Small Intestinal Bacterial Overgrowth and Non-Alcoholic Fatty Liver Disease: What Do We Know in 2023?
Abstract
:1. Introduction
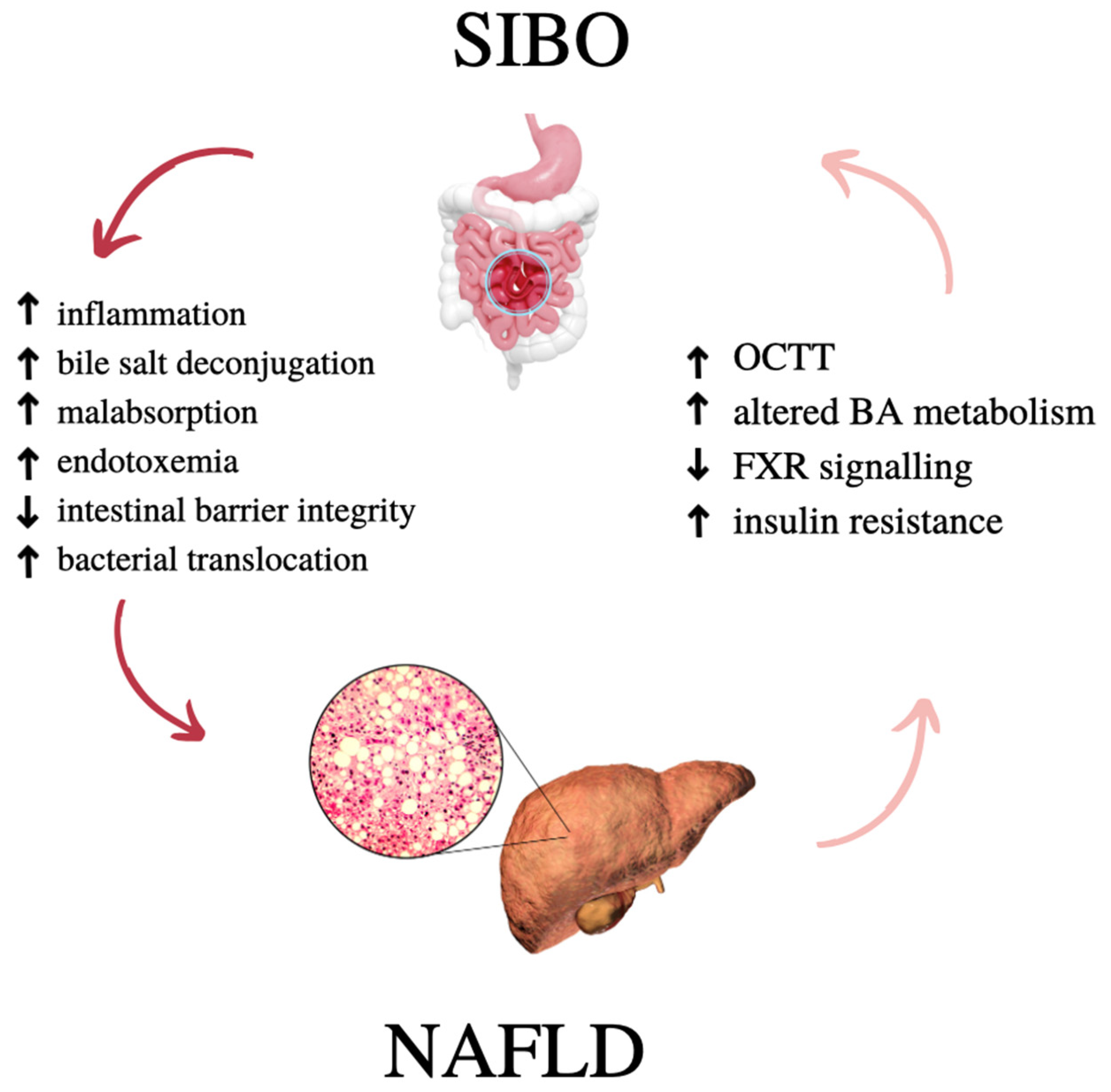
1.1. NAFLD and SIBO—What Do They Have in Common?
1.2. The Aim of the Study
2. Non-Alcoholic Fatty Liver Disease
2.1. Diagnosis
2.2. Treatment
3. Small Intestinal Bacterial Overgrowth
3.1. About SIBO
- Hydrogen SIBO. Usually associated with gastrointestinal symptoms such as bloating, excess gas, abdominal pain, diarrhea and weight loss [49].
- Methane SIBO, more often called IMO (intestinal methanogen overgrowth). IMO is a methanogen proliferation syndrome in the small intestine. The characteristic symptoms include abdominal pain, nausea, difficulty passing bowel movements and changing bowel habits. In this case, too much methane production is caused not by bacterial overgrowth but by the type of archaea, mainly Methanobrevibacter smithii. Due to the overgrowth of these archaea, the use of the term IMO seems more appropriate than “SIBO” or “Methane-SIBO”. Although methanogens are found in the small intestine, people who test positive for exhaled methane also have elevated levels of methanogens in their stools, suggesting that they may be present throughout the digestive tract [48,50].
- Hydrogen-sulfide SIBO. The characteristic symptoms are gas and stools with the smell of hydrogen sulfide, halitosis, chronic fatigue, headaches, and fibromyalgia. Symptoms worsen with sulfur-rich foods found in diets or supplements [52]. Moreover, patients in hydrogen–methane tests at each stage of the study report low levels of methane and hydrogen despite a number of symptoms [51,53].
