Effects of Undernutrition on Swallowing Function and Activities of Daily Living in Hospitalized Patients: Data from the Japanese Sarcopenic Dysphagia Database
Abstract
1. Introduction
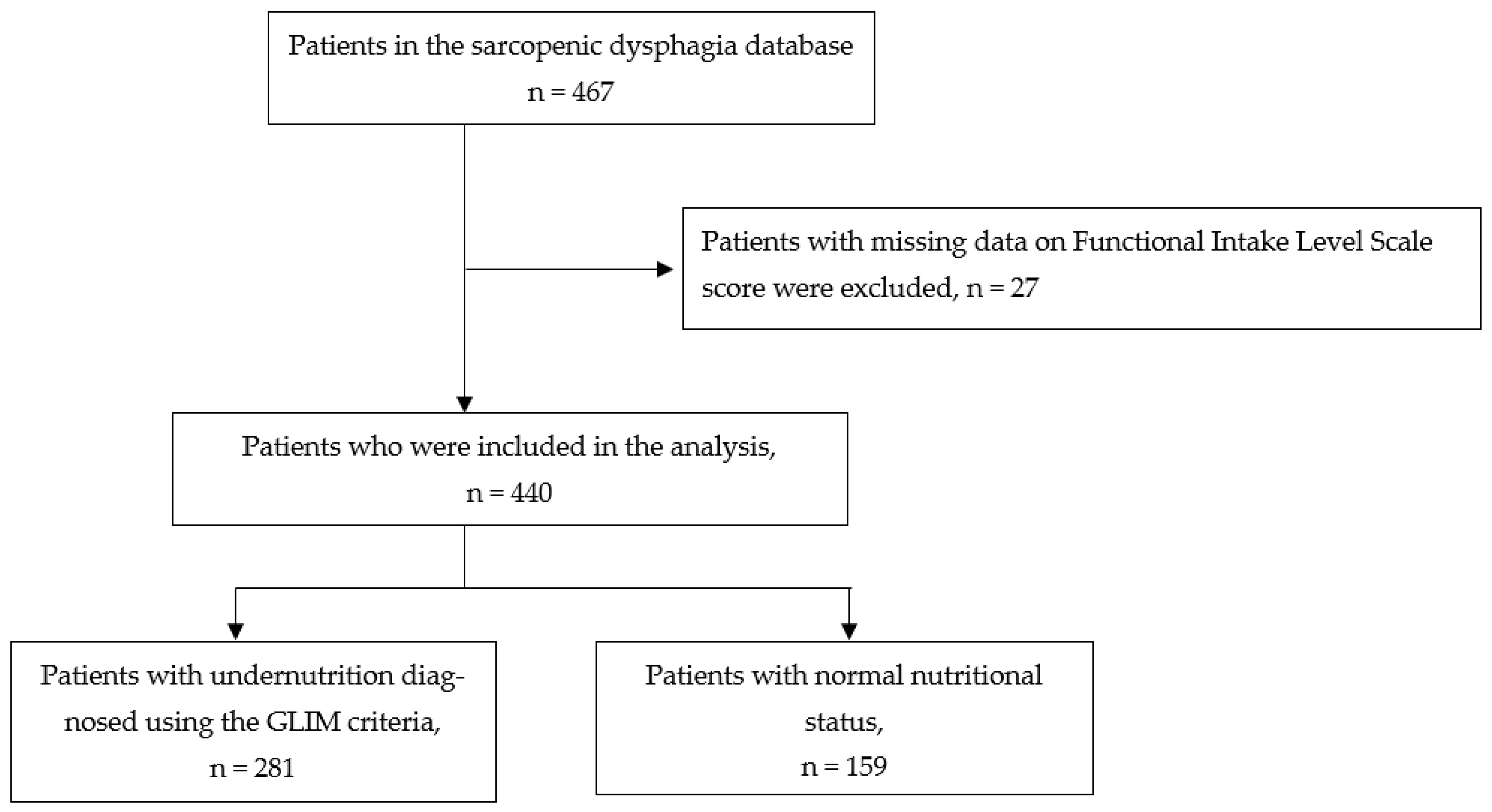
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Nutritional Assessment
2.4. Outcome
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Mori, T.; Nishioka, S.; Oshima, F.; Ogawa, S.; et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr. Gerontol. Int. 2019, 19, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.; Jayasekeran, V.; Pendleton, N.; Horan, M.; Jones, M.; Hamdy, S. Prevalence and symptom profiling of oropharyngeal dysphagia in a community dwelling of an elderly population: A self-reporting questionnaire survey. Dis. Esophagus. 2011, 24, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Stemple, J.; Merrill, R.M.; Thomas, L. Dysphagia in the elderly: Preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann. Otol. Rhinol. Laryngol. 2007, 116, 858–865. [Google Scholar] [CrossRef]
- Park, Y.H.; Han, H.R.; Oh, B.M.; Lee, J.; Park, J.A.; Yu, S.J.; Chang, H. Prevalence and associated factors of dysphagia in nursing home residents. Geriatr. Nurs. 2013, 34, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Peñalva-Arigita, A.; Prats, R.; Lecha, M.; Sansano, A.; Vila, L. Prevalence of dysphagia in a regional hospital setting: Acute care hospital and a geriatric sociosanitary care hospital: A cross-sectional study. Clin. Nutr. ESPEN 2019, 33, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Bomze, L.; Dehom, S.; Lao, W.P.; Thompson, J.; Lee, N.; Cragoe, A.; Luceno, C.; Crawley, B. Comorbid dysphagia and malnutrition in elderly hospitalized patients. Laryngoscope 2021, 131, 2441–2447. [Google Scholar] [CrossRef]
- Sura, L.; Madhavan, A.; Carnaby, G.; Crary, M.A. Dysphagia in the elderly: Management and nutritional considerations. Clin. Interv. Aging 2012, 7, 287–298. [Google Scholar]
- Tran, T.P.; Nguyen, L.T.; Kayashita, J.; Shimura, F.; Yamamoto, S. Nutritional status and feeding practice among dysphagic older adult inpatients in Vietnam. J. Nutr. Sci. Vitaminol. 2020, 66, 224–228. [Google Scholar] [CrossRef]
- Veldee, M.S.; Peth, L.D. Can protein-calorie malnutrition cause dysphagia? Dysphagia 1992, 7, 86–101. [Google Scholar] [CrossRef]
- Wakabayashi, H. Presbyphagia and sarcopenic dysphagia: Association between aging, sarcopenia, and deglutition disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar] [CrossRef]
