Current Status and Nutritional Value of Green Leaf Protein
Abstract
:1. Introduction
2. Conventional Protein Sources and Their Alternatives
2.1. Cultivated Protein
2.1.1. Cellular Agriculture of Animal Protein
2.1.2. Algal Bioreactors
2.1.3. Insect Protein Farms
2.2. Fermentation Protein (Traditional, Biomass, and Precision)
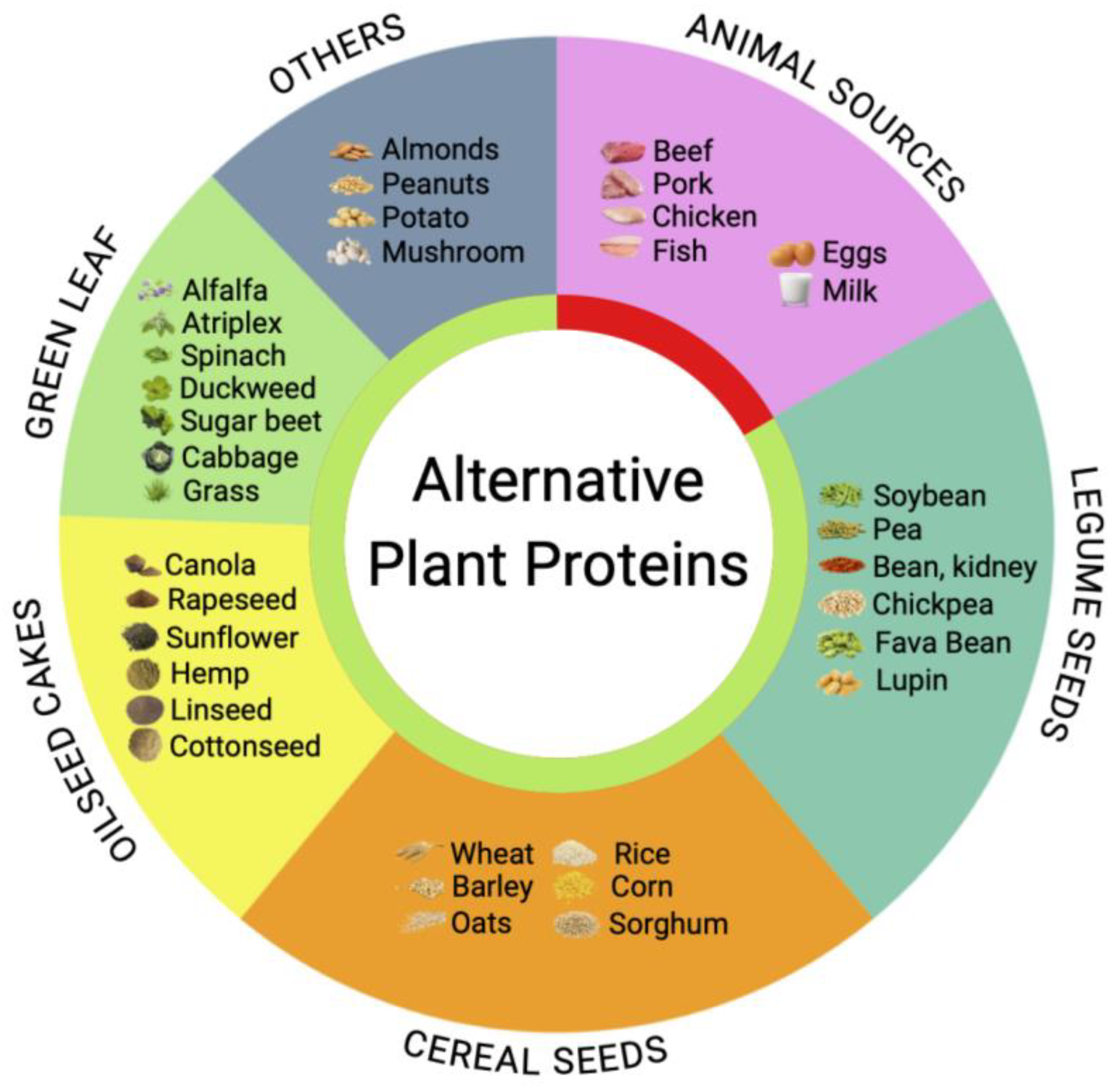
3. Plant-Based Alternatives and Undervaluation of Green Leaf Biomass
3.1. Green Leaf Protein, Brief History and Past Uses
3.2. Composition of Green Leaf Proteins
3.3. Extraction and Concentration
3.3.1. Thermal-Assisted Extraction
3.3.2. Alkaline Extraction
3.3.3. Enzyme-Assisted Extraction
3.3.4. Ultrasound-Assisted Extraction (Sonication)
3.3.5. Pulse Electric Field-Assisted Extraction (PEF, Electroporation)
3.3.6. Heat Precipitation
3.3.7. Acid Precipitation (Isoelectric)
3.3.8. Ultrafiltration
3.4. Scaleup and Technological Concerns
4. Leaf Protein Quality and Nutritional Outcomes
4.1. Functionality of Protein Ingredients
4.1.1. Solubility
4.1.2. Gelation
4.1.3. Foaming and Stability
4.1.4. Emulsifying Properties
4.2. Nutritional Aspects of Green Leaf Protein
4.2.1. Amino acid Composition
4.2.2. Digestibility and Antinutritional Components
4.2.3. Applications to Feed and Foraging Systems
4.2.4. Agrochemicals and Reuse of Treated Wastewater
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukase, E.; Martin, W. Economic Growth, Convergence, and World Food Demand and Supply. World Dev. 2020, 132, 104954. [Google Scholar] [CrossRef]
- Lonnie, M.; Laurie, I.; Myers, M.; Horgan, G.; Russell, W.R.; Johnstone, A.M. Exploring Health-Promoting Attributes of Plant Proteins as a Functional Ingredient for the Food Sector: A Systematic Review of Human Interventional Studies. Nutrients 2020, 12, 2291. [Google Scholar] [CrossRef] [PubMed]
- Komarnytsky, S.; Retchin, S.; Vong, C.I.; Lila, M.A. Gains and Losses of Agricultural Food Production: Implications for the Twenty-First Century. Annu. Rev. Food Sci. Technol. 2022, 13, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.P.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein Demand: Review of Plant and Animal Proteins Used in Alternative Protein Product Development and Production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Dietary Protein Intake and Human Health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization. The State of Food Insecurity in the World: Addressing Food Insecurity in Protracted Crises 2010; Food and Agriculture Organization: Rome, Italy, 2010. [Google Scholar]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [Green Version]
- Gilani, G.S.; Cockell, K.A.; Sepehr, E. Effects of Antinutritional Factors on Protein Digestibility and Amino Acid Availability in Foods. J. AOAC Int. 2005, 88, 967–987. [Google Scholar] [CrossRef] [Green Version]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Wang, Y.; Tuccillo, F.; Lampi, A.-M.; Knaapila, A.; Pulkkinen, M.; Kariluoto, S.; Coda, R.; Edelmann, M.; Jouppila, K.; Sandell, M.; et al. Flavor Challenges in Extruded Plant-Based Meat Alternatives: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2898–2929. [Google Scholar] [CrossRef]
- Chriki, S.; Hocquette, J.-F. The Myth of Cultured Meat: A Review. Front. Nutr. 2020, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22, 7513. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, H.L.; Teixeira de Mattos, M.J. Environmental Impacts of Cultured Meat Production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-Based Food and Protein Trend from a Business Perspective: Markets, Consumers, and the Challenges and Opportunities in the Future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.J.; Barnett, J.C. What’s in a Name? Consumer Perceptions of in Vitro Meat under Different Names. Appetite 2019, 137, 104–113. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron Bioavailability and Dietary Reference Values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [Green Version]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.D.R.; Leite, M.D.O.; Albino, L.F.T.; Martins, M.A. Microalgae Proteins: Production, Separation, Isolation, Quantification, and Application in Food and Feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- de Vries, M.; de Boer, I.J.M. Comparing Environmental Impacts for Livestock Products: A Review of Life Cycle Assessments. Livest. Sci. 2010, 128, 1–11. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Phong, W.N.; Show, P.L.; Ling, T.C.; Juan, J.C.; Ng, E.-P.; Chang, J.-S. Mild Cell Disruption Methods for Bio-Functional Proteins Recovery from Microalgae—Recent Developments and Future Perspectives. Algal. Res. 2018, 31, 506–516. [Google Scholar] [CrossRef]