3.2. Diagnosis
3.3. Treatment
4. Gut–Liver Axis
4.1. Gut–Liver Axis in Health and Disease
4.2. Intestinal Permeability and NAFLD
4.3. Pathogen-Associated Molecular Patterns and NAFLD
4.4. Bile Acid Metabolism and NAFLD
4.5. Clinical Implications
5. Gut Microbiota Changes in NAFLD and SIBO
- (a)
- Body weight—an increase in body weight contributes to a decrease in the diversity of the gut microbiota [92];
- (b)
- Anatomical and functional changes in the intestinal barrier (defined as the immune barrier, the intestinal vascular barrier, and the hepatic barrier). Intestinal dysbiosis, intensifying the translocation of bacteria through the portal vein to the liver (endotoxemia), enhances inflammatory responses in the liver [93],
- (c)
- Specific patterns pro-inflammatory compounds of the intestinal microbiome—PAMPs and MAMPs such as LPS, peptidoglycans and lipopeptides, microbial DNA, circulating proinflammatory cytokines (IL-1, IL-6, INF-γ, and TNF-α) may contribute to the inflammatory response and fibrosis in patients with NAFLD [87,94];
- (d)
- Could influence the metabolic and inflammatory state of the liver through the release of anti-inflammatory compounds (short chain fatty acids-SCFA) that bind to G protein-coupled receptors (GPCRs) induce hepatic lipids and glucose homeostasis, the regulation of intestinal integrity and intrinsic immune defenses [95].
6. Pro-, Pre-, and Symbiotic Therapy in NAFLD and SIBO
7. Nutrition—What Is beyond the Obvious?
7.1. Current State of Knowledge—Diet for SIBO and NAFLD
7.2. Mediterranean Diet
7.3. Low FODMAPs Diet
7.4. Ketogenic Diet
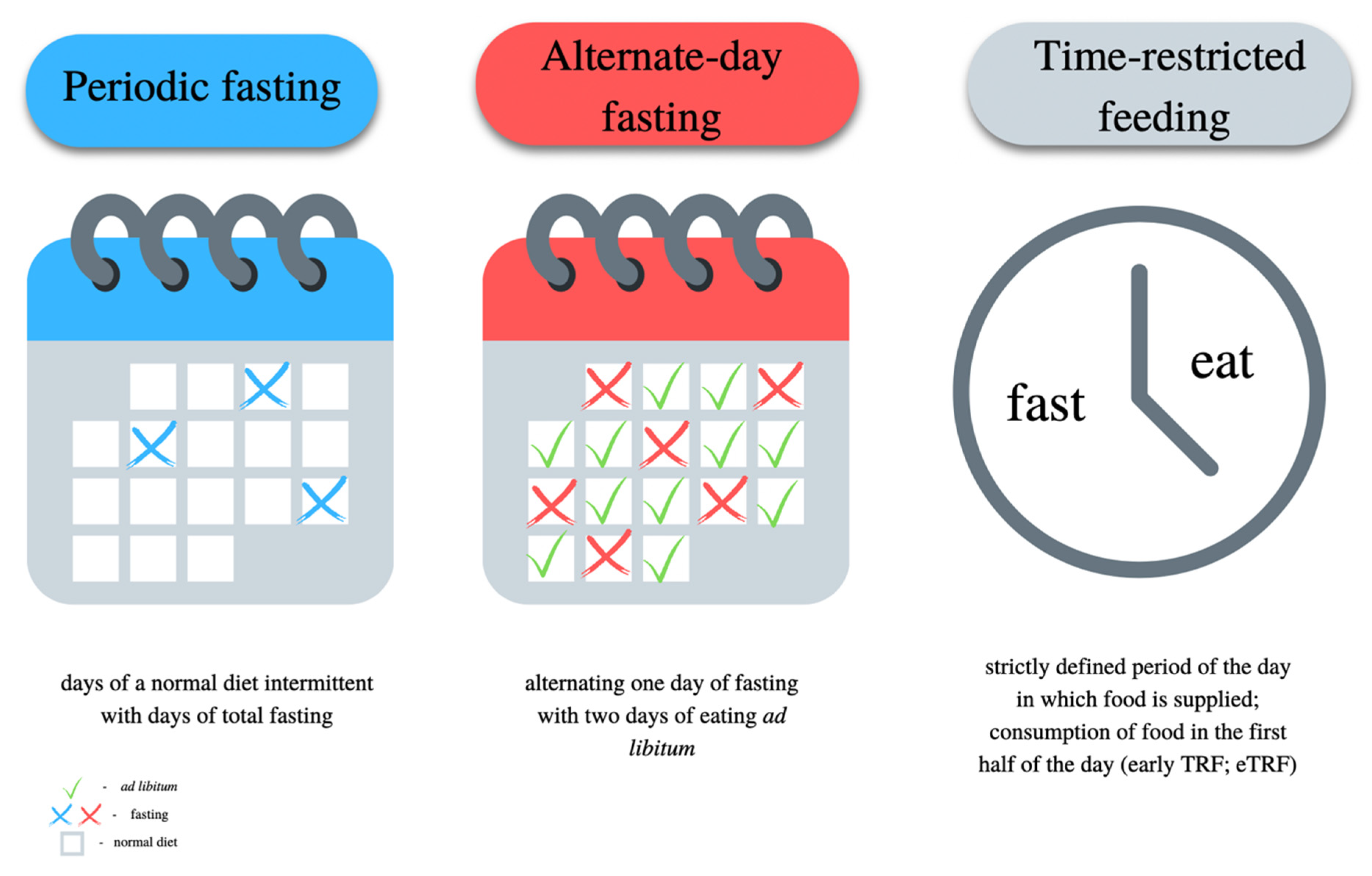
7.5. Intermittent Fasting
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Dhibi, M.; Brahmi, F.; Mnari, A.; Houas, Z.; Chargui, I.; Bchir, L.; Gazzah, N.; Alsaif, M.A.; Hammami, M. The intake of high fat diet with different trans fatty acid levels differentially induces oxidative stress and non alcoholic fatty liver disease (NAFLD) in rats. Nutr. Metab. 2011, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riazi, K.; Raman, M.; Taylor, L.; Swain, M.G.; Shaheen, A.A. Dietary Patterns and Components in Nonalcoholic Fatty Liver Disease (NAFLD): What Key Messages Can Health Care Providers Offer? Nutrients 2019, 11, 2878. [Google Scholar] [CrossRef] [Green Version]
- Lujan, P.V.; Esmel, E.V.; Meseguer, E.S. Overview of Non-Alcoholic Fatty Liver Disease (NAFLD) and the Role of Sugary Food Consumption and Other Dietary Components in Its Development. Nutrients 2021, 13, 1442. [Google Scholar] [CrossRef]
- Cheon, S.Y.; Song, J. Novel insights into non-alcoholic fatty liver disease and dementia: Insulin resistance, hyperammonemia, gut dysbiosis, vascular impairment, and inflammation. Cell Biosci. 2022, 12, 99. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kubota, N.; Yamauchi, T.; Kadowaki, T. Role of Insulin Resistance in MAFLD. Int. J. Mol. Sci. 2021, 22, 4156. [Google Scholar] [CrossRef] [PubMed]
- Vos, B.; Moreno, C.; Nagy, N.; Féry, F.; Cnop, M.; Vereerstraeten, P.; Devière, J.; Adler, M. Lean non-alcoholic fatty liver disease (Lean-NAFLD): A major cause of cryptogenic liver disease. Acta Gastro Enterol. Belg. 2011, 74, 389–394. [Google Scholar]
- Kuchay, M.S.; Martínez-Montoro, J.I.; Choudhary, N.S.; Fernández-García, J.C.; Ramos-Molina, B. Non-Alcoholic Fatty Liver Disease in Lean and Non-Obese Individuals: Current and Future Challenges. Biomedicines 2021, 9, 1346. [Google Scholar] [CrossRef]
- Stefan, N.; Schick, F.; Birkenfeld, A.L.; Häring, H.-U.; White, M.F. The role of hepatokines in NAFLD. Cell Metab. 2023, 35, 236–252. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- Stefan, N.; Schick, F.; Häring, H.-U. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017, 26, 292–300. [Google Scholar] [CrossRef]
- Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020, 8, 616–627. [Google Scholar] [CrossRef]
- Castaño-Rodríguez, N.; Mitchell, H.M.; Kaakoush, N.O. NAFLD, Helicobacter species and the intestinal microbiome. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Morya, R.K.; Bhadada, S.K.; Rana, S. Type 1 diabetes mellitus: Complex interplay of oxidative stress, cytokines, gastrointestinal motility and small intestinal bacterial overgrowth. Eur. J. Clin. Investig. 2018, 48, e13021. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Baba, C.S.; Ghoshal, U.; Alexander, G.; Misra, A.; Saraswat, V.A.; Choudhuri, G. Low-grade small intestinal bacterial overgrowth is common in patients with non-alcoholic steatohepatitis on quantitative jejunal aspirate culture. Indian J. Gastroenterol. 2017, 36, 390–399. [Google Scholar] [CrossRef]