- Ueshima, J.; Shimizu, A.; Maeda, K.; Uno, C.; Shirai, Y.; Sonoi, M.; Motokawa, K.; Egashira, F.; Kayashita, J.; Kudo, M.; et al. Nutritional management in adult patients with dysphagia: Position paper from Japanese working group on integrated nutrition for dysphagic people. J. Am. Med. Dir. Assoc. 2022, 23, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. JPEN J. Parenter. Enteral. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef]
- Ueshima, J.; Momosaki, R.; Shimizu, A.; Motokawa, K.; Sonoi, M.; Shirai, Y.; Uno, C.; Kokura, Y.; Shimizu, M.; Nishiyama, A.; et al. Nutritional assessment in adult patients with dysphagia: A scoping review. Nutrients 2021, 13, 778. [Google Scholar] [CrossRef] [PubMed]
- Riso, S.; Para, O.; Collo, A.; Campanini, M.; Rotunno, S.; Giorgetti, G.; Zanetti, M.; Manfellotto, D. FADOI and SINPE Clinical nutrition in internal medicine: An Italian survey by the scientific societies FADOI and SINPE. Nutrition 2022, 98, 111623. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Wakabayashi, H.; Fujimoto, M.; Obayashi, S.; Yamamoto, M.; Nishioka, S.; Momosaki, R. Association between malnutrition severity and swallowing function in convalescent rehabilitation wards: A multi-center cohort study in malnourished patients with sarcopenic dysphagia. J. Nutr. Health Aging 2022, 26, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ohno, T.; Nomoto, A.; Shigematsu, T.; Kayashita, J. Japanese Working Group on Sarcopenic Dysphagia Effect of low tongue pressure on nutritional status and improvement of swallowing function in sarcopenic dysphagia. Nutrition 2021, 90, 111295. [Google Scholar] [CrossRef]
- Mizuno, S.; Wakabayashi, H.; Fujishima, I.; Kishima, M.; Itoda, M.; Yamakawa, M.; Wada, F.; Kato, R.; Furiya, Y.; Nishioka, S.; et al. Construction and quality evaluation of the Japanese sarcopenic dysphagia database. J. Nutr. Health Aging 2021, 25, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and validity of a tool to measure the severity of dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom. Manag. 2013, 46, 201–206. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini nutritional assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, R.; Murakami, H.; Sanada, K.; Tanaka, N.; Sawada, S.S.; Tabata, I.; Higuchi, M.; Miyachi, M. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr. Gerontol. Int. 2015, 15, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Collin, C.; Wade, D.T.; Davies, S.; Horne, V. The Barthel ADL Index: A reliability study. Int. Disabil Stud. 1988, 10, 61–63. [Google Scholar] [CrossRef]