- Fleurence, J. The Enzymatic Degradation of Algal Cell Walls: A Useful Approach for Improving Protein Accessibility? J. Appl. Phycol. 1999, 11, 313–314. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Looy, H.; Dunkel, F.V.; Wood, J.R. How Then Shall We Eat? Insect-Eating Attitudes and Sustainable Foodways. Agric. Hum. Values 2014, 31, 131–141. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Cortes Ortiz, J.A.; Ruiz, A.T.; Morales-Ramos, J.A.; Thomas, M.; Rojas, M.G.; Tomberlin, J.K.; Yi, L.; Han, R.; Giroud, L.; Jullien, R.L. Chapter 6—Insect Mass Production Technologies. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 153–201. ISBN 978-0-12-802856-8. [Google Scholar]
- Kim, T.-K.; Yong, H.I.; Kim, Y.-B.; Kim, H.-W.; Choi, Y.-S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Käßer, L.; Harnischfeger, J.; Salzig, D.; Czermak, P. The Effect of Different Insect Cell Culture Media on the Efficiency of Protein Production by Spodoptera Frugiperda Cells. Electron. J. Biotechnol. 2022, 56, 54–64. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for Future Food Systems. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef]
- Pacheco, M.T.; Caballero-Córdoba, G.M.; Sgarbieri, V.C. Composition and Nutritive Value of Yeast Biomass and Yeast Protein Concentrates. J. Nutr. Sci. Vitam. 1997, 43, 601–612. [Google Scholar] [CrossRef] [Green Version]
- Tsai, L.B.; Mann, M.; Morris, F.; Rotgers, C.; Fenton, D. The Effect of Organic Nitrogen and Glucose on the Production of Recombinant Human Insulin-like Growth Factor in High Cell Density Escherichia Coli Fermentations. J. Ind. Microbiol. 1987, 2, 181–186. [Google Scholar] [CrossRef]
- Banks, M.; Johnson, R.; Giver, L.; Bryant, G.; Guo, M. Industrial Production of Microbial Protein Products. Curr. Opin. Biotechnol. 2022, 75, 102707. [Google Scholar] [CrossRef] [PubMed]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing Factors on Single-Cell Protein Production by Submerged Fermentation: A Review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Method Development to Increase Protein Enrichment During Dry Fractionation of Starch-Rich Legumes. Food Bioprocess. Technol. 2015, 8, 1495–1502. [Google Scholar] [CrossRef] [Green Version]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond Oil: Press Cakes and Meals Supplying Global Protein Requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Ganzhorn, J.U.; Arrigo- Nelson, S.J.; Carrai, V.; Chalise, M.K.; Donati, G.; Droescher, I.; Eppley, T.M.; Irwin, M.T.; Koch, F.; Koenig, A.; et al. The Importance of Protein in Leaf Selection of Folivorous Primates. Am. J. Primatol. 2017, 79, e22550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palatini, K.M.; Durand, P.-J.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Bitter Receptors and Glucose Transporters Interact to Control Carbohydrate and Immune Responses in the Gut. FASEB J. 2016, 30, 682.6. [Google Scholar] [CrossRef]
- Toda, Y.; Hayakawa, T.; Itoigawa, A.; Kurihara, Y.; Nakagita, T.; Hayashi, M.; Ashino, R.; Melin, A.D.; Ishimaru, Y.; Kawamura, S.; et al. Evolution of the Primate Glutamate Taste Sensor from a Nucleotide Sensor. Curr. Biol. 2021, 31, 4641–4649.e5. [Google Scholar] [CrossRef]
- Pirie, N.W. Leaf Protein and Its By-Products in Human and Animal Nutrition; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Enochian, R.V. Producing Pro-Xan (Leaf Protein Concentrate) from Alfalfa: Economics of an Emerging Technology [USA]; Department of Agriculture, Economics, Statistics, and Cooperatives Service: Washington, DC, USA, 1980. [Google Scholar]
- Shah, F.H.; Salam Sheikh, A.; Farrukh, N.; Rasool, A. A Comparison of Leaf Protein Concentrate Fortified Dishes and Milk as Supplements for Children with Nutritionally Inadequate Diets. Plant Food Hum. Nutr. 1980, 30, 245–258. [Google Scholar] [CrossRef]