- Achufusi, T.G.O.; Sharma, A.; Zamora, E.A.; Manocha, D. Small Intestinal Bacterial overgrowth: Comprehensive Review of Diagnosis, Prevention, and Treatment Methods. Cureus 2020, 12, e8860. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Ghoshal, U. Small Intestinal Bacterial Overgrowth and Other Intestinal Disorders. Gastroenterol. Clin. N. Am. 2017, 46, 103–120. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Gwee, K.-A. Post-infectious IBS, tropical sprue and small intestinal bacterial overgrowth: The missing link. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 435–441. [Google Scholar] [CrossRef]
- Rana, S.V.; Malik, A.; Bhadada, S.K.; Sachdeva, N.; Morya, R.K.; Sharma, G. Malabsorption, Orocecal Transit Time and Small Intestinal Bacterial Overgrowth in Type 2 Diabetic Patients: A Connection. Indian J. Clin. Biochem. 2017, 32, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0151829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessoku, T.; Kobayashi, T.; Imajo, K.; Tanaka, K.; Yamamoto, A.; Takahashi, K.; Kasai, Y.; Ozaki, A.; Iwaki, M.; Nogami, A.; et al. Endotoxins and Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 770986. [Google Scholar] [CrossRef]
- Fei, N.; Bruneau, A.; Zhang, Z.; Wang, R.; Wang, J.; Rabot, S.; Gérard, P.; Zhao, L. Endotoxin Producers Overgrowing in Human Gut Microbiota as the Causative Agents for Nonalcoholic Fatty Liver Disease. mBio 2020, 11, e03263-19. [Google Scholar] [CrossRef] [Green Version]
- Wigg, A.J.; Roberts-Thomson, I.C.; Dymock, R.B.; McCarthy, P.J.; Grose, R.H.; Cummins, A.G. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001, 48, 206–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Krogh-Madsen, R.; Møller, K.; Dela, F.; Kronborg, G.; Jauffred, S.; Pedersen, B.K. Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-α, and FFAs to low-dose endotoxemia in humans. Am. J. Physiol. Metab. 2004, 286, E766–E772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fianchi, F.; Liguori, A.; Gasbarrini, A.; Grieco, A.; Miele, L. Nonalcoholic Fatty Liver Disease (NAFLD) as Model of Gut–Liver Axis Interaction: From Pathophysiology to Potential Target of Treatment for Personalized Therapy. Int. J. Mol. Sci. 2021, 22, 6485. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Riordan, S.M.; Duncombe, V.M.; Thomas, M.C.; Nagree, A.; Bolin, T.D.; McIver, C.J.; Williams, R.E.; Wigg, A.J.; Cummins, A.G. Small intestinal bacterial overgrowth, intestinal permeability, and non-alcoholic steatohepatitis. Gut 2002, 50, 136–138. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, A.L.; Wunderlich, C.M.; Schneiders, L.; Vogt, M.C.; Woeste, M.A.; Belgardt, B.F.; Niessen, C.M.; Martiny, B.; Schauss, A.C.; Frommolt, P.; et al. Intestinal insulin/IGF1 signalling through FoxO1 regulates epithelial integrity and susceptibility to colon cancer. Nat. Metab. 2019, 1, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.C.; Bhagatwala, J. Small Intestinal Bacterial Overgrowth: Clinical Features and Therapeutic Management. Clin. Transl. Gastroenterol. 2019, 10, e00078. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [Green Version]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Imajo, K.; Toyoda, H.; Yasuda, S.; Suzuki, Y.; Sugimoto, K.; Kuroda, H.; Akita, T.; Tanaka, J.; Yasui, Y.; Tamaki, N.; et al. Utility of Ultrasound-Guided Attenuation Parameter for Grading Steatosis with Reference to MRI-PDFF in a Large Cohort. Clin. Gastroenterol. Hepatol. 2021, 20, 2533–2541.e7. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2017, 67, 328–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, N.; Nadeau, B.; Shannon, C.; Tincopa, M. Evaluation of Dietary Approaches for the Treatment of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Nutrients 2019, 11, 3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointest. Liver Dis. 2018, 27, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Ferolla, S.M.; Couto, C.A.; Costa-Silva, L.; Armiliato, G.N.A.; Pereira, C.A.S.; Martins, F.S.; Ferrari, M.D.L.A.; Vilela, E.G.; Torres, H.O.G.; Cunha, A.S.; et al. Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients 2016, 8, 397. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Halegoua-DeMarzio, D. Role of Probiotics in Non-alcoholic Fatty Liver Disease: Does Gut Microbiota Matter? Nutrients 2019, 11, 2837. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, D.A.; Ryan, P.M.; Monjaraz, E.M.T.; Mayans, J.A.R.; Quigley, E.M. Small Intestinal Bacterial Overgrowth in Children: A State-Of-The-Art Review. Front. Pediatr. 2019, 7, 363. [Google Scholar] [CrossRef] [Green Version]
