Using Capillary Whole Blood to Quantitatively Measure Ferritin: A Validation Trial of a Point-of-Care System
Abstract
:1. Introduction
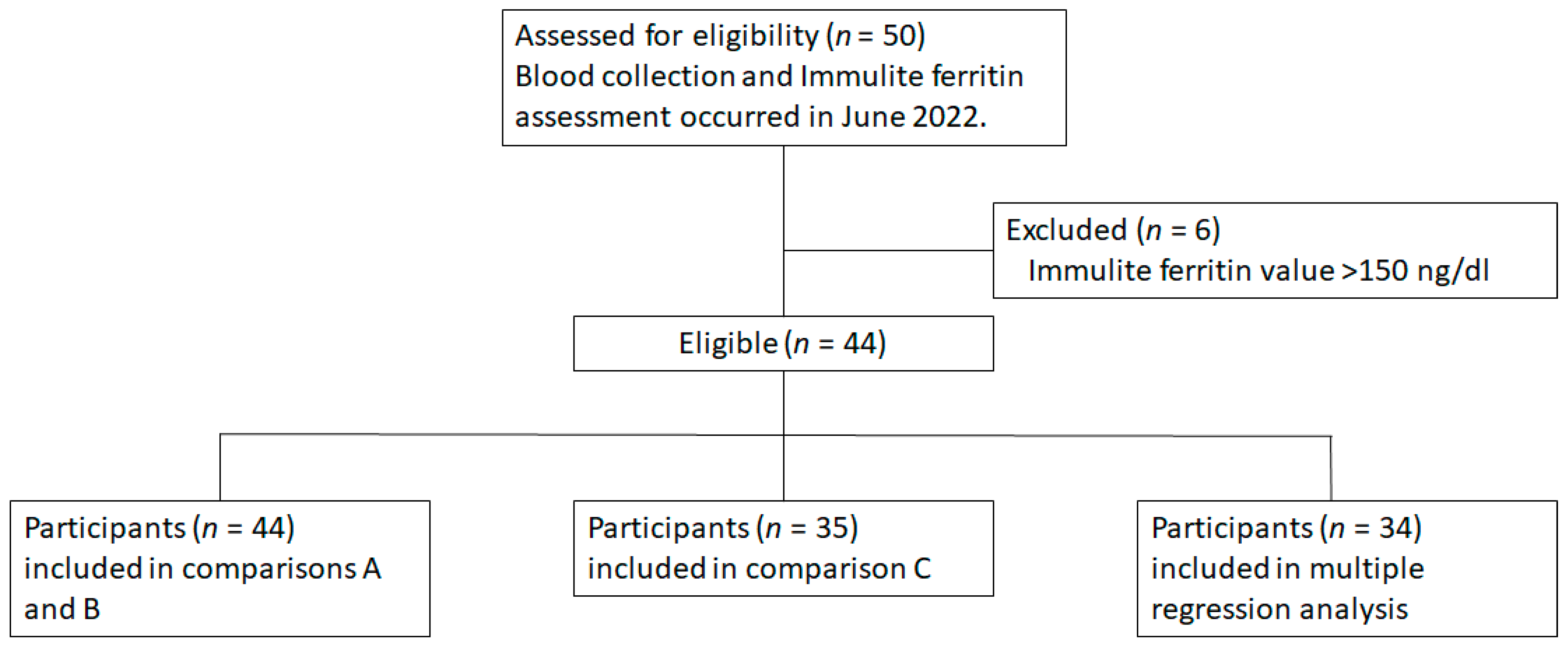
2. Materials and Methods
- A.
- How do the diagnostic devices compare under expected usage?