- Telek, L. Leaf Protein Extraction from Tropical Plants. Plants Potentials Extr. Protein 1983, 305, 78–110. [Google Scholar]
- Davys, M.N.; Richardier, C.; Kennedy, D.; Mathan, O.; Collin, S.; Subtil, J.; Bertin, E.; Davys, M. Leaf Concentrate and Other Benefits of Leaf Fractionation. In Combating Micronutrient Deficiencies: Food-Based Approaches; CABI: Wallingford, UK, 2010; pp. 338–365. ISBN 978-1-84593-714-0. [Google Scholar]
- Raven, J.A. Rubisco: Still the Most Abundant Protein of Earth? New Phytol. 2013, 198, 1–3. [Google Scholar] [CrossRef]
- Makino, A.; Sakuma, H.; Sudo, E.; Mae, T. Differences between Maize and Rice in N-Use Efficiency for Photosynthesis and Protein Allocation. Plant Cell Physiol. 2003, 44, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.H.; Nieuwland, M.; de Jong, G.A.H. Characterization of Heat-Set Gels from RuBisCO in Comparison to Those from Other Proteins. J. Agric. Food Chem. 2014, 62, 10783–10791. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Okolie, C.L.; Qian, H.; Ohanenye, I.C.; Agyei, D.; Aluko, R.E. Ribulose-1,5-Bisphosphate Carboxylase as a Sustainable and Promising Plant Source of Bioactive Peptides for Food Applications. Trends Food Sci. Technol. 2017, 69, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Nynäs, A.-L.; Newson, W.R.; Johansson, E. Protein Fractionation of Green Leaves as an Underutilized Food Source—Protein Yield and the Effect of Process Parameters. Foods 2021, 10, 2533. [Google Scholar] [CrossRef]
- Tamayo Tenorio, A.; Boom, R.M.; van der Goot, A.J. Understanding Leaf Membrane Protein Extraction to Develop a Food-Grade Process. Food Chem. 2017, 217, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Corona, A.; Ambye-Jensen, M.; Vega, G.C.; Hauschild, M.Z.; Birkved, M. Techno-Environmental Assessment of the Green Biorefinery Concept: Combining Process Simulation and Life Cycle Assessment at an Early Design Stage. Sci. Total Environ. 2018, 635, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Walter, W.M., Jr.; Purcell, A.E.; McCollum, G.K. Laboratory Preparation of a Protein-Xanthophyll Concentrate from Sweet Potato Leaves. J. Agric. Food Chem. 1978, 26, 1222–1226. [Google Scholar] [CrossRef]
- Martin, A.H.; Castellani, O.; de Jong, G.A.; Bovetto, L.; Schmitt, C. Comparison of the Functional Properties of RuBisCO Protein Isolate Extracted from Sugar Beet Leaves with Commercial Whey Protein and Soy Protein Isolates. J. Sci. Food Agric. 2019, 99, 1568–1576. [Google Scholar] [CrossRef]
- Colas, D.; Doumeng, C.; Pontalier, P.Y.; Rigal, L. Twin-Screw Extrusion Technology, an Original Solution for the Extraction of Proteins from Alfalfa (Medicago Sativa). Food Bioprod. Process. 2013, 91, 175–182. [Google Scholar] [CrossRef]
- Kerfai, S.; Fernández, A.; Mathé, S.; Alfenore, S.; Arlabosse, P. Production of Green Juice with an Intensive Thermo-Mechanical Fractionation Process. Part II: Effect of Processing Conditions on the Liquid Fraction Properties. Chem. Eng. J. 2011, 167, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Chen, J.; Xiong, Y.L. Structural and Emulsifying Properties of Soy Protein Isolate Subjected to Acid and Alkaline PH-Shifting Processes. J. Agric. Food Chem. 2009, 57, 7576–7583. [Google Scholar] [CrossRef]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.-B.; Chen, B.; Rao, J. Effect of Alkaline Extraction PH on Structure Properties, Solubility, and Beany Flavor of Yellow Pea Protein Isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef]
- Wang, C.; Tian, Z.; Chen, L.; Temelli, F.; Liu, H.; Wang, Y. Functionality of Barley Proteins Extracted and Fractionated by Alkaline and Alcohol Methods. Cereal Chem. 2010, 87, 597–606. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the Plant Protein Extraction: Mechanism and Recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Bals, B.; Dale, B.E. Economic Comparison of Multiple Techniques for Recovering Leaf Protein in Biomass Processing. Biotechnol. Bioeng 2011, 108, 530–537. [Google Scholar] [CrossRef]
- Zhang, C.; Sanders, J.P.M.; Bruins, M.E. Critical Parameters in Cost-Effective Alkaline Extraction for High Protein Yield from Leaves. Biomass Bioenergy 2014, 67, 466–472. [Google Scholar] [CrossRef]