- Nishioka, S.; Okamoto, T.; Takayama, M.; Urushihara, M.; Watanabe, M.; Kiriya, Y.; Shintani, K.; Nakagomi, H.; Kageyama, N. Malnutrition risk predicts recovery of full oral intake among older adult stroke patients undergoing enteral nutrition: Secondary analysis of a multicentre survey (the APPLE study). Clin. Nutr. 2017, 36, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Wakabayashi, H. Interaction between malnutrition and physical disability in older adults: Is there a malnutrition-disability cycle? Nutr. Rev. 2023, 10, 191–205. [Google Scholar] [CrossRef]
- Butler, S.G.; Stuart, A.; Leng, X.; Wilhelm, E.; Rees, C.; Williamson, J.; Kritchevsky, S.B. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Takaki, M.; Akagi, J. Decreased skeletal muscle mass and risk factors of sarcopenic dysphagia: A prospective observational cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1290–1294. [Google Scholar] [CrossRef]
- Wojzischke, J.; van Wijngaarden, J.; van den Berg, C.; Cetinyurek-Yavuz, A.; Diekmann, R.; Luiking, Y.; Bauer, J. Nutritional status and functionality in geriatric rehabilitation patients: A systematic review and meta-analysis. Eur. Geriatr. Med. 2020, 11, 195–207. [Google Scholar] [CrossRef]
- Kokura, Y.; Momosaki, R. Prevalence of malnutrition assessed by the GLIM criteria and association with Activities of Daily Living in older residents in an integrated facility for medical and long-term care. Nutrients 2022, 14, 3656. [Google Scholar] [CrossRef] [PubMed]
- Kalm, L.M.; Semba, R.D. They starved so that others be better fed: Remembering Ancel Keys and the Minnesota experiment. J. Nutr. 2005, 135, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Kurkcu, M.; Meijer, R.I.; Lonterman, S.; Muller, M.; de van der Schueren, M.A.E. The association between nutritional status and frailty characteristics among geriatric outpatients. Clin. Nutr. ESPEN 2018, 23, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ohno, T.; Nomoto, A.; Kayashita, J.; Mori, N. the Japanese Working Group on Sarcopenic Dysphagia Nutritional management enhances the recovery of swallowing ability in older patients with sarcopenic dysphagia. Nutrients 2021, 11, 596. [Google Scholar] [CrossRef]
- Hudson, H.M.; Daubert, C.R.; Mills, R.H. The interdependency of protein-energy malnutrition, aging, and dysphagia. Dysphagia 2000, 15, 31–38. [Google Scholar] [CrossRef]
- Shimazu, S.; Yoshimura, Y.; Kudo, M.; Nagano, F.; Bise, T.; Shiraishi, A.; Sunahara, T. Frequent and personalized nutritional support leads to improved nutritional status, activities of daily living, and dysphagia after stroke. Nutrition 2021, 83, 111091. [Google Scholar] [CrossRef]
- Wakabayashi, H. Rehabilitation nutrition in general and family medicine. J. Gen. Fam Med. 2017, 18, 153–154. [Google Scholar] [CrossRef]