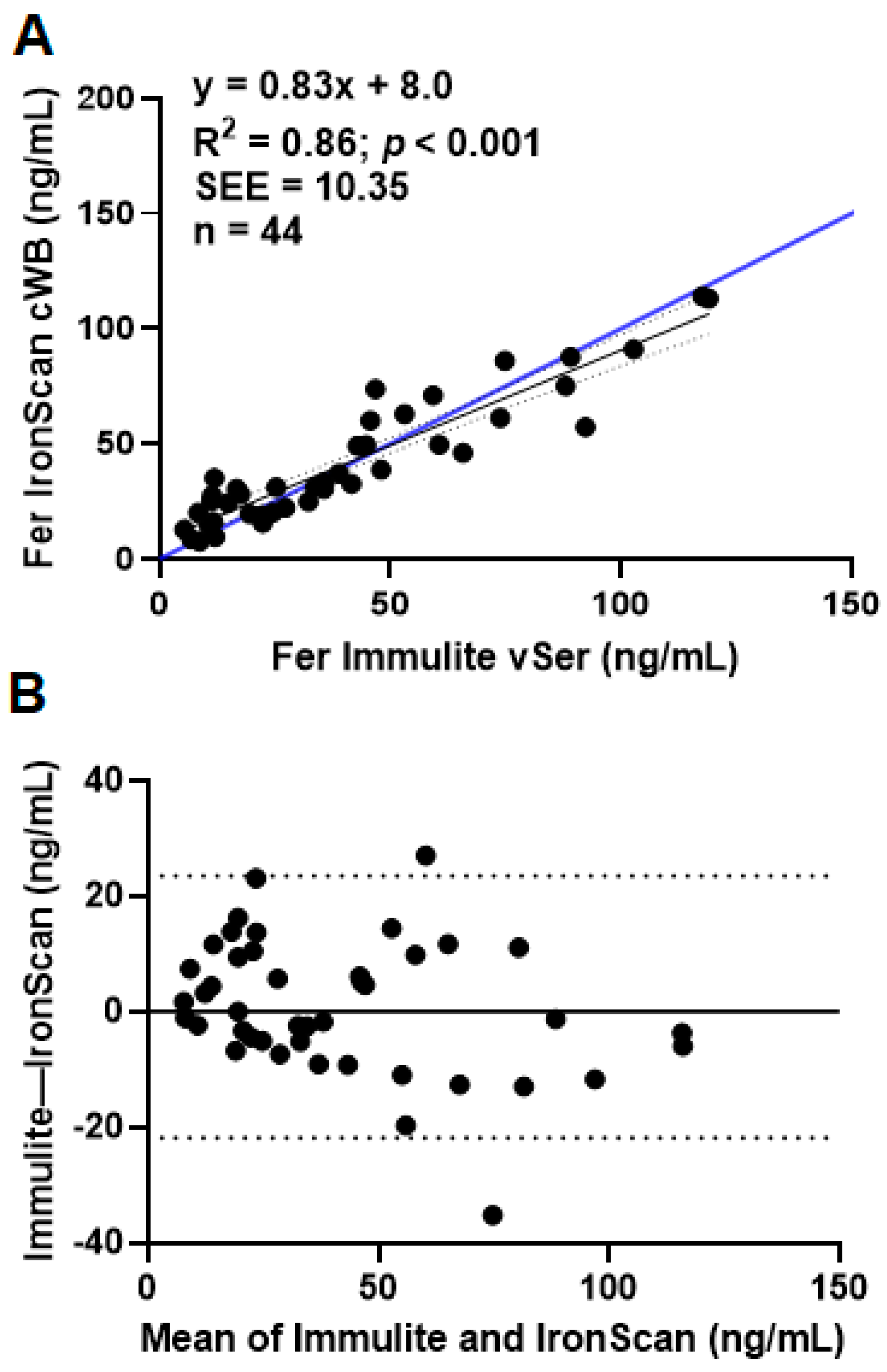
- IronScan™ cWB blood vs. gold standard vSer in Immulite
- B.
- How do the diagnostic devices compare when using the same collection site (venous)?
- IronScan™ vWB whole blood vs. gold standard vSer in Immulite
- C.
- How do the diagnostic devices compare when using the same collection site and form of blood (vSer)?
