Systematic Review and Meta-Analysis: Malnutrition and In-Hospital Death in Adults Hospitalized with COVID-19
Highlights
- Malnutrition in adults hospitalized with COVID-19 nearly quadrupled the risk of in-hospital death.
- Meta-analysis of studies from four continents shows a malnutrition prevalence of 52.6% among COVID-19 patients.
- The risk of death was significantly elevated across studies using various malnutrition screening methods.
- The findings underscore the urgent need for routine nutrition screening in hospitalized COVID-19 patients.
- Since the findings are derived from studies performed in nine countries over four continents with data from 354,332 patients, they should be generalizable.
Abstract
:1. Introduction
COVID-19
2. Methods
2.1. Protocol and Registration
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Databases
2.5. Search Words
2.6. Study Quality
2.7. Search Strategy
2.8. Data Extraction
2.9. Data Analysis
3. Results
3.1. Selection of Studies
3.2. Description of Included Studies
4. Discussion
4.1. Limitations
4.2. Implications
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J. Parenter. Enteral Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totland, T.; Krogh, H.; Smedshaug, G.; Tornes, R.; Bye, A.; Paur, I. Harmonization and standardization of malnutrition screening for all adults—A systematic review initiated by the Norwegian Directorate of Health. Clin. Nutr. ESPEN 2022, 52, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Omura, T.; Araki, A. Skeletal muscle as a treatment target for older adults with diabetes mellitus: The importance of a multimodal intervention based on functional category. Geriatr. Gerontol. Int. 2022, 22, 110–120. [Google Scholar] [CrossRef]
- Tan, V.M.; Pang, B.W.; Lau, L.K.; Jabbar, K.A.; Seah, W.T.; Chen, K.K.; Ng, T.P.; Wee, S.L. Malnutrition and Sarcopenia in Community-Dwelling Adults in Singapore: Yishun Health Study. J. Nutr. Health Aging 2021, 25, 374–381. [Google Scholar] [CrossRef] [PubMed]
- De van der Schueren, M.A.E.; Borkent, J.W.; Spaans, G.W.; Nijhof, A.; Manders, M. GLIM in nursing homes; practical implications. Clin. Nutr. 2022, 41, 2442–2445. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Maeda, K.; Fujimoto, Y.; Nonogaki, T.; Ishida, Y.; Ohta, R.; Shimizu, A.; Ueshima, J.; Nagano, A.; Fukushima, R. Prognostic implications of the global leadership initiative on malnutrition criteria as a routine assessment modality for malnutrition in hospitalized patients at a university hospital. Clin. Nutr. 2022, 42, 166–172. [Google Scholar] [CrossRef]
- Silva, N.; Cardoso, L.; Muniz, C.; Prestes, I.; Pena, G. Failure to achieve proteic goals in non-critical patients increases risk for death: Old discussion, ongoing problem. Nutrition 2020, 77, 110894. [Google Scholar] [CrossRef]