- Kammes, K.L.; Bals, B.D.; Dale, B.E.; Allen, M.S. Grass Leaf Protein, a Coproduct of Cellulosic Ethanol Production, as a Source of Protein for Livestock. Anim. Feed Sci. Technol. 2011, 164, 79–88. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Simultaneous Effect of Vacuum and Ultrasound Assisted Enzymatic Extraction on the Recovery of Plant Protein and Bioactive Compounds from Sesame Bran. J. Food Compos. Anal. 2020, 87, 103424. [Google Scholar] [CrossRef]
- Sari, Y.W.; Mulder, W.J.; Sanders, J.P.M.; Bruins, M.E. Towards Plant Protein Refinery: Review on Protein Extraction Using Alkali and Potential Enzymatic Assistance. Biotechnol. J. 2015, 10, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gat, Y.; Arya, S.; Kumar, V.; Panghal, A.; Kumar, A. A Review on Microbial Alkaline Protease: An Essential Tool for Various Industrial Approaches. Ind. Biotechnol. 2019, 15, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Wang, X.; Wang, Z.; Wu, Y.; Chen, J. Studies on Tea Protein Extraction Using Alkaline and Enzyme Methods. Food Chem. 2008, 107, 929–938. [Google Scholar] [CrossRef]
- Dotsenko, G.; Lange, L. Enzyme Enhanced Protein Recovery from Green Biomass Pulp. Waste Biomass Valor 2017, 8, 1257–1264. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.-S.; Tian, Y.-J.; He, Y.-Z.; Li, L.; Hu, S.-Q.; Li, B. Optimisation of Ultrasonic-Assisted Protein Extraction from Brewer’s Spent Grain. Czech J. Food Sci. 2010, 28, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Singla, M.; Sit, N. Application of Ultrasound in Combination with Other Technologies in Food Processing: A Review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Bao, T.; Zheng, X.; Chen, W.; Wang, J. A Recyclable Protein Resource Derived from Cauliflower By-Products: Potential Biological Activities of Protein Hydrolysates. Food Chem. 2017, 221, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Khaksar, F.B.; Pagan, J.; Ibarz, A. Application of Ultrasound-Ultrafiltration-Assisted Alkaline Isoelectric Precipitation (UUAAIP) Technique for Producing Alfalfa Protein Isolate for Human Consumption: Optimization, Comparison, Physicochemical, and Functional Properties. Food Res. Int. 2020, 130, 108907. [Google Scholar] [CrossRef] [PubMed]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell Disruption for Microalgae Biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Queiroz, C.; Mendes Lopes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol Oxidase: Characteristics and Mechanisms of Browning Control. Food Rev. Int. 2008, 24, 361–375. [Google Scholar] [CrossRef]
- Gachovska, T.; Raghavan, M. Pulsed Electric Field Assisted Juice Extraction from Alfalfa. Can. Biosyst. Eng. 2006, 48, 33. [Google Scholar]
- Tamayo Tenorio, A.; Gieteling, J.; de Jong, G.A.H.; Boom, R.M.; van der Goot, A.J. Recovery of Protein from Green Leaves: Overview of Crucial Steps for Utilisation. Food Chem. 2016, 203, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Damborg, V.K.; Jensen, S.K.; Weisbjerg, M.R.; Adamsen, A.P.; Stødkilde, L. Screw-Pressed Fractions from Green Forages as Animal Feed: Chemical Composition and Mass Balances. Anim. Feed Sci. Technol. 2020, 261, 114401. [Google Scholar] [CrossRef]
- Santamaria-Fernandez, M.; Ambye-Jensen, M.; Damborg, V.K.; Lübeck, M. Demonstration-Scale Protein Recovery by Lactic Acid Fermentation from Grass Clover—A Single Case of the Production of Protein Concentrate and Press Cake Silage for Animal Feeding Trials. Biofuels Bioprod. Biorefining 2019, 13, 502–513. [Google Scholar] [CrossRef]
- Tanambell, H.; Møller, A.H.; Corredig, M.; Dalsgaard, T.K. RuBisCO from Alfalfa—Native Subunits Preservation through Sodium Sulfite Addition and Reduced Solubility after Acid Precipitation Followed by Freeze-Drying. LWT 2022, 154, 112682. [Google Scholar] [CrossRef]
- Kader, J.C.; Julienne, M.; Vergnolle, C. Purification and Characterization of a Spinach-Leaf Protein Capable of Transferring Phospholipids from Liposomes to Mitochondria or Chloroplasts. Eur. J. Biochem. 1984, 139, 411–416. [Google Scholar] [CrossRef]