- IronScan™ vSer vs. gold standard vSer in Immulite
3. Results
3.1. Subject Characteristics
3.1.1. Comparison A: Diagnostic Device Comparison under Expected Usage
3.1.2. Comparison B: IronScan™ versus Immulite Using Whole Blood or Serum from the Same Venous Collection Site
3.1.3. Comparison C: IronScan™ versus Immulite Using Serum Obtained from the Same Venous Collection Site
3.1.4. Multiple Regression Analysis of All IronScan™ Comparisons to Immulite 2000 Xpi
3.1.5. Identification of Iron Deficiency Using the WHO or Clinical Cutoffs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haas, J.D.; Brownlie, T. Iron Deficiency and Reduced Work Capacity: A Critical Review of the Research to Determine a Causal Relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef] [Green Version]
- Pasricha, S.-R.; Low, M.; Thompson, J.; Farrell, A.; De-Regil, L.-M. Iron Supplementation Benefits Physical Performance in Women of Reproductive Age: A Systematic Review and Meta-Analysis. J. Nutr. 2014, 144, 906–914. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Wimbley, T.D.; Graham, D.Y. Diagnosis and Management of Iron Deficiency Anemia in the 21st Century. Ther. Adv. Gastroenterol. 2011, 4, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Abu-Ouf, N.M.; Jan, M.M. The Impact of Maternal Iron Deficiency and Iron Deficiency Anemia on Child’s Health. Saudi Med. J. 2015, 36, 146. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Iron Deficiency in Pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A Systematic Analysis of Global Anemia Burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Peyrin-Biroulet, L.; Williet, N.; Cacoub, P. Guidelines on the Diagnosis and Treatment of Iron Deficiency across Indications: A Systematic Review. Am. J. Clin. Nutr. 2015, 102, 1585–1594. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Guideline on the Use of Ferritin Concentrations to Assess Iron Status in Individual Populations; World Health Organization: Geneva, Switzerland, 2020; p. 62. [Google Scholar]
- Goldman, M.; Uzicanin, S.; Osmond, L.; Yi, Q.-L.; Scalia, V.; O’Brien, S.F. Two-Year Follow-up of Donors in a Large National Study of Ferritin Testing: Ferritin Study Follow-Up. Transfusion 2018, 58, 2868–2873. [Google Scholar] [CrossRef]
- Teichman, J.; Nisenbaum, R.; Lausman, A.; Sholzberg, M. Suboptimal Iron Deficiency Screening in Pregnancy and the Impact of Socioeconomic Status in a High-Resource Setting. Blood Adv. 2021, 5, 4666–4673. [Google Scholar] [CrossRef]
- Oatley, H.; Borkhoff, C.M.; Chen, S.; Macarthur, C.; Persaud, N.; Birken, C.S.; Maguire, J.L.; Parkin, P.C.; TARGet Kids! Collaboration. Screening for Iron Deficiency in Early Childhood Using Serum Ferritin in the Primary Care Setting. Pediatrics 2018, 142, e20182095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carsley, S.; Fu, R.; Borkhoff, C.M.; Reid, N.; Baginska, E.; Birken, C.S.; Maguire, J.L.; Hancock-Howard, R.; Parkin, P.C.; Coyte, P.C. Iron Deficiency Screening for Children at 18 Months: A Cost-Utility Analysis. CMAJ Open 2019, 7, E689–E698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mawani, M.; Aziz Ali, S. Iron Deficiency Anemia among Women of Reproductive Age, an Important Public Health Problem: Situation Analysis. Reprod. Syst. Sex. Disord. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Srinivasan, B. IronPhone_ Mobile Device-Coupled Point-of-Care Diagnostics for Assessment of Iron Status by Quantification of Serum Ferritin. Biosens. Bioelectron. 2018, 99, 115–121. [Google Scholar] [CrossRef]
- Albrecht, K.; Lotz, J.; Frommer, L.; Lackner, K.J.; Kahaly, G.J. A Rapid Point-of-Care Assay Accurately Measures Vitamin D. J. Endocrinol. Investig. 2021, 44, 2485–2492. [Google Scholar] [CrossRef]
- U.S. Department of Health & Human Services. U.S. Centers for Disease Control and Prevention Notice of Specific List for Categorization of Laboratory Test Systems, Assays, and Examinations Complexity. Fed. Regist. 1999, 64, 55190–551564. [Google Scholar]