- Hegelund, M.H.; Ryrsø, C.K.; Ritz, C.; Dungu, A.M.; Sejdic, A.; Jensen, A.V.; Hansen, N.M.; Mølgaard, C.; Krogh-Madsen, R.; Lindegaard, B.; et al. Are Undernutrition and Obesity Associated with Post-Discharge Mortality and Re-Hospitalization after Hospitalization with Community-Acquired Pneumonia? Nutrients 2022, 14, 4906. [Google Scholar] [CrossRef]
- Cheong, C.; Yap, P.; Yap, K.; Ng, T. Associations of Inflammatory, Metabolic, Malnutrition, and Frailty Indexes with Multimorbidity Incidence and Progression, and Mortality Impact: Singapore Longitudinal Aging Study. Gerontology 2023, 6, 1–12. [Google Scholar] [CrossRef]
- Paur, I.; Smedshaug, G.B.; Haugum, B.; Bye, A.; Eliassen, E.; Flottorp, T.L.; Juul, H.J.; Mowe, M.; Nakken, T.; Ore, S.; et al. The Norwegian Directorate of Health recommends malnutrition screening tool (MST) for all adults. Clin. Nutr. ESPEN 2022, 52, 28–31. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. JPEN J. Parenter. Enteral Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y.; Morley, J.E.; Chumlea, W.; Salva, A.; Rubenstein, K.Z.; et al. Overview of the MNA—Its history and challenges. J. Nutr. Health Aging 2006, 10, 456–463; discussion 63–65. [Google Scholar] [PubMed]
- Kondrup, J.; Rasmussen, H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Scott, A. Screening for malnutrition in the community: The MUST tool. Br. J. Community Nurs. 2008, 13, 406–412. [Google Scholar] [CrossRef]
- Salinas, M.; Flores, E.; Blasco, A.; López-Garrigós, M.; Puche, C.; Asencio, A.; Leiva-Salinas, C. CONUT: A tool to assess nutritional status. First application in a primary care population. Diagnosis 2020, 8, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Nishigoori, S.; Uchiyama, S.; Asai, K.; Shimizu, W. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score. Heart Vessels 2018, 33, 134–144. [Google Scholar] [CrossRef]
- Power, L.; Mullally, D.; Gibney, E.; Clarke, M.; Visser, M.; Volkert, D.; Bardon, L.; de van der Schueren, M.A.; Corish, C.A.; MaNuEL Consortium. A review of the validity of malnutrition screening tools used in older adults in community and healthcare settings—A MaNuEL study. Clin. Nutr. ESPEN 2018, 24, 1–13. [Google Scholar] [CrossRef] [Green Version]
- El Chaar, D.; Mattar, L.; Fakih El Khoury, C. AND/ASPEN and the GLIM malnutrition diagnostic criteria have a high degree of criterion validity and reliability for the identification of malnutrition in a hospital setting: A single-center prospective study. JPEN J. Parenter. Enteral Nutr. 2022, 46, 1061–1070. [Google Scholar] [CrossRef]
- Clarke-Deelder, E.; Rokicki, S.; McGovern, M.; Birabwa, C.; Cohen, J.; Waiswa, P.; Abbo, C. Levels of depression, anxiety, and psychological distress among Ugandan adults during the first wave of the COVID-19 pandemic: Cross-sectional evidence from a mobile phone-based population survey. Glob. Ment. Health 2022, 9, 274–284. [Google Scholar] [CrossRef]