- Coldebella, P.F.; Gomes, S.D.; Evarini, J.A.; Cereda, M.P.; Coelho, S.R.M.; Coldebella, A. Evaluation of Protein Extraction Methods to Obtain Protein Concentrate from Cassava Leaf. Eng. Agríc. 2013, 33, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, L.; Weiss, J. Alternative Protein Sources as Technofunctional Food Ingredients. Annu. Rev. Food Sci. Technol. 2021, 12, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the Functional Properties of Pea, Chickpea and Lentil Protein Concentrates Processed Using Ultrafiltration and Isoelectric Precipitation Techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea Protein Isolates: Structure, Extraction, and Functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Zydney, A.L. Protein Separations Using Membrane Filtration: New Opportunities for Whey Fractionation. Int. Dairy J. 1998, 8, 243–250. [Google Scholar] [CrossRef]
- Muneer, F.; Hovmalm, H.P.; Svensson, S.-E.; Newson, W.R.; Johansson, E.; Prade, T. Economic Viability of Protein Concentrate Production from Green Biomass of Intermediate Crops: A Pre-Feasibility Study. J. Clean. Prod. 2021, 294, 126304. [Google Scholar] [CrossRef]
- Ghaly, A.; Alkoaik, F. Extraction of Protein from Common Plant Leaves for Use as Human Food. Am. J. Appl. Sci. 2010, 7, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Nieuwland, M.; Geerdink, P.; Engelen-Smit, N.P.E.; van der Meer, I.M.; America, A.H.P.; Mes, J.J.; Kootstra, A.M.J.; Henket, J.T.M.M.; Mulder, W.J. Isolation and Gelling Properties of Duckweed Protein Concentrate. ACS Food Sci. Technol. 2021, 1, 908–916. [Google Scholar] [CrossRef]
- Zhang, C.; Sanders, J.P.M.; Xiao, T.T.; Bruins, M.E. How Does Alkali Aid Protein Extraction in Green Tea Leaf Residue: A Basis for Integrated Biorefinery of Leaves. PLoS ONE 2015, 10, e0133046. [Google Scholar] [CrossRef]
- Huang, K.H.; Tao, M.C.; Boulet, M.; Riel, R.R.; Julien, J.P.; Brisson, G.J. A Process for the Preparation of Leaf Protein Concentrates Based on the Treatment of Leaf Juices with Polar Solvents. Can. Inst. Food Technol. J. 1971, 4, 85–90. [Google Scholar] [CrossRef]
- Knuckles, B.E.; Edwards, R.H.; Kohler, G.O.; Whitney, L.F. Flocculants in the Separation of Green and Soluble White Protein Fractions from Alfalfa. J. Agric. Food Chem. 1980, 28, 32–36. [Google Scholar] [CrossRef]
- Hartman, G.H.; Akeson, W.R.; Stahmann, M.A. Leaf Protein Concentrate Prepared by Spray-Drying. J. Agric. Food Chem. 1967, 15, 74–79. [Google Scholar] [CrossRef]
- Sullivan, M.L.; Hatfield, R.D. Polyphenol Oxidase and O-Diphenols Inhibit Postharvest Proteolysis in Red Clover and Alfalfa. Crop Sci. 2006, 46, 662–670. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Ye, A.; Ferrua, M.J. Aspects of Food Structures in the Digestive Tract. Curr. Opin. Food Sci. 2015, 3, 85–93. [Google Scholar] [CrossRef]
- Zayas, J.F. Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; ISBN 978-3-642-63856-5. [Google Scholar]
- Foegeding, E.A.; Davis, J.P. Food Protein Functionality: A Comprehensive Approach. Food Hydrocoll. 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Reetu; Punia, S.; Dhakane-Lad, J.; Singh, S.; Dhumal, S.; Pradhan, P.C.; Bhushan, B.; et al. Functional Characterization of Plant-Based Protein to Determine Its Quality for Food Applications. Food Hydrocoll. 2022, 123, 106986. [Google Scholar] [CrossRef]
- Kaur, L.; Lamsar, H.; López, I.F.; Filippi, M.; Ong Shu Min, D.; Ah-Sing, K.; Singh, J. Physico-Chemical Characteristics and In Vitro Gastro-Small Intestinal Digestion of New Zealand Ryegrass Proteins. Foods 2021, 10, 331. [Google Scholar] [CrossRef]
- Saricaoglu, F.T.; Gul, O.; Besir, A.; Atalar, I. Effect of High Pressure Homogenization (HPH) on Functional and Rheological Properties of Hazelnut Meal Proteins Obtained from Hazelnut Oil Industry by-Products. J. Food Eng. 2018, 233, 98–108. [Google Scholar] [CrossRef]
- Li, R.; Cui, Q.; Wang, G.; Liu, J.; Chen, S.; Wang, X.; Wang, X.; Jiang, L. Relationship between Surface Functional Properties and Flexibility of Soy Protein Isolate-Glucose Conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, J.; Andrade, J.; Rababah, T.M.; Almajwal, A.; Abulmeaty, M.M.; Feng, H. Modifying the Physicochemical Properties of Pea Protein by PH-Shifting and Ultrasound Combined Treatments. Ultrason Sonochem. 2017, 38, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Klost, M.; Drusch, S. Functionalisation of Pea Protein by Tryptic Hydrolysis—Characterisation of Interfacial and Functional Properties. Food Hydrocoll. 2019, 86, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Lamsal, B.P.; Koegel, R.G.; Gunasekaran, S. GELATION OF ALFALFA SOLUBLE LEAF PROTEINS. Trans. ASAE 2005, 48, 2229–2235. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture Extrusion Cooking of Pea Protein Isolates: Raw Material Characteristics, Extruder Responses, and Texture Properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Nivala, O.; Mäkinen, O.E.; Kruus, K.; Nordlund, E.; Ercili-Cura, D. Structuring Colloidal Oat and Faba Bean Protein Particles via Enzymatic Modification. Food Chem. 2017, 231, 87–95. [Google Scholar] [CrossRef]
