Naringenin Attenuates Isoprenaline-Induced Cardiac Hypertrophy by Suppressing Oxidative Stress through the AMPK/NOX2/MAPK Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Echocardiography
2.3. Cell Isolation and Culture
2.4. Masson Trichrome Staining and Immunofluorescence Staining
2.5. Oxidative Stress Detection
2.6. Real-Time Quantitative PCR Analysis
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
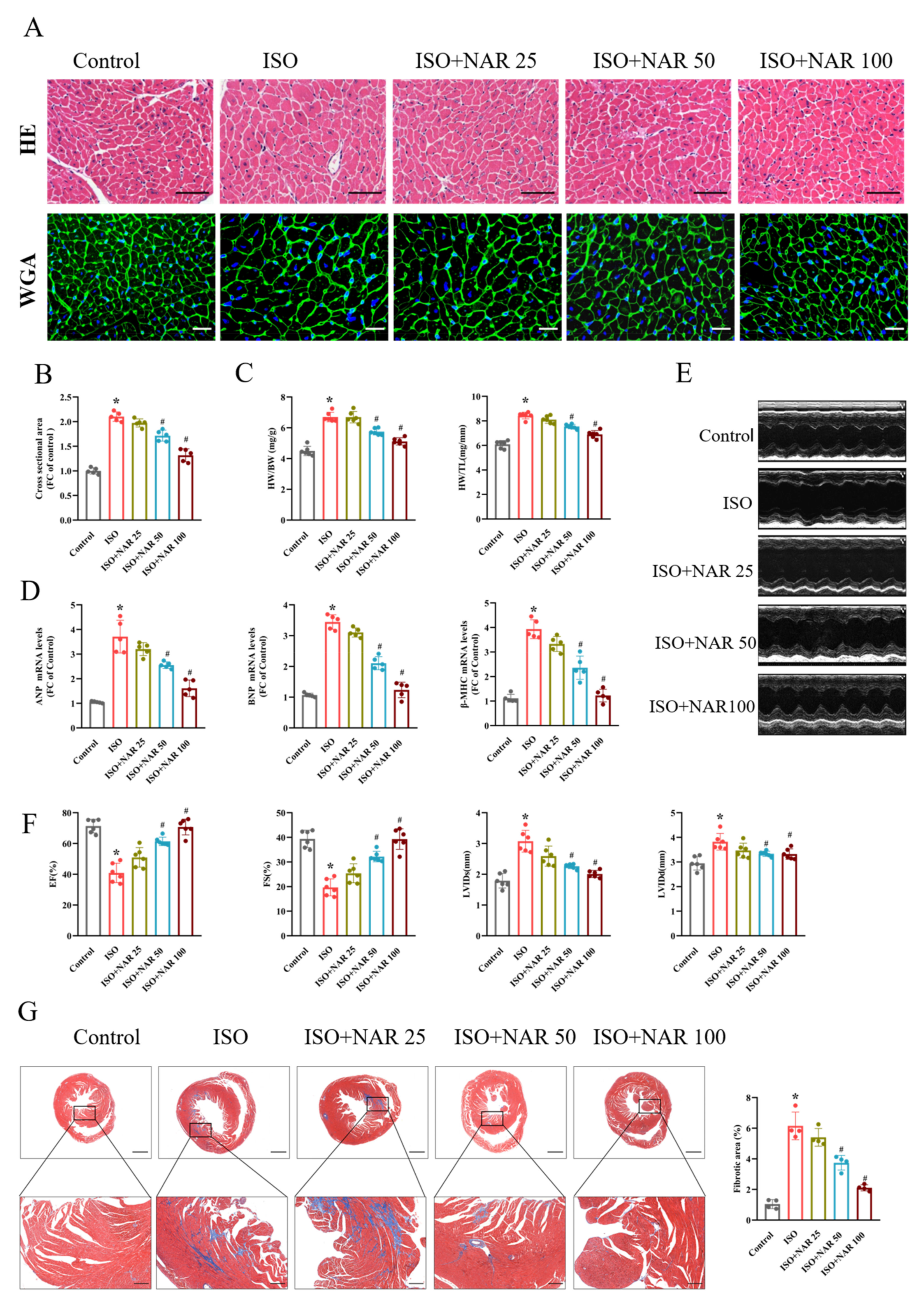
3.1. Naringenin Attenuated Isoprenaline (ISO)-Induced Cardiac Hypertrophy
3.2. Naringenin Ameliorated ISO-Induced Cardiomyocyte Hypertrophy by Inhibiting Oxidative Stress through AMPK/NOX2/MAPK Signaling Pathway
3.3. Inhibition of AMPK Blocked the Anti-Hypertrophic Effects of Naringenin on ISO-Induced Cardiac Hypertrophy In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Su, Y.; Zhao, Y.; Sheng, X.; Tong, R.; Ying, X.; Gao, L.; Ji, Q.; Gao, Y.; Yan, Y.; et al. Melatonin differentially regulates pathological and physiological cardiac hypertrophy: Crucial role of circadian nuclear receptor RORalpha signaling. J. Pineal. Res. 2019, 67, e12579. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Cong, S.; Chan, X.; Yap, E.P.; Yu, F.; Hausenloy, D.J. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radic. Biol. Med. 2021, 166, 297–312. [Google Scholar] [CrossRef]
- Faria, A.; Persaud, S.J. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol. Ther. 2017, 172, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Vashi, R.; Patel, B.M. NRF2 in Cardiovascular Diseases: A Ray of Hope! J. Cardiovasc. Transl. Res. 2021, 14, 573–586. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Chrysohoou, C.; Vogiatzi, G.; Magkas, N.; Bournelis, I.; Bampali, S.; Gruson, D.; Tousoulis, D. Vitamins in Heart Failure: Friend or Enemy? Curr. Pharm. Des. 2017, 23, 3731–3742. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.Y.; Li, H.B. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.H. Naringinases: Occurrence, characteristics, and applications. Appl. Microbiol. Biotechnol. 2011, 90, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Ciumarnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Rachisan, A.L.; Negrean, V.; Perne, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Lin, J.H.; Hammes, H.P.; Zhang, C. Flavonoids in Treatment of Chronic Kidney Disease. Molecules 2022, 27, 2365. [Google Scholar] [CrossRef]
- Hamsalakshmi; Alex, A.M.; Arehally Marappa, M.; Joghee, S.; Chidambaram, S.B. Therapeutic benefits of flavonoids against neuroinflammation: A systematic review. Inflammopharmacology 2022, 30, 111–136. [Google Scholar] [CrossRef]
- Meng, L.M.; Ma, H.J.; Guo, H.; Kong, Q.Q.; Zhang, Y. The cardioprotective effect of naringenin against ischemia-reperfusion injury through activation of ATP-sensitive potassium channel in rat. Can. J. Physiol. Pharmacol. 2016, 94, 973–978. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Li, H.; Chen, F.; Shi, J. Naringin ameliorates diabetic nephropathy by inhibiting NADPH oxidase 4. Eur. J. Pharmacol. 2017, 804, 1–6. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Z.; Yuan, Y.; Li, F.; Liu, Y.; Ma, Z.; Liao, H.; Bian, Z.; Zhang, Y.; Zhou, H.; et al. Naringenin attenuates pressure overload-induced cardiac hypertrophy. Exp. Ther. Med. 2015, 10, 2206–2212. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.E.; Nabavi, S.F.; Daglia, M.; Tenore, G.C.; Mansouri, K.; Nabavi, S.M. Naringenin and atherosclerosis: A review of literature. Curr. Pharm. Biotechnol. 2015, 16, 245–251. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, M.; Cao, Y.; Zhang, Z.; Jiang, B.; Tian, F.; Feng, J.; Dou, Y.; Gorospe, M.; Zheng, M.; et al. HuR regulates phospholamban expression in isoproterenol-induced cardiac remodelling. Cardiovasc. Res. 2020, 116, 944–955. [Google Scholar] [CrossRef]