- Duong, K.N.C.; Le Bao, T.N.; Nguyen, P.T.L.; Van, T.V.; Lam, T.P.; Gia, A.P.; Anuratpanich, L.; Van, B.V. Psychological Impacts of COVID-19 During the First Nationwide Lockdown in Vietnam: Web-Based, Cross-Sectional Survey Study. JMIR Form. Res. 2020, 4, e24776. [Google Scholar] [CrossRef]
- Sameer, A.; Khan, M.; Nissar, S.; Banday, M. Assessment of Mental Health and Various Coping Strategies among general population living Under Imposed COVID-Lockdown Across world: A Cross-Sectional Study. Ethics Med. Public Health 2020, 15, 100571. [Google Scholar] [CrossRef] [PubMed]
- Geda, N.; Feng, C.; Peters, B. Suicidal ideation among Canadian adults during the COVID-19 pandemic: The role of psychosocial factors and substance use behaviours. BMC Psychiatry 2022, 22, 711. [Google Scholar] [CrossRef] [PubMed]
- Zalsman, G.; Levy, Y.; Sommerfeld, E.; Segal, A.; Assa, D.; Ben-Dayan, L.; Valevski, A.; Mann, J.J. Suicide-related calls to a national crisis chat hotline service during the COVID-19 pandemic and lockdown. J. Psychiatr. Res. 2021, 139, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Junus, A.; Li, T.; Yip, P. Temporal and spatial trends in suicide-related visits before and during the COVID-19 pandemic in the US, 2018–2021. J. Affect. Disord. 2023, 324, 24–35. [Google Scholar] [CrossRef]
- Abdulla, Z.A.R.A.; Almahmood, H.O.; Alghasra, R.R.; Alherz, Z.A.S.; Alsharifa, H.A.G.; Qamber, S.J.; Alomar, N.A.; Almajed, F.E.; Almahroos, T.R.; Alnajjas, Z.A.; et al. Prevalence and associated factors of binge eating disorder among Bahraini youth and young adults: A cross-sectional study in a self-selected convenience sample. J. Eat. Disord. 2023, 11, 5. [Google Scholar] [CrossRef]
- Ra, J. Consumption of sugar-sweetened beverages and fast foods deteriorates adolescents’ mental health. Front. Nutr. 2022, 9, 1058190. [Google Scholar] [CrossRef]
- Summers, C.; Do Vale, M.L.; Haines, L.; Armes, S.; Bradfield, J.; Crocombe, D.; Ray, S. A web-based survey assessing perceived changes in diet, physical activity and sleeping behaviours in adults with type 1 and type 2 diabetes during the COVID-19 pandemic in the UK. BMJ Nutr. Prev. Health 2022, 5, 137–144. [Google Scholar] [CrossRef]
- Han, S.; Jang, H.; Ko, Y. COVID-19-related anxiety and lifestyle changes. Front. Public Health 2022, 10, 886137. [Google Scholar] [CrossRef]
- Kaufman-Shriqui, V.; Navarro, D.; Raz, O.; Boaz, M. Dietary changes and anxiety during the coronavirus pandemic: A multinational survey. Eur. J. Clin. Nutr. 2022, 76, 84–92. [Google Scholar] [CrossRef]
- Correia, M.; Waitzberg, D. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Malnutrition and in-Hospital Death in Adults Hospitalized with COVID-19. PROSPERO 2023 CRD42023392009. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023392009 (accessed on 31 January 2023).
- Zhang, K.; Gui, H.; Cong, J.; He, P. A modified nutrition risk screening 2002 predicts the risk of death among hospitalized patients with COVID-19. Clin. Nutr. ESPEN 2022, 52, 365–370, Epub 17 September 2022. [Google Scholar] [CrossRef] [PubMed]