- Maeda, K.; Akagi, J. Treatment of sarcopenic dysphagia with rehabilitation and nutritional support: A comprehensive approach. J. Acad. Nutr. Diet. 2016, 116, 573–577. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Uwano, R. Rehabilitation nutrition for possible sarcopenic dysphagia after lung cancer surgery: A case report. Am. J. Phys. Med. Rehabil. 2016, 95, e84–e89. [Google Scholar] [CrossRef]
- Zanetti, G.; Merati, G. Interaction between photosystem I and ferredoxin. Identification by chemical cross-linking of the polypeptide which binds ferredoxin. Eur. J. Biochem. 1987, 169, 143–146. [Google Scholar] [CrossRef]
| All Patients n = 440 | Patients with Undernutrition Diagnosed Using the GLIM Criteria n = 281 | Patients with Normal Nutritional Status n = 159 | p-Value | |
|---|---|---|---|---|
| Age, years, mean ± SD | 80.2 ± 10.9 | 81.8 ± 10.5 | 77.3 ± 11.0 | <0.001 (a) |
| Sex, female, n (%) | 224 (50.9) | 152 (54.1) | 72 (45.3) | 0.091 (b) |
| Prehospitalization facilities, n (%) | 0.223 (c) | |||
| Acute-care hospitals | 187 (42.5) | 125 (44.5) | 62 (39.0) | |
| Rehabilitation hospitals | 209 (47.5) | 124 (44.1) | 85 (53.5) | |
| Long-term care hospitals | 41 (9.3) | 30 (10.7) | 11 (6.9) | |
| Others | 3 (0.7) | 2 (0.7) | 1 (0.6) | |
| Body mass index (kg/m2), mean ± SD | 20.2 ± 3.7 | 19.6 ± 3.5 | 21.4 ± 3.8 | <0.001 (a) |
| Calf circumference (cm), median (IQR) | 28 (25, 31) | 27 (25, 30) | 30 (26, 33) | <0.001 (d) |
| Handgrip strength (kg), median (IQR) | 12.0 (6.3, 19.0) | 11.0 (6.0, 17.5) | 14.2 (8.1, 22.1) | <0.001 (d) |
| C-reactive protein levels (mg/dL), median (IQR) | 0.7 (0.2, 2.9) | 1.0 (0.3, 5.2) | 0.2 (0.1, 0.7) | <0.001 (d) |
| Serum albumin levels (g/dL), median (IQR) | 3.4 (3.0, 3.7) | 3.3 (2.9, 3.6) | 3.5 (3.3, 3.9) | <0.001 (d) |
| Charlson Comorbidity Index, median (IQR) | 2 (0, 4) | 2 (1, 4) | 2 (0, 3) | <0.001 (d) |
| Patients with Undernutrition Diagnosed Using the GLIM Criteria n = 281 | Patients with Normal Nutritional Status n = 159 | p-Value | |
|---|---|---|---|
| Phenotypic criteria, presence, n (%) | |||
| Weight loss | 41 (9.4) | 7 (1.6) | <0.001 (a) |
| Low body mass index | 158 (36.0) | 53 (12.1) | <0.001 (a) |
| Reduced muscle mass | 276 (63.0) | 118 (26.9) | <0.001 (a) |
| Etiologic criteria, presence, n (%) | |||
| Reduced food intake or assimilation | 160 (36.4) | 8 (1.8) | <0.001 (a) |
| Disease burden/inflammation | 170 (38.7) | 13 (3.0) | <0.001 (a) |
| Patients with Undernutrition Diagnosed Using the GLIM Criteria n = 281 | Patients with Normal Nutritional Status n = 159 | p-Value | |
|---|---|---|---|