- Li, L.; Fu, W.; Gong, X.; Chen, Z.; Tang, L.; Yang, D.; Liao, Q.; Xia, X.; Wu, H.; Liu, C.; et al. The role of G protein-coupled receptor kinase 4 in cardiomyocyte injury after myocardial infarction. Eur. Heart J. 2021, 42, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.X.; Kimura, S.; Nishiyama, A.; Shokoji, T.; Rahman, M.; Yao, L.; Nagai, Y.; Fujisawa, Y.; Miyatake, A.; Abe, Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc. Res. 2005, 65, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Saleem, N.; Goswami, S.K. Activation of adrenergic receptor in H9c2 cardiac myoblasts co-stimulates Nox2 and the derived ROS mediate the downstream responses. Mol. Cell. Biochem. 2017, 436, 167–178. [Google Scholar] [CrossRef]
- Prasad, A.; Mahmood, A.; Gupta, R.; Bisoyi, P.; Saleem, N.; Naga Prasad, S.V.; Goswami, S.K. In cardiac muscle cells, both adrenergic agonists and antagonists induce reactive oxygen species from NOX2 but mutually attenuate each other’s effects. Eur. J. Pharmacol. 2021, 908, 174350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Kee, H.J.; Bai, L.; Kim, M.K.; Kee, S.J.; Jeong, M.H. Selective HDAC8 Inhibition Attenuates Isoproterenol-Induced Cardiac Hypertrophy and Fibrosis via p38 MAPK Pathway. Front. Pharmacol. 2021, 12, 677757. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell. Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell. Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Ou-Yang, Q.; Wang, L.; Li, T.; Xie, X.; Liu, J. AdipoRon prevents l-thyroxine or isoproterenol-induced cardiac hypertrophy through regulating the AMPK-related pathway. Acta Biochim. Biophys. Sin. 2019, 51, 20–30. [Google Scholar] [CrossRef]
- Nyane, N.A.; Tlaila, T.B.; Malefane, T.G.; Ndwandwe, D.E.; Owira, P.M.O. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. Eur. J. Pharmacol. 2017, 803, 103–111. [Google Scholar] [CrossRef]
- Matsushima, S.; Kuroda, J.; Ago, T.; Zhai, P.; Park, J.Y.; Xie, L.H.; Tian, B.; Sadoshima, J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res. 2013, 112, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Chen, X.F.; Wang, N.Y.; Wang, X.M.; Liang, S.T.; Zheng, W.; Lu, Y.B.; Zhao, X.; Hao, D.L.; Zhang, Z.Q.; et al. SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation 2017, 136, 2051–2067. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T.; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 2326–2333. [Google Scholar]
- Al-Khudairy, L.; Flowers, N.; Wheelhouse, R.; Ghannam, O.; Hartley, L.; Stranges, S.; Rees, K. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2017, 3, CD011114. [Google Scholar] [CrossRef] [Green Version]
- Mason, S.A.; Keske, M.A.; Wadley, G.D. Effects of Vitamin C Supplementation on Glycemic Control and Cardiovascular Risk Factors in People with Type 2 Diabetes: A GRADE-Assessed Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2021, 44, 618–630. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muniz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Xiao, H. AMPK and cardiac remodelling. Sci. China Life Sci. 2018, 61, 14–23. [Google Scholar] [CrossRef]