- Ponce, J.; Anzalone, A.J.; Bailey, K.; Sayles, H.; Timmerman, M.; Jackson, M.; McClay, J.; Hanson, C. National COVID Cohort Collaborative (N3C) Consortium. Impact of malnutrition on clinical outcomes in patients diagnosed with COVID-19. JPEN J. Parenter Enteral. Nutr. 2022, 46, 1797–1807, Epub 21 June 2022. [Google Scholar] [CrossRef]
- da Silva, C.L.; Sousa, T.M.M.; de Sousa Junior, J.B.; Nakano, E.Y. Nutritional factors associated with mortality in hospitalized patients with COVID-19. Clin. Nutr. Open Sci. 2022, 45, 17–26, Epub 20 August 2022. [Google Scholar] [CrossRef]
- Gregoriano, C.; Voelkle, M.; Koch, D.; Hauser, S.I.; Kutz, A.; Mueller, B.; Schuetz, P. Association of Different Malnutrition Parameters and Clinical Outcomes among COVID-19 Patients: An Observational Study. Nutrients 2022, 14, 3449. [Google Scholar] [CrossRef]
- Shabanpur, M.; Pourmahmoudi, A.; Nicolau, J.; Veronese, N.; Roustaei, N.; Jahromi, A.J.; Hosseinikia, M. The importance of nutritional status on clinical outcomes among both ICU and Non-ICU patients with COVID-19. Clin. Nutr. ESPEN 2022, 49, 225–231, Epub 21 April 2022. [Google Scholar] [CrossRef]
- Aktan, A.; Güzel, T.; Demir, M.; Özbek, M. The effect of nutritional scores on mortality in COVID-19 patients. Rev. Assoc. Med. Bras. 2022, 68, 1096–1102. [Google Scholar] [CrossRef]
- Nunes, E.C.; Marcon, S.; Oliveira, P.E.; Loss, S.H. Nutritional profile and outcomes of noncritical hospitalized patients with COVID-19 in a large tertiary hospital in southern Brazil. Rev. Assoc. Med. Bras. 2022, 68, 1216–1220. [Google Scholar] [CrossRef]
- Polat, O.; Yuruyen, M.; Sonmezoz, G.B.; Kansu, A.D.; Erismis, B.; Karendere, F.; Kocoglu, H.; Karabela, S.; Yasar, K.K. Malnutrition risk frequency and independent risk factors associated with mortality in hospitalized elderly patients with COVID-19 in Turkey. Asia Pac. J. Clin. Nutr. 2022, 31, 355–361. [Google Scholar] [CrossRef]
- Nicolau, J.; Ayala, L.; Sanchís, P.; Olivares, J.; Dotres, K.; Soler, A.G.; Rodríguez, I.; Gómez, L.A.; Masmiquel, L. Influence of nutritional status on clinical outcomes among hospitalized patients with COVID-19. Clin. Nutr. ESPEN 2021, 43, 223–229, Epub 29 April 2021. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Formisano, E.; Klersy, C.; Ferretti, V.; Ferrari, A.; Demontis, S.; Mascheroni, A.; Masi, S.; Crotti, S.; Lobascio, F.; et al. NUTRI-COVID19 Collaborative Working Group. Nutritional parameters associated with prognosis in non-critically ill hospitalized COVID-19 patients: The NUTRI-COVID19 study. Clin Nutr. 2022, 41, 2980–2987, Epub 25 June 2021. [Google Scholar] [CrossRef] [PubMed]
- Kananen, L.; Eriksdotter, M.; Boström, A.M.; Kivipelto, M.; Annetorp, M.; Metzner, C.; Bäck Jerlardtz, V.; Engström, M.; Johnson, P.; Lundberg, L.G.; et al. Body mass index and Mini Nutritional Assessment-Short Form as predictors of in-geriatric hospital mortality in older adults with COVID-19. Clin Nutr. 2022, 41, 2973–2979, Epub 23 December 2021. [Google Scholar] [CrossRef] [PubMed]