- Khoshini, R.; Dai, S.-C.; Lezcano, S.; Pimentel, M. A Systematic Review of Diagnostic Tests for Small Intestinal Bacterial Overgrowth. Dig. Dis. Sci. 2008, 53, 1443–1454. [Google Scholar] [CrossRef]
- Leite, G.; Morales, W.; Weitsman, S.; Celly, S.; Parodi, G.; Mathur, R.; Barlow, G.M.; Sedighi, R.; Millan, M.J.V.; Rezaie, A.; et al. The duodenal microbiome is altered in small intestinal bacterial overgrowth. PLoS ONE 2020, 15, e0234906. [Google Scholar] [CrossRef]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, M.; Saad, R.J.; Long, M.D.; Rao, S.S.C. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef]
- Takakura, W.; Pimentel, M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome—An Update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Z.; Dong, W.X.; Zhang, X.; Zhu, S.W.; Liu, Z.J.; Duan, L.P. The diagnostic value of hydrogen sulfide breath test for small intestinal bacterial overgrowth. Zhonghua Nei Ke Za Zhi 2021, 60, 356–361. [Google Scholar]
- Shayto, R.H.; Mrad, R.A.; Sharara, A.I. Use of rifaximin in gastrointestinal and liver diseases. World J. Gastroenterol. 2016, 22, 6638–6651. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Cao, B.; Tian, Q. The effect of small intestinal bacterial overgrowth on minimal hepatic encephalopathy in patients with cirrhosis. Arch. Med. Sci. 2016, 3, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Cao, B.; Tian, Q. Effects of SIBO and rifaximin therapy on MHE caused by hepatic cirrhosis. Int. J. Clin. Exp. Med. 2015, 8, 2954–2957. [Google Scholar]
- Thompson, J.R. Is irritable bowel syndrome an infectious disease? World J. Gastroenterol. 2016, 22, 1331–1334. [Google Scholar] [CrossRef]
- Grzybowska, W.; Wojcik, A.; Tyski, S. Ocena oddzialywania neomycyny z antybiotykiem innej grupy na wybrane szczepy bakteryjne. Med. Doświadczalna I Mikrobiol. 2004, 56, 187–198. [Google Scholar]
- Ghoshal, U.; Shukla, R.; Srivastava, D.; Ghoshal, U.C. Irritable Bowel Syndrome, Particularly the Constipation-Predominant Form, Involves an Increase in Methanobrevibacter smithii, Which Is Associated with Higher Methane Production. Gut Liver 2016, 10, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, M.; Chow, E.J.; Lin, H.C. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: A double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 2003, 98, 412–419. [Google Scholar] [CrossRef]
- Low, K.; Hwang, L.; Hua, J.; Zhu, A.; Morales, W.; Pimentel, M. A Combination of Rifaximin and Neomycin Is Most Effective in Treating Irritable Bowel Syndrome Patients with Methane on Lactulose Breath Test. J. Clin. Gastroenterol. 2010, 44, 547–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansorg, R.; Rath, P.-M.; Runde, V.; Beelen, D.W. Influence of intestinal decontamination using metronidazole on the detection of methanogenic Archaea in bone marrow transplant recipients. Bone Marrow Transplant. 2003, 31, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An Update on Metabolism, Structure–Cytotoxicity and Resistance Mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baughn, A.D.; Malamy, M.H. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 2004, 427, 441–444. [Google Scholar] [CrossRef]
- Zhong, C.; Qu, C.; Wang, B.; Liang, S.; Zeng, B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth: A Meta-Analysis and Systematic Review of Current Evidence. J. Clin. Gastroenterol. 2017, 51, 300–311. [Google Scholar] [CrossRef]
- Martín-Mateos, R.; Albillos, A. The Role of the Gut-Liver Axis in Metabolic Dysfunction-Associated Fatty Liver Disease. Front. Immunol. 2021, 12, 660179. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [Green Version]
- Rahman, K.; Desai, C.; Iyer, S.S.; Thorn, N.E.; Kumar, P.; Liu, Y.; Smith, T.; Neish, A.S.; Li, H.; Tan, S.; et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology 2016, 151, 733–746.e12. [Google Scholar] [CrossRef] [Green Version]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, K.; Kodama, Y.; Inokuchi, S.; Schnabl, B.; Aoyama, T.; Ohnishi, H.; Olefsky, J.M.; Brenner, D.A.; Seki, E. Toll-Like Receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1β in Mice. Gastroenterology 2010, 139, 323–334.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Engstler, A.J.; Aumiller, T.; Degen, C.; Dürr, M.; Weiss, E.; Maier, I.B.; Schattenberg, J.M.; Jin, C.J.; Sellmann, C.; Bergheim, I. Insulin resistance alters hepatic ethanol metabolism: Studies in mice and children with non-alcoholic fatty liver disease. Gut 2016, 65, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Fernández-Real, J.-M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.A.; Schoonjans, K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef]