| Functional Intake Level Scale score, median (IQR) | |||
| Admission | 7 (5, 8) | 7 (2, 7) | <0.001 (a) |
| Follow-up | 8 (7, 8) | 8 (7, 8) | 0.262 (a) |
| Change | 1 (0, 5) | 0 (0, 2) | <0.001 (a) |
| Barthel Index, median (IQR) | |||
| Admission | 30 (10, 50) | 25 (5, 50) | 0.224 (a) |
| Follow-up | 50 (20, 70) | 60 (30, 90) | <0.001 (a) |
| Change | 10 (0, 28) | 20 (5, 40) | <0.001 (a) |
| Change in the Functional Intake Level Scale Score | Change in the Barthel Index Score | |||||||
|---|---|---|---|---|---|---|---|---|
| B | 95% Confidence Interval | p-Value | B | 95% Confidence Interval | p-Value | |||
| Lower | Upper | Lower | Upper | |||||
| Age | −0.051 | −0.072 | −0.029 | <0.001 | −0.201 | −0.416 | 0.014 | 0.067 |
| Sex | −0.392 | −0.849 | 0.064 | 0.092 | −0.277 | −4.857 | 4.303 | 0.905 |
| Charlson Comorbidity Index | −0.098 | −0.220 | 0.024 | 0.116 | −1.859 | −3.085 | −0.633 | <0.003 |
| Prehospitalization facilities | −1.159 | −1.497 | −0.821 | <0.001 | −0.547 | −3.938 | 2.844 | 0.751 |
| Undernutrition diagnosed using the GLIM criteria | −0.633 | −1.099 | −0.167 | <0.008 | −8.414 | −13.089 | −3.739 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, S.; Kokura, Y.; Maeda, K.; Nishioka, S.; Momosaki, R.; Matsuoka, H.; Tomii, Y.; Sugita, S.; Shimizu, K.; Esashi, N.; et al. Effects of Undernutrition on Swallowing Function and Activities of Daily Living in Hospitalized Patients: Data from the Japanese Sarcopenic Dysphagia Database. Nutrients 2023, 15, 1291. https://doi.org/10.3390/nu15051291
Abe S, Kokura Y, Maeda K, Nishioka S, Momosaki R, Matsuoka H, Tomii Y, Sugita S, Shimizu K, Esashi N, et al. Effects of Undernutrition on Swallowing Function and Activities of Daily Living in Hospitalized Patients: Data from the Japanese Sarcopenic Dysphagia Database. Nutrients. 2023; 15(5):1291. https://doi.org/10.3390/nu15051291
Chicago/Turabian StyleAbe, Sayaka, Yoji Kokura, Keisuke Maeda, Shinta Nishioka, Ryo Momosaki, Hiroki Matsuoka, Yasuomi Tomii, Shinnosuke Sugita, Kenta Shimizu, Nanami Esashi, and et al. 2023. "Effects of Undernutrition on Swallowing Function and Activities of Daily Living in Hospitalized Patients: Data from the Japanese Sarcopenic Dysphagia Database" Nutrients 15, no. 5: 1291. https://doi.org/10.3390/nu15051291
APA StyleAbe, S., Kokura, Y., Maeda, K., Nishioka, S., Momosaki, R., Matsuoka, H., Tomii, Y., Sugita, S., Shimizu, K., Esashi, N., & Wakabayashi, H. (2023). Effects of Undernutrition on Swallowing Function and Activities of Daily Living in Hospitalized Patients: Data from the Japanese Sarcopenic Dysphagia Database. Nutrients, 15(5), 1291. https://doi.org/10.3390/nu15051291