- Vong, T.; Yanek, L.R.; Wang, L.; Yu, H.; Fan, C.; Zhou, E.; Oh, S.J.; Szvarca, D.; Kim, A.; Potter, J.J.; et al. Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19. Nutrients 2022, 14, 1310. [Google Scholar] [CrossRef]
- Abate, S.; Chekole, Y.; Estifanos, M.; Abate, K.; Kabthymer, R. Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 43, 174–183. [Google Scholar] [CrossRef]
- Raj, K.; Yeruva, K.; Jyotheeswara Pillai, K.; Kumar, P.; Agrawal, A.; Chandna, S.; Khuttan, A.; Tripathi, S.; Akella, R.; Gudi, T.R.; et al. Population Risk Factors for Severe Disease and Mortality in COVID-19 in the United States during the Pre-Vaccine Era: A Retrospective Cohort Study of National Inpatient Sample. Med. Sci. 2022, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Graziano, E.; Peghin, M.; De Martino, M.; De Carlo, C.; Da Porto, A.; Bulfone, L.; Casarsa, V.; Sozio, E.; Fabris, M.; Cifù, A.; et al. The impact of body composition on mortality of COVID-19 hospitalized patients: A prospective study on abdominal fat, obesity paradox and sarcopenia. Clin. Nutr. ESPEN 2022, 51, 437–444. [Google Scholar] [CrossRef]
- Silva, F.M.; Lima, J.; Teixeira, P.P.; Grezzana, G.B.; Figueiro, M.; Colombo, T.; Souto, K.; Stein, A.T. Risk of bias and certainty of evidence on the association between obesity and mortality in patients with SARS-CoV-2: An umbrella review of meta-analyses. Clin. Nutr. ESPEN 2023, 53, 13–25. [Google Scholar] [CrossRef]
- Da Porto, A.; Tascini, C.; Peghin, M.; Sozio, E.; Colussi, G.; Casarsa, V.; Bulfone, L.; Graziano, E.; De Carlo, C.; Catena, C.; et al. Prognostic Role of Malnutrition Diagnosed by Bioelectrical Impedance Vector Analysis in Older Adults Hospitalized with COVID-19 Pneumonia: A Prospective Study. Nutrients 2021, 13, 4085. [Google Scholar] [CrossRef]
- Kang, M.G.; Choi, J.Y.; Yoo, H.J.; Park, S.Y.; Kim, Y.; Kim, J.Y.; Kim, S.W.; Kim, C.H.; Kim, K.I. Impact of malnutrition evaluated by the mini nutritional assessment on the prognosis of acute hospitalized older adults. Front. Nutr. 2023, 9, 1046985. [Google Scholar] [CrossRef]
- Lenti, M.V.; Croce, G.; Brera, A.S.; Ballesio, A.; Padovini, L.; Bertolino, G.; Sabatino, A.D.; Klersy, C.; Corazza, G.R. Rate and risk factors of in-hospital and early post-discharge mortality in patients admitted to an internal medicine ward. Clin. Med. 2023, 231, 16–23. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; Humphreys, M.H.; Kopple, J.D. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003, 63, 793–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, N.M.; Pai, P.C.; Chuang, C.C.; Chuang, W.C.; Tseng, C.K.; Chang, K.P.; Yen, T.C.; Lin, J.D.; Chang, J.T. Overweight and obesity predict better overall survival rates in cancer patients with distant metastases. Cancer Med. 2016, 5, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Bannister, W.P.; Mast, T.C.; de Wit, S.; Gerstoft, J.; Wiese, L.; Milinkovic, A.; Hadziosmanovic, V.; Clarke, A.; Rasmussen, L.D.; Lacombe, K.; et al. Changes in body mass index and clinical outcomes after initiation of contemporary antiretroviral regimens. AIDS 2022, 36, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Fehler, P.; Zielińska, M.; Uchmanowicz, B.; Juárez-Vela, R.; Lewandowski, Ł.; Zieliński, S.; Czapla, M. Do Body Mass Index and Nutritional Risk Score 2002 Influence the In-Hospital Mortality of Patients Following Cardiac Arrest? Nutrients 2023, 15, 436. [Google Scholar] [CrossRef]
- Kasapoglu, U.; Gok, A.; Delen, L.; Ozer, A. Comparison of nutritional risk status assessment tools in predicting 30-day survival in critically ill COVID-19 pneumonia patients. Ann. Saudi Med. 2022, 42, 236–245. [Google Scholar] [CrossRef]
- Inzitari, M.; Arnal, C.; Ribera, A.; Hendry, A.; Cesari, M.; Roca, S.; Pérez, L.M. Comprehensive Geriatric Hospital at Home: Adaptation to Referral and Case-Mix Changes During the COVID-19 Pandemic. J. Am. Med. Dir. Assoc. 2023, 24, 3–9.e1. [Google Scholar] [CrossRef]
- Jeleff, M.; Traugott, M.; Jirovsky-Platter, E.; Jordakieva, G.; Kutalek, R. Occupational challenges of healthcare workers during the COVID-19 pandemic: A qualitative study. BMJ Open 2022, 12, e054516. [Google Scholar] [CrossRef]

| Citation | Study Design | Number of Patients | Malnutrition Measure | Country | Publication Date |
|---|---|---|---|---|---|
| Zhang K, Gui H, Cong J, He P. A modified nutrition risk screening 2002 predicts the risk of death among hospitalized patients with COVID-19. Clin Nutr ESPEN. 2022; 52:365–370 [34]. | Prospective | 678 | Modified NRS; NRS 2002; MNA-SF; MUST | China | Dec-22 |
| Ponce J, Anzalone AJ, Bailey K, Sayles H, Timmerman M, Jackson M, McClay J, Hanson C; National COVID Cohort Collaborative (N3C) Consortium. Impact of malnutrition on clinical outcomes in patients diagnosed with COVID-19. JPEN J Parenter Enteral Nutr. 2022; 46: 1797–1807 [35]. | Cross sectional | 343,188 | Diagnostic code in electronic medical record | USA | Nov-22 |
| da Silva CL, Sousa TMM, de Sousa Junior JB, Nakano EY. Nutritional factors associated with mortality in hospitalized patients with COVID-19. Clin Nutr Open Sci. 2022; 45:17–26 [36]. | Historical prospective | 222 | NRS 2002 | Brazil | Aug-22 |
| Gregoriano C, Voelkle M, Koch D, Hauser SI, Kutz A, Mueller B, Schuetz P. Association of Different Malnutrition Parameters and Clinical Outcomes among COVID-19 Patients: An Observational Study. Nutrients. 2022;14:3449 [37]. | Prospective | 305 | NRS 2002; BMI; albumin | Switzerland | Aug-22 |
| Shabanpur M, Pourmahmoudi A, Nicolau J, Veronese N, Roustaei N, Jahromi AJ, Hosseinikia M. The importance of nutritional status on clinical outcomes among both ICU and Non-ICU patients with COVID-19. Clin Nutr ESPEN. 2022; 49:225–231 [38]. | Prospective | 400 | NRS 2002 | Iran | Jun-22 |
