 | n = 157 | ||
| Mortality, No. (%) | 9 (5.5) | 9 (5.7) | 0.558 |
| NEC stage III, No. (%) | 1 (0.6) | 4 (3.0) | 0.125 |
| BPD, No. (%) | 13 (7.9) | 4 (2.7) | 0.033 * |
| Culture-proven sepsis, No. (%) | 12 (7.4) | 28 (18.7) | 0.002 * |
| Retinopathy of prematurity, No. (%) | 34 (20.9) | 16 (10.5) | 0.009 * |
| Periventricular leukomalacia, No. (%) | 4 (2.5) | 4 (2.9) | 0.552 |
| Intraventricular hemorrhage, No. (%) | 12 (7.4) | 10 (6.7) | 0.493 |
| Patent ductus arteriosus, No. (%) | 39 (23.9) | 35 (23.2) | 0.491 |
| Severe anemia, No. (%) | 43 (26.4) | 41 (27.2) | 0.489 |
| Overall morbidity, No (%) | 51 (31.1) | 62 (40.3) | 0.056 |
| Variables | ß | S.E. | Wald | p Value | Odds Ratio (OR) | 95 C.I for OR | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| GA ° | 1.765 | 0.618 | 8.166 | 0.004 * | 5.840 | 1.74 | 19.59 |
| Group assignment | 1.095 | 0.310 | 1.492 | 0.000 * | 1.038 | 1.62 | 5.48 |
| Antenatal corticosteroids ± | 0.037 | 0.310 | 0.014 | 0.905 | 1.038 | 0.56 | 1.90 |
| Culture-proven sepsis | 1.503 | 0.393 | 14.492 | 0.000 * | 4.496 | 2.08 | 9.71 |
| Variables | ß | S.E. | Wald | p Value | Odds Ratio (OR) | 95 C.I for OR | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| GA ° | 1.867 | 1.077 | 3.003 | 0.083 | 6.467 | 0.783 | 53.418 |
| Group assignment | 0.266 | 0.285 | 0.876 | 0.349 | 1.305 | 0.747 | 2.280 |
| Hyperglycemia | 1.620 | 0.602 | 7.229 | 0.007 * | 5.052 | 1.551 | 16.452 |
| Sepsis± | 0.655 | 0.607 | 1.165 | 0.280 | 1.926 | 0.586 | 6.332 |
| BPDξ | 0.432 | 0.743 | 0.338 | 0.561 | 1.541 | 0.359 | 6.613 |
| Growth at 12 Months | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Unstandardized parameters | |||
| Weight, g | 8691 (8403 to 8978) | 9309 (8944 to 9674) | 0.010 * |
| Length, cm | 72.76 (72.01 to 73.50) | 74.58 (73.59 to 75.57) | 0.004 * |
| CC, cm | 45.19 (44.87 to 45.51) | 45.86 (45.44 to 46.28) | 0.013 * |
| BMI, Kg/m² | 16.40 (15.97 to 16.83) | 16.63 (16.20 to 17.05) | 0.492 |
| Standardized Percentiles | |||
| Weight, g | 37.55 (31.63 to 43.47) | 56.00 (47.97 to 64.03) | 0.000 * |
| Length, cm | 39.79 (33.28 to 46.30) | 64.68 (56.57 to 72.78) | 0.000 * |
| CC, cm | 46.44 (40.34 to 52.53) | 67.44 (60.44 to 74.44) | 0.000 * |
| Standardized Z-Score | |||
| Weight, g | −0.86 (−1.58 to −1.15) | 0.22 (−0.91 to 0.53) | 0.025 * |
| Length, cm | −1.29 (−1.97 to −0.60) | 0.55 (0.22 to 0.88) | 0.000 * |
| CC, cm | 1.57 (0.50 to 2.64) | 0.66 (0.39 to 0.93) | 0.196 |
| BMI, Kg/m² | −0.37 (−0.79 to 0.03) | −0.07 (−0.37 to 0.22) | 0.298 |
| Weight/Length, kg/cm | −0.17 (−0.38 to 0.03) | 0.01 (−0.28 to 0.30) | 0.315 |
| Weight Z-Score | T | S.E. | ß | p Value | 95 C.I for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Model 1 | ||||||
| Gestational age | −0.464 | 0.158 | −0.405 | 0.004 | −0.776 | −0.151 |
| Birth weight Z-Score | −0.093 | 0.301 | −0.035 | 0.759 | −0.688 | 0.503 |
| Energy intake 1 | −0.008 | 0.002 | −0.594 | 0.000 * | −0.012 | −0.004 |
| Model 2 | ||||||
| Gestational age | −0.450 | 0.137 | −0.393 | 0.001 * | −0.720 | −0.180 |
| Weight 36 wks Z-Score | −0.062 | 0.242 | −0.270 | 0.797 | −0.541 | 0.416 |
| Energy intake 1 | −0.008 | 0.002 | −0.585 | 0.000 * | −0.011 | −0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Chiara, M.; Laccetta, G.; Regoli, D.; Dito, L.; Spiriti, C.; De Santis, B.; Travaglia, E.; Prota, R.; Parisi, P.; Brunelli, R.; et al. Delayed Macronutrients’ Target Achievement in Parenteral Nutrition Reduces the Risk of Hyperglycemia in Preterm Newborn: A Randomized Controlled Trial. Nutrients 2023, 15, 1279. https://doi.org/10.3390/nu15051279
Di Chiara M, Laccetta G, Regoli D, Dito L, Spiriti C, De Santis B, Travaglia E, Prota R, Parisi P, Brunelli R, et al. Delayed Macronutrients’ Target Achievement in Parenteral Nutrition Reduces the Risk of Hyperglycemia in Preterm Newborn: A Randomized Controlled Trial. Nutrients. 2023; 15(5):1279. https://doi.org/10.3390/nu15051279
Chicago/Turabian StyleDi Chiara, Maria, Gianluigi Laccetta, Daniela Regoli, Lucia Dito, Caterina Spiriti, Benedetta De Santis, Elisa Travaglia, Rita Prota, Pasquale Parisi, Roberto Brunelli, and et al. 2023. "Delayed Macronutrients’ Target Achievement in Parenteral Nutrition Reduces the Risk of Hyperglycemia in Preterm Newborn: A Randomized Controlled Trial" Nutrients 15, no. 5: 1279. https://doi.org/10.3390/nu15051279