- Nissen, S.H.; Schmidt, J.M.; Gregersen, S.; Hammershøj, M.; Møller, A.H.; Danielsen, M.; Stødkilde, L.; Nebel, C.; Dalsgaard, T.K. Increased Solubility and Functional Properties of Precipitated Alfalfa Protein Concentrate Subjected to PH Shift Processes. Food Hydrocoll. 2021, 119, 106874. [Google Scholar] [CrossRef]
- Amagliani, L.; Silva, J.V.C.; Saffon, M.; Dombrowski, J. On the Foaming Properties of Plant Proteins: Current Status and Future Opportunities. Trends Food Sci. Technol. 2021, 118, 261–272. [Google Scholar] [CrossRef]
- Lawal, O.S.; Adebowale, K.O. Effect of Acetylation and Succinylation on Solubility Profile, Water Absorption Capacity, Oil Absorption Capacity and Emulsifying Properties of Mucuna Bean (Mucuna Pruriens) Protein Concentrate. Nahrung 2004, 48, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of Plant Proteins for Improved Functionality: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Drusch, S.; Klost, M.; Kieserling, H. Current Knowledge on the Interfacial Behaviour Limits Our Understanding of Plant Protein Functionality in Emulsions. Curr. Opin. Colloid Interface Sci. 2021, 56, 101503. [Google Scholar] [CrossRef]
- Tang, C.-H. Emulsifying Properties of Soy Proteins: A Critical Review with Emphasis on the Role of Conformational Flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Damgaard, H.; Greve-Poulsen, M.; Larsen, L.B.; Hammershøj, M. Foam and Emulsion Properties of Potato Protein Isolate and Purified Fractions. Food Hydrocoll. 2018, 74, 367–378. [Google Scholar] [CrossRef]
- Hojilla-Evangelista, M.P.; Selling, G.W.; Hatfield, R.; Digman, M. Extraction, Composition, and Functional Properties of Dried Alfalfa (Medicago Sativa L.) Leaf Protein. J. Sci. Food Agric. 2017, 97, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.; Colt, M. Nutrient Value of Leaf vs. Seed. Front. Chem. 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghamdi, M.; Gutierrez, J.; Komarnytsky, S. Essential Minerals and Metabolic Adaptation of Immune Cells. Nutrients 2023, 15, 123. [Google Scholar] [CrossRef]
- Moore, E.M.; Wagner, C.; Komarnytsky, S. The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front. Pharm. 2020, 11, 785. [Google Scholar] [CrossRef]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein Quality Evaluation Twenty Years after the Introduction of the Protein Digestibility Corrected Amino Acid Score Method. Br. J. Nutr. 2012, 108 (Suppl. S2), S183–S211. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, N.; Wildman, S.G. Fraction I Protein. Annu. Rev. Plant Physiol. 1970, 21, 325–358. [Google Scholar] [CrossRef]
- Stødkilde, L.; Damborg, V.K.; Jørgensen, H.; Lærke, H.N.; Jensen, S.K. Digestibility of Fractionated Green Biomass as Protein Source for Monogastric Animals. Animal 2019, 13, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, R.; Altamirano, S.B.; Moretti, R.H. Nutritional Characteristics of Cassava (Manihot Esculenta Crantz) Leaf Protein Concentrates Obtained by Ultrafiltration and Acidic Thermocoagulation. Plant Foods Hum. Nutr. 1994, 45, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Matheis, G.; Whitaker, J.R. Modification of Proteins by Polyphenol Oxidase and Peroxidase and Their Products. J. Food Biochem. 1984, 8, 137–162. [Google Scholar] [CrossRef]
- Rawel, H.M.; Kroll, J.; Riese, B. Reactions of Chlorogenic Acid with Lysozyme: Physicochemical Characterization and Proteolytic Digestion of the Derivatives. J. Food Sci. 2000, 65, 1091–1098. [Google Scholar] [CrossRef]