| Aktan A, Güzel T, Demir M, Özbek M. The effect of nutritional scores on mortality in COVID-19 patients. Rev Assoc Med Bras (1992). 2022; 68:1096–1102 [39]. | Retrospective | 1488 | CONUT, PNI | Turkey | Aug-22 |
| Nunes EC, Marcon S, Oliveira PE, Loss SH. Nutritional profile and outcomes of noncritical hospitalized patients with COVID-19 in a large tertiary hospital in southern Brazil. Rev Assoc Med Bras (1992). 2022; 68: 1216–1220 [40]. | Cross sectional | 526 | NRS 2002, diet acceptance (% food consumed) | Brazil | Sep-22 |
| Polat O, Yuruyen M, Sonmezoz GB, Kansu AD, Erismis B, Karendere F, Kocoglu H, Karabela S, Yasar KK. Malnutrition risk frequency and independent risk factors associated with mortality in hospitalized elderly patients with COVID-19 in Turkey. Asia Pac J Clin Nutr. 2022; 31:355–361 [41]. | Cross sectional | 451 | NRS 2002 | Turkey | Mar-22 |
| Nicolau J, Ayala L, Sanchís P, Olivares J, Dotres K, Soler AG, Rodríguez I, Gómez LA, Masmiquel L. Influence of nutritional status on clinical outcomes among hospitalized patients with COVID-19. Clin Nutr ESPEN. 2021; 43:223–229 [42]. | Cross sectional | 75 | SGA | Spain | Apr-21 |
| Caccialanza R, Formisano E, Klersy C, Ferretti V, Ferrari A, Demontis S, Mascheroni A, Masi S, Crotti S, Lobascio F, Cerutti N, Orlandoni P, Dalla Costa C, Redaelli E, Fabbri A, Malesci A, Corrao S, Bordandini L, Cereda E; NUTRI-COVID19 Collaborative Working Group. Nutritional parameters associated with prognosis in non-critically ill hospitalized COVID-19 patients: The NUTRI-COVID19 study. Clin Nutr. 2022; 41:2980–2987 [43]. | Prospective | 1391 | Self-reported reduction of food intake 3–5 days prior to hospitalization | Italy | Dec-22 |
| Kananen L, Eriksdotter M, Boström AM, Kivipelto M, Annetorp M, Metzner C, Bäck Jerlardtz V, Engström M, Johnson P, Lundberg LG, Åkesson E, Sühl Öberg C, Hägg S, Religa D, Jylhävä J, Cederholm T. Body mass index and Mini Nutritional Assessment-Short Form as predictors of in-geriatric hospital mortality in older adults with COVID-19. Clin Nutr. 2022; 41:2973–2979 [44]. | Retrospective | 1297 | MNA, BMI | Sweden | Dec-22 |
| Vong T, Yanek LR, Wang L, Yu H, Fan C, Zhou E, Oh SJ, Szvarca D, Kim A, Potter JJ, Mullin GE. Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19. Nutrients. 2022;14:1310 [45]. | Retrospective | 4311 | MUST | USA | Mar-22 |
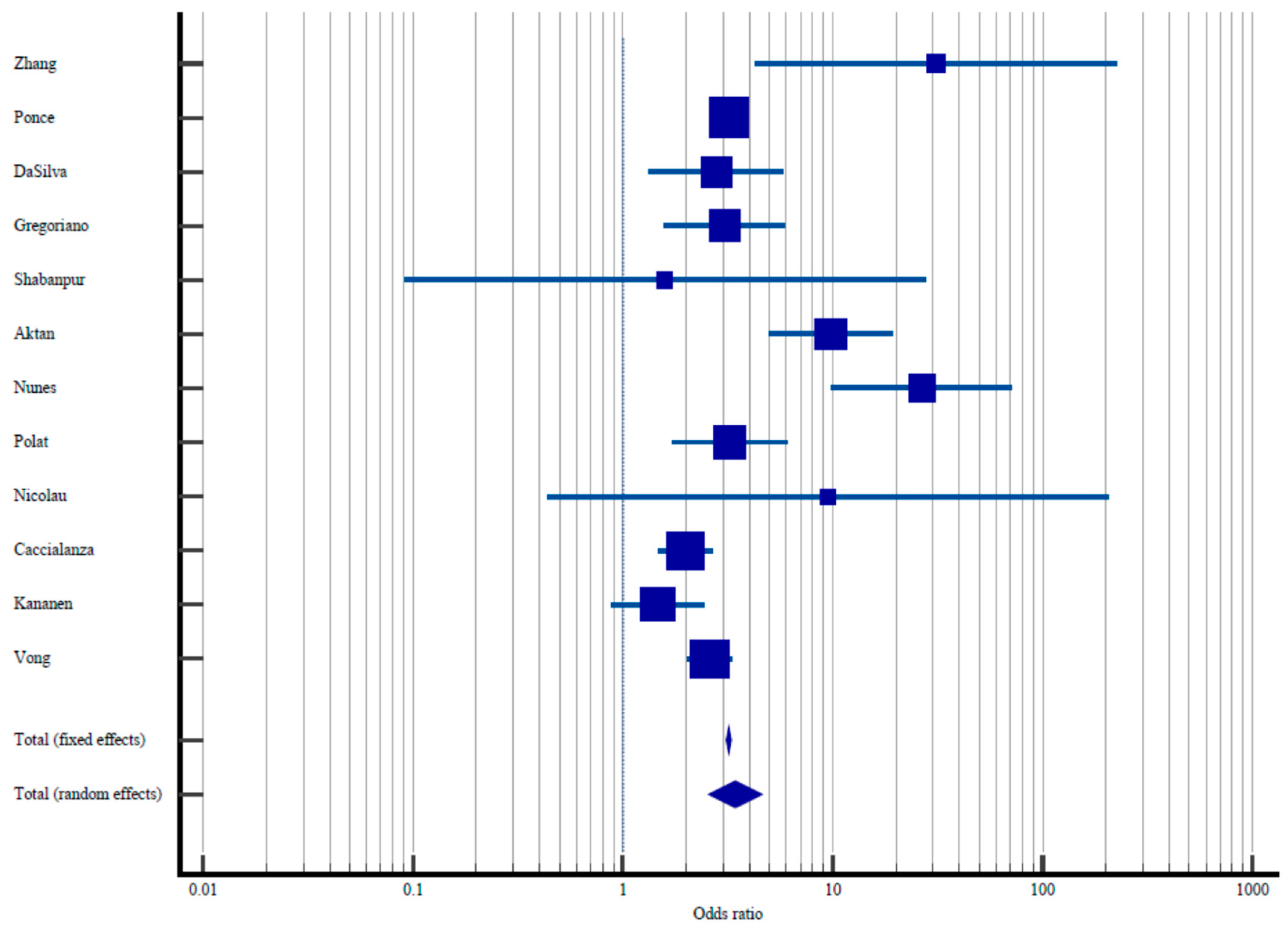
| Study | Sample Size | Proportion with Elevated Malnutrition Risk/Diagnosis of Malnutrition | Odds Ratio | 95% CI Odds Ratio | % Weight Fixed | % Weight Random |
|---|---|---|---|---|---|---|
| Zhang et al. [34] | 67/484 | 1/194 | 31.0 | 4.27–225.03 | 0.021 | 1.95 |
| Ponce et al. [35] | 7455/26,917 | 33,831/316,217 | 3.19 | 3.11–3.29 | 97.85 | 16.06 |
| DaSilva et al. [36] | 40/137 | 11/85 | 2.77 | 1.33–5.77 | 0.15 | 8.09 |