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives. J. Gastroenterol. 2020, 55, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Carr, R.M.; Reid, A.E. FXR Agonists as Therapeutic Agents for Non-alcoholic Fatty Liver Disease. Curr. Atheroscler. Rep. 2015, 17, 500. [Google Scholar] [CrossRef]
- Sinal, C.J.; Tohkin, M.; Miyata, M.; Ward, J.M.; Lambert, G.; Gonzalez, F.J. Targeted Disruption of the Nuclear Receptor FXR/BAR Impairs Bile Acid and Lipid Homeostasis. Cell 2000, 102, 731–744. [Google Scholar] [CrossRef] [Green Version]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021, 33, 1671–1684.e4. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Traussnigg, S.A.; Trauner, M. Nuclear Receptor Modulation for the Treatment of Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2016, 36, 69–86. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Xue, R.; Su, L.; Lai, S.; Wang, Y.; Zhao, D.; Fan, J.; Chen, W.; Hylemon, P.B.; Zhou, H. Bile Acid Receptors and the Gut–Liver Axis in Nonalcoholic Fatty Liver Disease. Cells 2021, 10, 2806. [Google Scholar] [CrossRef] [PubMed]
- Smits, S.A.; Leach, J.; Sonnenburg, E.D.; Gonzalez, C.G.; Lichtman, J.S.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Elias, J.E.; et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017, 357, 802–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Schnabl, B. Roles for the mycobiome in liver disease. Liver Int. 2022, 42, 729–741. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; De Angelis, M.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.-H.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Da Silva, H.E.; Teterina, A.; Comelli, E.M.; Taibi, A.; Arendt, B.M.; Fischer, S.E.; Lou, W.; Allard, J.P. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci. Rep. 2018, 8, 1466. [Google Scholar] [CrossRef] [Green Version]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnabl, B.; Brenner, D.A. Interactions between the Intestinal Microbiome and Liver Diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luther, J.; Garber, J.J.; Khalili, H.; Dave, M.; Bale, S.S.; Jindal, R.; Motola, D.L.; Luther, S.; Bohr, S.; Jeoung, S.W.; et al. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 222–232.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Luo, K.; Li, J.; Li, Y.; Zhang, Y.; Yuan, Z.; Xu, Q.; Wu, X. Role of Intestinal Microbes in Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 12661. [Google Scholar] [CrossRef]
- Koutnikova, H.; Genser, B.; Monteiro-Sepulveda, M.; Faurie, J.-M.; Rizkalla, S.; Schrezenmeir, J.; Clément, K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2019, 9, e017995. [Google Scholar] [CrossRef]
- Sharpton, S.R.; Sharpton, S.R.; Maraj, B.; Maraj, B.; Harding-Theobald, E.; Harding-Theobald, E.; Vittinghoff, E.; Vittinghoff, E.; Terrault, N.A.; Terrault, N.A. Gut microbiome–targeted therapies in nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Am. J. Clin. Nutr. 2019, 110, 139–149. [Google Scholar] [CrossRef]
- Yang, R.; Shang, J.; Zhou, Y.; Liu, W.; Tian, Y.; Shang, H. Effects of probiotics on nonalcoholic fatty liver disease: A systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1401–1409. [Google Scholar] [CrossRef]
- Muramatsu, D.; Okabe, M.; Takaoka, A.; Kida, H.; Iwai, A. Aureobasidium pullulans produced β-glucan is effective to enhance Kurosengoku soybean extract induced Thrombospondin-1 expression. Sci. Rep. 2017, 7, 2831. [Google Scholar] [CrossRef]
- Bustos Fernández, L.M.; Man, F.; Lasa, J.S. Impact of Saccharomyces boulardii CNCM I-745 on bacterial overgrowth and composition of intestinal microbiota in IBS-D patients: Results of a randomized pilot study. Dig. Dis. 2023. [Google Scholar] [CrossRef]
- Francque, S.M.; Marchesini, G.; Kautz, A.; Walmsley, M.; Dorner, R.; Lazarus, J.V.; Zelber-Sagi, S.; Hallsworth, K.; Busetto, L.; Frühbeck, G.; et al. Non-alcoholic fatty liver disease: A patient guideline. JHEP Rep. 2021, 3, 100322. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef] [PubMed]
- Abstracts—APASL 2013. Hepatol. Int. 2013, 7, 1–754. [CrossRef] [PubMed] [Green Version]