- Habicht, J.P.; Meyers, L.D.; Brownie, C. Indicators for Identifying and Counting the Improperly Nourished. Am. J. Clin. Nutr. 1982, 35, 1241–1254. [Google Scholar] [CrossRef]
- Haas, J.D.; Habicht, J.P. Growth and Growth Charts in the Assessment of Preschool Nutritional Status. Diet Dis. Tradit. Dev. Soc. 1990, 160–183. [Google Scholar]
- Pootrakul, P.; Skikne, B.S.; Cook, J.D. The Use of Capillary Blood for Measurements of Circulating Ferritin. Am. J. Clin. Nutr. 1983, 37, 307–310. [Google Scholar] [CrossRef]
- Quezada-Pinedo, H.G.; Cassel, F.; Duijts, L.; Muckenthaler, M.U.; Gassmann, M.; Jaddoe, V.W.V.; Reiss, I.K.M.; Vermeulen, M.J. Maternal Iron Status in Pregnancy and Child Health Outcomes after Birth: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2221. [Google Scholar] [CrossRef]
- Tran, K.; McCormack, S. Screening and Treatment of Obstetric Anemia: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technology in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Cable, R.G.; Glynn, S.A.; Kiss, J.E.; Mast, A.E.; Steele, W.R.; Murphy, E.L.; Wright, D.J.; Sacher, R.A.; Gottschall, J.L.; Tobler, L.H.; et al. Iron Deficiency in Blood Donors: The REDS-II Donor Iron Status Evaluation (RISE) Study. Transfusion 2012, 52, 702–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baart, A.M.; van Noord, P.A.H.; Vergouwe, Y.; Moons, K.G.M.; Swinkels, D.W.; Wiegerinck, E.T.; de Kort, W.L.A.M.; Atsma, F. High Prevalence of Subclinical Iron Deficiency in Whole Blood Donors Not Deferred for Low Hemoglobin: Iron Deficiency among Blood Donors. Transfusion 2013, 53, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Rigas, A.S.; Sørensen, C.J.; Pedersen, O.B.; Petersen, M.S.; Thørner, L.W.; Kotzé, S.; Sørensen, E.; Magnussen, K.; Rostgaard, K.; Erikstrup, C.; et al. Predictors of Iron Levels in 14,737 D Anish Blood Donors: Results from the D Anish Blood Donor Study. Transfusion 2014, 54, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Salvin, H.E.; Pasricha, S.-R.; Marks, D.C.; Speedy, J. Iron Deficiency in Blood Donors a National Cross-sectional Study.Pdf. Transfusion 2014, 54, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Uzicanin, S.; Osmond, L.; Scalia, V.; O’Brien, S.F. A Large National Study of Ferritin Testing in Canadian Blood Donors: A Large National Study of Ferritin Testing. Transfusion 2017, 57, 564–570. [Google Scholar] [CrossRef] [PubMed]


| Mean ± SD | Range | |
|---|---|---|
| Age, year | 33.8 ± 11.0 | 19–61 |
| Height, cm | 168.5 ± 7.8 | 150–178 |
| Weight, kg | 70.1 ± 15.3 | 52–106 |
| BMI, kg/m2 | 24.7 ± 4.6 | 19–41 |
| Mean ± SD | Range | |
|---|---|---|
| Ferritin from Immulite vSer, ng/mL | 41.1 ± 30.7 | 5.4–119.0 |
| Ferritin from IronScan™ cWB, ng/mL | 40.7 ± 27.3 | 7.6–114.0 |
| Ferritin from IronScan™ vWB, ng/mL | 56.3 ± 40.1 | 5.0–154.0 |
| Ferritin from IronScan™ vSer, ng/mL | 46.1 ± 32.5 | 7.2–137.6 |
| Sensitivity (%) | Specificity (%) | |
|---|---|---|
| ≤12 ng/mL (clinical) | 33 | 100 |
| ≤30 ng/mL (WHO) | 85 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiddler, J.L.; McBurney, M.I.; Haas, J.D. Using Capillary Whole Blood to Quantitatively Measure Ferritin: A Validation Trial of a Point-of-Care System. Nutrients 2023, 15, 1305. https://doi.org/10.3390/nu15061305
Fiddler JL, McBurney MI, Haas JD. Using Capillary Whole Blood to Quantitatively Measure Ferritin: A Validation Trial of a Point-of-Care System. Nutrients. 2023; 15(6):1305. https://doi.org/10.3390/nu15061305
Chicago/Turabian StyleFiddler, Joanna L., Michael I. McBurney, and Jere D. Haas. 2023. "Using Capillary Whole Blood to Quantitatively Measure Ferritin: A Validation Trial of a Point-of-Care System" Nutrients 15, no. 6: 1305. https://doi.org/10.3390/nu15061305