- Amer, B.; Juul, L.; Møller, A.H.; Møller, H.S.; Dalsgaard, T.K. Improved Solubility of Proteins from White and Red Clover—Inhibition of Redox Enzymes. Int. J. Food Sci. Technol. 2021, 56, 302–311. [Google Scholar] [CrossRef]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Shepon, A.; Eshel, G.; Noor, E.; Milo, R. Energy and Protein Feed-to-Food Conversion Efficiencies in the US and Potential Food Security Gains from Dietary Changes. Environ. Res. Lett. 2016, 11, 105002. [Google Scholar] [CrossRef]
- Norman, H.C.; Freind, C.; Masters, D.G.; Rintoul, A.J.; Dynes, R.A.; Williams, I.H. Variation within and between Two Saltbush Species in Plant Composition and Subsequent Selection by Sheep. Aust. J. Agric. Res. 2004, 55, 999–1007. [Google Scholar] [CrossRef]
- Carlsson, R.; Clarke, E.M.W. Atriplex Hortensis L. as a Leafy Vegetable, and as a Leaf Protein Concentrate Plant. Plant Food Hum. Nutr. 1983, 33, 127–133. [Google Scholar] [CrossRef]
- Damborg, V.K.; Stødkilde, L.; Jensen, S.K.; Weisbjerg, M.R. Protein Value and Degradation Characteristics of Pulp Fibre Fractions from Screw Pressed Grass, Clover, and Lucerne. Anim. Feed. Sci. Technol. 2018, 244, 93–103. [Google Scholar] [CrossRef]
- Hayes, T.B.; Hansen, M. From Silent Spring to Silent Night: Agrochemicals and the Anthropocene. Elem. Sci. Anthr. 2017, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Gnanaprakasam, P.D.; Vanisree, A.J. Recurring Detrimental Impact of Agrochemicals on the Ecosystem, and a Glimpse of Organic Farming as a Possible Rescue. Environ. Sci. Pollut. Res. 2022, 29, 75103–75112. [Google Scholar] [CrossRef] [PubMed]

| Protein Quality | Essential Amino Acid Composition (% Protein) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein Type | 100 g Fresh Weight | %Digestibility | %Biological Value | %Net utilization | % PDC AAS | % DI AAS | Arg * | Cys * | His ** | Ile ** | Leu ** | Lys ** | Met ** | Phe ** | Tre ** | Trp ** | Tyr * | Val ** | Met + Cys | Phe + Tyr |
| Animal sources | ||||||||||||||||||||
| Beef | 22.7 | 92% | 80% | 73% | 92% | 112% | 6.3 | 1.4 | 2.7 | 4.9 | 8.5 | 8.3 | 2.4 | 3.8 | 4.0 | 1.3 | 3.0 | 5.3 | 3.8 | 6.8 |
| Pork | 16.9 | 98% | - | - | 70% | - | 6.6 | 1.3 | 2.8 | 4.8 | 7.0 | 7.2 | 2.4 | 4.1 | 5.0 | 1.3 | 2.8 | 4.2 | 3.7 | 7.0 |
| Chicken | 20.8 | 95% | 79% | 80% | 91% | 108% | 7.0 | 1.2 | 4.7 | 3.8 | 12.2 | 5.6 | 6.8 | 4.7 | 3.3 | 7.4 | 3.8 | 5.5 | 8.1 | 8.7 |
| Fish a | 17.8 | 94% | 67% | 64% | 106% | 100% | 4.5 | 2.0 | 2.3 | 3.1 | 4.7 | 6.2 | 3.6 | 5.5 | 3.4 | 0.9 | 5.7 | 3.8 | 5.6 | 11.2 |
| Eggs a | 12.6 | 98% | 100% | 94% | 100% | 113% | 5.4 | 2.2 | 2.3 | 5.7 | 8.5 | 6.9 | 3.4 | 5.8 | 4.6 | 1.2 | 3.9 | 6.4 | 5.6 | 9.7 |
| Milk a | 3.3 | 96% | 91% | 82% | 100% | 114% | 3.33 | 0.9 | 3.6 | 4.0 | 8.8 | 7.7 | 2.9 | 4.6 | 4.8 | 1.4 | 5.0 | 4.7 | 3.7 | 9.6 |
| Whey PI | 0.9 | 100% | 104% | 92% | 100% | 125% | 1.8 | 2.1 | 1.3 | 5.6 | 10.3 | 9.7 | 1.7 | 2.6 | 7.9 | 1.9 | 2.7 | 5.9 | 3.8 | 5.3 |
| Pulse (legume) seeds | ||||||||||||||||||||
| Soybean a | 13.0 | 97% | 73% | 66% | 100% | 100% | 6.2 | 2.1 | 3.0 | 5.3 | 7.1 | 6.1 | 2.7 | 3.9 | 3.7 | 7.6 | 4.1 | 5.2 | 4.8 | 8.0 |
| Soy flour a | 37.8 | 80% | - | - | 93% | 105% | 7.6 | 1.5 | 2.6 | 4.9 | 7.8 | 6.4 | 1.4 | 5.2 | 3.6 | 1.4 | 3.8 | 4.7 | 2.9 | 9.0 |
| Soy PI a | 88.3 | 98% | 74% | 61% | 100% | 98% | 7.6 | 1.2 | 2.6 | 4.8 | 7.7 | 6.0 | 1.3 | 5.2 | 3.6 | 1.3 | 3.7 | 4.7 | 2.5 | 8.9 |
| Pea, yellow | 22.3 | 87% | 64% | 56% | 78% | 65% | 8.4 | 1.4 | 2.5 | 4.2 | 7.1 | 7.2 | 1.0 | 4.7 | 3.8 | 0.9 | 3.1 | 4.8 | 2.4 | 7.8 |
| Pea PI | - | 99% | 65% | - | 89% | - | 7.4 | 0.7 | 2.0 | 3.8 | 7.2 | 5.8 | 0.7 | 4.6 | 3.0 | 0.7 | 3.2 | 4.0 | 1.4 | 7.8 |
| Bean, kidney | 22.5 | 64% | - | - | 68% | 59% | 5.7 | 0.9 | 2.7 | 4.5 | 7.6 | 5.5 | 1.2 | 5.1 | 3.4 | 7.5 | 4.2 | 5.0 | 2.1 | 9.3 |
| Chickpea | 20.5 | 89% | 68% | 58% | 74% | - | 14.1 | 0.6 | 4.5 | 5.1 | 8.8 | 10.5 | 1.4 | 5.1 | 3.6 | 1.0 | 3.6 | 5.0 | 2.0 | 8.7 |
| Fava bean | 26.1 | 95% | - | - | 69% | - | 9.0 | 1.2 | 2.6 | 4.1 | 7.1 | 6.3 | 0.8 | 4.0 | 3.5 | 0.8 | 2.7 | 4.6 | 2.0 | 6.7 |
| Lupin | 36.2 | 76% | 83% | - | 81% | - | 11.0 | 1.5 | 2.7 | 4.2 | 6.9 | 4.7 | 0.7 | 4.0 | 3.4 | 0.8 | 3.6 | 3.9 | 2.2 | 7.6 |
| Cereal seeds | ||||||||||||||||||||
| Wheat, grain a | 9.6 | 86% | 80% | - | 42% | 54% | 2.4 | 0.7 | 1.4 | 3.0 | 5.0 | 1.1 | 0.7 | 3.7 | 1.8 | 0.3 | 2.4 | 2.3 | 1.4 | 6.1 |
| Barley, grain | 12.5 | 99% | 81% | - | 61% | 51% | 6.0 | 1.5 | 2.2 | 3.6 | 4.6 | 0.8 | 0.7 | 3.6 | 1.9 | 0.7 | 1.6 | 3.5 | 2.2 | 5.2 |