- Zeybel, M.; Walsh, M.J.; Mann, D.A.; Mann, J. AASLD Abstracts. Hepatology 2012, 56, 191A–1144A. [Google Scholar] [CrossRef] [Green Version]
- Marchesini, G.; Bugianesi, E.; Burra, P.; Marra, F.; Miele, L.; Alisi, A.; Vajro, P.; Masarone, M.; Petta, S.; Persico, M.; et al. Non-alcoholic fatty liver disease in adults 2021: A clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Del Negro, V.; Angeletti, P.M.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health. Biofactors 2013, 39, 335–342. [Google Scholar] [CrossRef]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Van Hul, M. Mediterranean diet, gut microbiota and health: When age and calories do not add up! Gut 2020, 69, 1167–1168. [Google Scholar] [CrossRef] [Green Version]
- Frontiers|Gut Microbiome Composition in Non-Human Primates Consuming a Western or Mediterranean Diet|Nutrition. Available online: https://www.frontiersin.org/articles/10.3389/fnut.2018.00028/full (accessed on 25 June 2022).
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef]
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary macronutrients and the gut microbiome: A precision nutrition approach to improve cardiometabolic health. Gut 2022, 71, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Mayr, H.L.; Thomas, C.J. The anti-inflammatory effects of a Mediterranean diet: A review. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Bartimoccia, S.; Cammisotto, V.; Nocella, C.; Del Ben, M.; D’Amico, A.; Castellani, V.; Baratta, F.; Pignatelli, P.; Loffredo, L.; Violi, F.; et al. Extra Virgin Olive Oil Reduces Gut Permeability and Metabolic Endotoxemia in Diabetic Patients. Nutrients 2022, 14, 2153. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean Dietary Pattern as the Diet of Choice for Non-Alcoholic Fatty Liver Disease: Evidence and Plausible Mechanisms. Available online: https://pubmed.ncbi.nlm.nih.gov/28371239/ (accessed on 13 September 2022).
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef]
- Meir, A.Y.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Sultan, N.; Varney, J.E.; Halmos, E.P.; Biesiekierski, J.R.; Yao, C.K.; Muir, J.G.; Gibson, P.R.; Tuck, C.J. How to Implement the 3-Phase FODMAP Diet into Gastroenterological Practice. J. Neurogastroenterol. Motil. 2022, 28, 343–356. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef]
- Ankersen, D.V.; Weimers, P.; Bennedsen, M.; Haaber, A.B.; Fjordside, E.L.; Beber, M.E.; Lieven, C.; Saboori, S.; Vad, N.; Rannem, T.; et al. Long-Term Effects of a Web-Based Low-FODMAP Diet Versus Probiotic Treatment for Irritable Bowel Syndrome, Including Shotgun Analyses of Microbiota: Randomized, Double-Crossover Clinical Trial. J. Med. Internet Res. 2021, 23, e30291. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Kasti, A.; Petsis, K.; Lambrinou, S.; Katsas, K.; Nikolaki, M.; Papanikolaou, I.S.; Hatziagelaki, E.; Triantafyllou, K. A Combination of Mediterranean and Low-FODMAP Diets for Managing IBS Symptoms? Ask Your Gut! Microorganisms 2022, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Churuangsuk, C.; Lean, M.E.J.; Combet, E. Low and reduced carbohydrate diets: Challenges and opportunities for type 2 diabetes management and prevention. Proc. Nutr. Soc. 2020, 79, 498–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossoff, E.H.; Rho, J.M. Ketogenic diets: Evidence for short- and long-term efficacy. Neurotherapeutics 2009, 6, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Cahill, G.F., Jr. Fuel Metabolism in Starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Sherwood, L.M.; Parris, E.E.; Cahill, G.F. Starvation in Man. N. Engl. J. Med. 1970, 282, 668–675. [Google Scholar] [CrossRef]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; Van Der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef] [Green Version]
- Fuehrlein, B.S.; Rutenberg, M.S.; Silver, J.N.; Warren, M.W.; Theriaque, D.W.; Duncan, G.E.; Stacpoole, P.W.; Brantly, M.L. Differential Metabolic Effects of Saturated Versus Polyunsaturated Fats in Ketogenic Diets. J. Clin. Endocrinol. Metab. 2004, 89, 1641–1645. [Google Scholar] [CrossRef] [Green Version]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Esmaili, S.; Rogers, G.B.; Bugianesi, E.; Petta, S.; Marchesini, G.; Bayoumi, A.; Metwally, M.; Azardaryany, M.K.; Coulter, S.; et al. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology 2020, 71, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Napoleão, A.; Fernandes, L.; Miranda, C.; Marum, A. Effects of Calorie Restriction on Health Span and Insulin Resistance: Classic Calorie Restriction Diet vs. Ketosis-Inducing Diet. Nutrients 2021, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, H.-B.; Crawford, P. Ketone bodies as epigenetic modifiers. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 260–266. [Google Scholar] [CrossRef]