| Gregoriano et al. [37] | 20/76 | 24/229 | 3.05 | 1.57–5.92 | 0.19 | 8.89 |
| Shabanpur et al. [38] | 21/387 | 0/13 | 1.58 | 0.09–27.55 | 0.01 | 1.00 |
| Aktan et al. [39] | 316/1225 | 9/263 | 9.81 | 4.99–19.31 | 0.18 | 8.72 |
| Nunes et al. [40] | 26/107 | 5/419 | 26,58 | 9.91–71.2 | 0.084 | 5.76 |
| Polat et al. [41] | 65/292 | 13/159 | 3.22 | 1.711–6.04 | 0.21 | 9.29 |
| Nicolau et al. [42] | 2/27 | 0/48 | 9.51 | 0.44–205.69 | 0.009 | 0.87 |
| Caccialanza et al. [43] | 291/984 | 71/407 | 1.99 | 1.49–2.66 | 0.98 | 13.92 |
| Kananen et al. [44] | 126/2070 | 19/227 | 1.46 | 0.88–2.42 | 0.32 | 10.94 |
| Vong et al. [45] | 102/403 | 453/3908 | 2.59 | 2.02–3.30 | 1.35 | 14.48 |
| Total (fixed effects) | 8531/32,109 | 34,437/322,169 | 3.19 | 3.11–3.29 | 100.00 | 100.00 |
| Total (random effects) | 8531/32,109 | 34,437/322,169 | 3.43 | 2.59–5.46 | 100.00 | 100.00 |
| Study | Sample Size | Proportion with Elevated Malnutrition Risk/Diagnosis of Malnutrition | 95% CI Odds Ratio | % Weight Fixed | % Weight Random |
|---|---|---|---|---|---|
| Zhang et al. [34] | 678 | 71.39 | 62.82–74.76 | 0.19 | 8.35 |
| Ponce et al. [35] | 343,188 | 7.84 | 7.75–7.93 | 98.05 | 8.36 |
| DaSilva et al. [36] | 222 | 61.71 | 54.97–68.14 | 0.06 | 8.31 |
| Gregoriano et al. [37] | 305 | 24.92 | 20.16–30.17 | 0.09 | 8.33 |
| Shabanpur et al. [38] | 400 | 96.75 | 94.51–98.26 | 0.11 | 8.33 |
| Aktan et al. [39] | 1488 | 82.33 | 80.29–84.23 | 0.43 | 8.36 |
| Nunes et al. [40] | 526 | 20.34 | 16.98–24.04 | 0.15 | 8.34 |
| Polat et al. [41] | 451 | 64.75 | 60.14–69.16 | 0.13 | 8.34 |
| Nicolau et al. [42] | 75 | 36.00 | 25.23–47.91 | 0.02 | 8.21 |
| Caccialanza et al. [43] | 1391 | 70.74 | 68.27–73.12 | 0.40 | 8.36 |
| Kananen et al. [44] | 1297 | 82.49 | 80.32–84.53 | 0.37 | 8.36 |
| Vong et al. [45] | 4311 | 9.35 | 8.49–10.26 | 1.22 | 8.36 |
| Total (fixed effects) | 354,332 | 8.63 | 8.53–8.71 | 100.00 | 100.00 |
| Total (random effects) | 354,332 | 52.61 | 29.50–75.14 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boaz, M.; Kaufman-Shriqui, V. Systematic Review and Meta-Analysis: Malnutrition and In-Hospital Death in Adults Hospitalized with COVID-19. Nutrients 2023, 15, 1298. https://doi.org/10.3390/nu15051298
Boaz M, Kaufman-Shriqui V. Systematic Review and Meta-Analysis: Malnutrition and In-Hospital Death in Adults Hospitalized with COVID-19. Nutrients. 2023; 15(5):1298. https://doi.org/10.3390/nu15051298
Chicago/Turabian StyleBoaz, Mona, and Vered Kaufman-Shriqui. 2023. "Systematic Review and Meta-Analysis: Malnutrition and In-Hospital Death in Adults Hospitalized with COVID-19" Nutrients 15, no. 5: 1298. https://doi.org/10.3390/nu15051298
APA StyleBoaz, M., & Kaufman-Shriqui, V. (2023). Systematic Review and Meta-Analysis: Malnutrition and In-Hospital Death in Adults Hospitalized with COVID-19. Nutrients, 15(5), 1298. https://doi.org/10.3390/nu15051298