| Oats, grain | 13.5 | 90% | - | - | - | 77% | 9.7 | 1.7 | 3.6 | 4.4 | 9.1 | 3.7 | 1.7 | 5.5 | 4.3 | 3.6 | 2.6 | 6.0 | 5.4 | 8.1 |
| Rice, white | 6.8 | 92% | - | - | 63% | 64% | 5.9 | 0.2 | 1.6 | 2.3 | 5.7 | 4.7 | 0.3 | 3.7 | 2.3 | 1.0 | 2.6 | 2.7 | 0.5 | 6.3 |
| Rice, brown | 7.5 | 79% | - | - | - | - | 7.6 | 1.2 | 2.6 | 4.2 | 8.3 | 3.8 | 2.2 | 5.1 | 3.7 | 1.3 | 3.8 | 5.8 | 3.4 | 8.9 |
| Corn, grain | 9.4 | - | - | - | 60% | 48% | 1.7 | 0.3 | 1.1 | 1.7 | 8.8 | 1.0 | 1.1 | 3.4 | 1.8 | 0.6 | 2.7 | 2.1 | 1.4 | 6.1 |
| Corn, distillers | 27.1 | - | - | - | - | - | 3.4 | 2.0 | 2.4 | 3.5 | 12.0 | 2.6 | 1.9 | 4.6 | 3.2 | 0.5 | 4.1 | 4.4 | 3.9 | 8.7 |
| Sorghum, grain | 10.6 | - | - | - | 20% | 29% | 4.1 | 1.7 | 1.9 | 3.1 | 13.0 | 2.0 | 1.2 | 5.0 | 3.2 | 1.8 | 4.0 | 4.3 | 2.9 | 9.0 |
| Oilseed cakes or meals | ||||||||||||||||||||
| Canola | 39.0 | - | - | - | - | - | 5.9 | 2.5 | 2.6 | 4.0 | 6.8 | 5.6 | 2.0 | 3.9 | 4.2 | 1.2 | 2.9 | 4.9 | 4.5 | 6.8 |
| Rapeseed | 38.3 | - | - | - | 92% | 70% | 6.1 | 2.3 | 2.6 | 4.0 | 6.7 | 5.5 | 2.1 | 3.9 | 4.4 | 1.3 | 3.1 | 5.1 | 4.4 | 7.0 |
| Sunflower | 37.7 | - | - | - | 99% | 97% | 8.5 | 1.7 | 2.5 | 4.1 | 6.2 | 3.5 | 2.3 | 4.4 | 3.6 | 1.2 | 2.4 | 4.9 | 4 | 6.8 |
| Hemp | 33.4 | 87% | - | - | 48% | - | 12.4 | 1.8 | 3.0 | 3.9 | 6.9 | 3.9 | 2.4 | 4.7 | 3.8 | 1.1 | 3.2 | 5.1 | 4.2 | 7.9 |
| Flax | 34.2 | - | - | - | - | - | 2.81 | 2.0 | 2.7 | 3.7 | 5.8 | 3.6 | 1.0 | 5.2 | 3.7 | 0.5 | 2.4 | 4.7 | 3 | 7.6 |
| Cotton | 45.0 | 85% | - | - | - | - | 11.1 | 1.6 | 2.9 | 3.2 | 5.9 | 4.2 | 1.4 | 5.1 | 3.3 | 1.1 | 2.9 | 4.2 | 3 | 8.0 |
| Green leaf or forage crops | ||||||||||||||||||||
| Alfalfa | 5.2 | 76% | - | 57% | 72% | 4.4 | 1.8 | 3.0 | 4.8 | 6.9 | 4.8 | 1.9 | 3.9 | 1.7 | 1.3 | 3.5 | 4.1 | 3.7 | 7.2 | |
| Spinach | 2.9 | 74% | - | 51% | - | 5.6 | 1.3 | 3.0 | 3.7 | 7.0 | 5.5 | 1.2 | 4.5 | 4.0 | 1.6 | 6.1 | 5.0 | 2.4 | 10.5 | |
| Sugar beet | 2.2 | 72% | - | - | - | - | 5.3 | 0.3 | 1.7 | 6.2 | 6.8 | 5.8 | 1.5 | 6.0 | 4.2 | 0.7 | 3.3 | 5.6 | 1.8 | 9.3 |
| Cabbage | 1.0 | 82% | - | - | 57% | - | 4.0 | 2.4 | 1.4 | 3.1 | 4.1 | 2.1 | 4.2 | 2.8 | 3.4 | 1.0 | 2.1 | 4.6 | 6.6 | 4.9 |
| Lettuce | 1.1 | 91% | 77% | - | 19% | - | 6.3 | 1.3 | 1.9 | 4.5 | 5.9 | 6.4 | 1.5 | 5.3 | 4.7 | 6.1 | 1.9 | 6.9 | 2.8 | 7.3 |
| Sweet potato | 2.5 | 73% | - | 92% | 70% | - | 6.0 | 3.8 | 1.4 | 3.7 | 8.6 | 3.6 | 1.1 | 7.0 | 5.0 | 0.9 | 4.1 | 5.7 | 4.9 | 11.1 |
| Cassava | 1.8 | 68% | 57% | 40% | - | - | 5.9 | 3.6 | 2.2 | 5.2 | 10.5 | 6.2 | 1.0 | 5.7 | 5.1 | 1.0 | 3.3 | 5.3 | 4.6 | 9.0 |
| Duckweed | 3.0 | 65% | - | - | 45% | 75% | 6.6 | 1.2 | 1.6 | 3.6 | 6.6 | 4.7 | 1.4 | 4.4 | 3.5 | 1.4 | 2.8 | 4.5 | 2.6 | 7.2 |
| Grass, orch. | 4.0 | 69% | - | - | - | - | 0.9 | 0.2 | 0.3 | 0.8 | 1.4 | 0.8 | 0.3 | 0.9 | 0.7 | 0.2 | 0.5 | 1.0 | 0.3 | 1.5 |
| Others (nuts, tubers, etc.) | ||||||||||||||||||||
| Almonds a | 21.2 | - | - | - | 23% | - | 9.3 | 0.2 | 2.1 | 2.7 | 5.8 | 2.4 | 0.4 | 4.5 | 1.9 | 0.9 | 1.2 | 3.2 | 0.7 | 5.4 |
| Peanuts a | 25.8 | 95% | 54% | 47% | 52% | 43% | 10.6 | 1.1 | 2.2 | 2.9 | 6.0 | 3.4 | 1.0 | 4.7 | 0.1 | 0.8 | 3.4 | 3.6 | 2.1 | 8.1 |
| Potato | 2.1 | 82% | - | - | 82% | - | 3.3 | 0.3 | 1.4 | 3.1 | 6.7 | 4.8 | 1.3 | 4.2 | 4.1 | 0.1 | 3.8 | 3.7 | 1.6 | 8 |
| Brewer’s yeast | 48.6 | - | - | - | - | - | 4.4 | 0.9 | 2.0 | 4.6 | 6.2 | 6.3 | 1.5 | 3.6 | 4.4 | 1.1 | 2.7 | 4.9 | 2.4 | 6.3 |
| Mushroom, butt. | 3.1 | - | - | - | - | - | 4.1 | 0.1 | 1.6 | 4.6 | 7.9 | 8.1 | 1.4 | 4.7 | 5.6 | 0.1 | 2.9 | 5.9 | 1.4 | 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balfany, C.; Gutierrez, J.; Moncada, M.; Komarnytsky, S. Current Status and Nutritional Value of Green Leaf Protein. Nutrients 2023, 15, 1327. https://doi.org/10.3390/nu15061327
Balfany C, Gutierrez J, Moncada M, Komarnytsky S. Current Status and Nutritional Value of Green Leaf Protein. Nutrients. 2023; 15(6):1327. https://doi.org/10.3390/nu15061327
Chicago/Turabian StyleBalfany, Connor, Janelle Gutierrez, Marvin Moncada, and Slavko Komarnytsky. 2023. "Current Status and Nutritional Value of Green Leaf Protein" Nutrients 15, no. 6: 1327. https://doi.org/10.3390/nu15061327
APA StyleBalfany, C., Gutierrez, J., Moncada, M., & Komarnytsky, S. (2023). Current Status and Nutritional Value of Green Leaf Protein. Nutrients, 15(6), 1327. https://doi.org/10.3390/nu15061327