- Wree, A.; Eguchi, A.; McGeough, M.D.; Pena, C.A.; Johnson, C.D.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014, 59, 898–910. [Google Scholar] [CrossRef] [Green Version]
- Holmer, M.; Lindqvist, C.; Petersson, S.; Moshtaghi-Svensson, J.; Tillander, V.; Brismar, T.B.; Hagström, H.; Stål, P. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet—A randomised controlled trial. JHEP Rep. 2021, 3, 100256. [Google Scholar] [CrossRef]
- Cunha, G.M.; Guzman, G.; De Mello, L.L.C.; Trein, B.; Spina, L.; Bussade, I.; Prata, J.M.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients with Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cenci, L.; Grimaldi, K.A. Effect of ketogenic mediterranean diet with phytoextracts and low carbohydrates/high-protein meals on weight, cardiovascular risk factors, body composition and diet compliance in Italian council employees. Nutr. J. 2011, 10, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Guisado, J.; Muñoz-Serrano, A.; Alonso-Moraga, A. Spanish Ketogenic Mediterranean diet: A healthy cardiovascular diet for weight loss. Nutr. J. 2008, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Paoli, A.; Moro, T.; Bosco, G.; Bianco, A.; Grimaldi, K.A.; Camporesi, E.; Mangar, D. Effects of n-3 Polyunsaturated Fatty Acids (ω-3) Supplementation on Some Cardiovascular Risk Factors with a Ketogenic Mediterranean Diet. Mar. Drugs 2015, 13, 996–1009. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Guisado, J.; Muñoz-Serrano, A. The Effect of the Spanish Ketogenic Mediterranean Diet on Nonalcoholic Fatty Liver Disease: A Pilot Study. J. Med. Food 2011, 14, 677–680. [Google Scholar] [CrossRef]
- Memel, Z.N.; Wang, J.; Corey, K.E. Intermittent Fasting as a Treatment for Nonalcoholic Fatty Liver Disease: What Is the Evidence? Clin. Liver Dis. 2022, 19, 101–105. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obsil, T.; Obsilova, V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 2008, 27, 2263–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Peroxisome Proliferator-Activated Receptors (PPAR). Antiproliferative Properties. Available online: https://pubmed.ncbi.nlm.nih.gov/21734325/ (accessed on 2 October 2022).
- Patikorn, C.; Roubal, K.; Veettil, S.K.; Chandran, V.; Pham, T.; Lee, Y.Y.; Giovannucci, E.L.; Varady, K.A.; Chaiyakunapruk, N. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-Analyses of Randomized Clinical Trials. JAMA Netw. Open 2021, 4, e2139558. [Google Scholar] [CrossRef]
- Różański, G.; Pheby, D.; Newton, J.L.; Murovska, M.; Zalewski, P.; Słomko, J. Effect of Different Types of Intermittent Fasting on Biochemical and Anthropometric Parameters among Patients with Metabolic-Associated Fatty Liver Disease (MAFLD)—A Systematic Review. Nutrients 2022, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Aliasghari, F.; Izadi, A.; Gargari, B.P.; Ebrahimi, S. The Effects of Ramadan Fasting on Body Composition, Blood Pressure, Glucose Metabolism, and Markers of Inflammation in NAFLD Patients: An Observational Trial. J. Am. Coll. Nutr. 2017, 36, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Arabi, S.M.; Zarifi, S.H.; Nematy, M.; Safarian, M. The effect of Ramadan fasting on non-alcoholic fatty liver disease (NAFLD) patients. J. Nutr. Fasting Health 2015, 3, 74–80. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Suggested Antibiotics for the Treatment of Small Intestinal Bacterial Overgrowth | |
|---|---|
| Drug | Dosage |
| Rifaximin | 550 mg three times per day; 61–78% efficacy |
| Amoxicillin/clavulanic acid | 875 mg twice daily; 50% efficacy |
| Ciprofloxacin | 500 mg twice daily; 43–100% efficacy |
| Doxycycline | 100 mg one-two times daily (efficacy not defined) |
| Metronidazole | 250 mg three times per day; 43–87% efficacy |
| Neomycin | 500 mg twice daily; 33–55% efficacy |
| Norfloxacin | 400 mg once daily; 30–100% efficacy |
| Tetracycline | 250 mg once daily; 87.5% efficacy |
| Trimethoprim-sulfamethoxazole | 160 mg/80 mg twice daily; 95% efficacy |
| NAFLD | NASH | Function Features [85] | |
| Increased in NAFLD Bacteria Fungi | Phylum: Proteobacteria [88] Family: Lactobacillaceae [89] Genus: Bacteroides [90] Ruminococcus [90] Lactobacillus [89] Species: E. coli [88], Klebsiella pneumoniae Streptococcus anginosus Veillonella atypica Talaromyces, Paraphaeosphaeria, Lycoperdon, Curvularia, Phialemoniopsis, Paraboeremia, Sarcinomyces, Cladophialophora, Sordaria | Ruminococcus, Blautia, Dorea [91] C. albicans, Mucor sp., Cyberlindnera jadinii, Penicillium sp., unknown Pleosporales, Babjeviella inositovora and Candida argentea | Oxidative damage γ-Aminobutyric acid biosynthesis Dentrification Ethanol production Lipopolysaccharide and peptidoglycan biosynthesis Branched-chain amino acid (BCAAs) and aromatic amino acid (AAA) biosynthesis |
| Decreased in NAFLD Bacteria Fungi | Phylum: Actinobacteria [91], Bacteroidetes [91], Firmicutes Genus: Oscillobacter, Prevotella [91], Ruminococcus [90], Coprococcus, Species: Faecalibacterium prausnitzii [89] Coprococcus comes Leptosphaeria, Pseudopithomyces, Fusicolla | Bacteroidetes [91] | Haem biosynthesis |
| Diet | Ketosis | Kcal per Day | % Macronutrients in Total Calories per Day | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHO | Source | Protein | Source | Fats | Source | Fiber | Source | |||
| Mediterranean diet | No | Individual, but >1000 kcal per day | >50% energy | whole grain, vegetables, seeds, nuts, starch, fruits | 15–20% energy | lean meat, fish, eggs, legumes, lean dairy products, fermented dairy products, seafood, tofu | 20–35% energy | olive o oil, fish oil, nuts, seeds, unrefined vegetable oils | 30–40 g/day | all vegetables and fruits, nuts, seeds, whole grain, resistant starch, cruciferous, green leafy vegetables, fermented foods, all legumes |
| Low FODMAPs diet | No | Individual, but >1000 kcal per day | >40% energy | low FODMAPSs: whole grain, rice, oats, vegetables, seeds, nuts, fruits | 15–20% energy | lean meat, fish, eggs, legumes, lean dairy products, fermented dairy products, seafood, tofu | 20–35% energy | olive o oil, fish oil, nuts, seeds, unrefined vegetable oils | Varies, but mostly <25 g/day | all except for fructooligosaccharides, oligosaccharides, disaccharides, monosaccharides and polyols |
| Standard ketogenic diet | Yes | Individual, but >1000 kcal per day | 20–30 g/day | some vegetable, some fruits, nuts, seeds | 15–20% energy | fatty meat, fatty dairy products, eggs, giblets, fish, seafood, sausages | >60–70% energy | butter, lard, animal fats, nuts, seeds, coconut oil, some MCT oil, little or no vegetable fats | <25 g/day | some vegetables and fruits low in carbohydrates, nuts, seeds, cruciferous and green leafy vegetables, fermented foods |
| VLCKD | Yes | <800 kcal/day | 20 g/day | little vegetable, some fruits, nuts, seeds | 15% energy | fatty meat, fatty dairy products, eggs, giblets, fish, seafood, sausages | >70% energy | butter, lard, animal fats, nuts, seeds, coconut oil, some MCT oil, little or no vegetable fats | <25 g/day | some vegetables and fruits low in carbohydrates, nuts, seeds, cruciferous and green leafy vegetables, fermented foods |
| Mediterranean ketogenic diet | Yes | Individual, but >1000 kcal per day | 50–80 g/day | vegetables, seeds, nuts, fruits | 15–20% energy | lean meat, fish, eggs, legumes, lean dairy products, fermented dairy products, seafood, tofu | >50% energy | olive o oil, fish oil, nuts, seeds, unrefined vegetable oils, MCT oil | 30–40 g/day | most vegetables and fruits low in carbohydrates, nuts, seeds, cruciferous and green leafy vegetables, fermented foods, some legumes |
| Intermittent fasting | Spontaneous | Individual, but usually >1000 kcal per day | Varies | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudan, A.; Kozłowska-Petriczko, K.; Wunsch, E.; Bodnarczuk, T.; Stachowska, E. Small Intestinal Bacterial Overgrowth and Non-Alcoholic Fatty Liver Disease: What Do We Know in 2023? Nutrients 2023, 15, 1323. https://doi.org/10.3390/nu15061323
Gudan A, Kozłowska-Petriczko K, Wunsch E, Bodnarczuk T, Stachowska E. Small Intestinal Bacterial Overgrowth and Non-Alcoholic Fatty Liver Disease: What Do We Know in 2023? Nutrients. 2023; 15(6):1323. https://doi.org/10.3390/nu15061323
Chicago/Turabian StyleGudan, Anna, Katarzyna Kozłowska-Petriczko, Ewa Wunsch, Tomasz Bodnarczuk, and Ewa Stachowska. 2023. "Small Intestinal Bacterial Overgrowth and Non-Alcoholic Fatty Liver Disease: What Do We Know in 2023?" Nutrients 15, no. 6: 1323. https://doi.org/10.3390/nu15061323
APA StyleGudan, A., Kozłowska-Petriczko, K., Wunsch, E., Bodnarczuk, T., & Stachowska, E. (2023). Small Intestinal Bacterial Overgrowth and Non-Alcoholic Fatty Liver Disease: What Do We Know in 2023? Nutrients, 15(6), 1323. https://doi.org/10.3390/nu15061323