- Gelinas, R.; Mailleux, F.; Dontaine, J.; Bultot, L.; Demeulder, B.; Ginion, A.; Daskalopoulos, E.P.; Esfahani, H.; Dubois-Deruy, E.; Lauzier, B.; et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat. Commun. 2018, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Zou, M.H. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic. Biol. Med. 2012, 52, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.M.; Dong, X.; Xue, X.D.; Zhang, J.; Li, Z.; Wu, H.J.; Yang, Z.L.; Yang, Y.; Wang, H.S. Naringenin improves mitochondrial function and reduces cardiac damage following ischemia-reperfusion injury: The role of the AMPK-SIRT3 signaling pathway. Food Funct. 2019, 10, 2752–2765a. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Cao, Z.; Li, W.; Liu, R.; Chen, Y.; Li, C.; Song, Y.; Liu, G.; Hu, J.; et al. Naringenin ameliorates homocysteine induced endothelial damage via the AMPKalpha/Sirt1 pathway. J. Adv. Res. 2021, 34, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, M.; Zhang, S.; Fan, Y.; Tan, Y.; Guo, Z.; Chen, L.; Bai, L.; Jiang, D.; Hao, X.; Li, X.; et al. The Expression of microRNA in Adult Rat Heart with Isoproterenol-Induced Cardiac Hypertrophy. Cells 2020, 9, 1173. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, J.; Long, Z.; Wang, C.; Wang, L.; Sun, P.; Li, P.; Wang, T. Hydrogen (H(2)) Inhibits Isoproterenol-Induced Cardiac Hypertrophy via Antioxidative Pathways. Front. Pharmacol. 2016, 7, 392. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.H.; Ma, C.H.; Yue, Z.Q.; Yao, X.; Mao, C.M. Protective effect of naringenin against lipopolysaccharide-induced injury in normal human bronchial epithelium via suppression of MAPK signaling. Inflammation 2015, 38, 195–204. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Lau, F.H.; Lin, Y.; Stephens, J.M.; Johnson, W.D.; Coulter, A.A. Naringenin Promotes Thermogenic Gene Expression in Human White Adipose Tissue. Obesity 2019, 27, 103–111. [Google Scholar] [CrossRef]
- Naeini, F.; Namkhah, Z.; Tutunchi, H.; Rezayat, S.M.; Mansouri, S.; Yaseri, M.; Hosseinzadeh-Attar, M.J. Effects of naringenin supplementation on cardiovascular risk factors in overweight/obese patients with nonalcoholic fatty liver disease: A pilot double-blind, placebo-controlled, randomized clinical trial. Eur. J. Gastroenterol. Hepatol. 2022, 34, 345–353. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; He, B.; Zhang, C.; He, Y.; Xia, T.; Zeng, C. Naringenin Attenuates Isoprenaline-Induced Cardiac Hypertrophy by Suppressing Oxidative Stress through the AMPK/NOX2/MAPK Signaling Pathway. Nutrients 2023, 15, 1340. https://doi.org/10.3390/nu15061340
Li Y, He B, Zhang C, He Y, Xia T, Zeng C. Naringenin Attenuates Isoprenaline-Induced Cardiac Hypertrophy by Suppressing Oxidative Stress through the AMPK/NOX2/MAPK Signaling Pathway. Nutrients. 2023; 15(6):1340. https://doi.org/10.3390/nu15061340
Chicago/Turabian StyleLi, Yu, Bo He, Chao Zhang, Yanji He, Tianyang Xia, and Chunyu Zeng. 2023. "Naringenin Attenuates Isoprenaline-Induced Cardiac Hypertrophy by Suppressing Oxidative Stress through the AMPK/NOX2/MAPK Signaling Pathway" Nutrients 15, no. 6: 1340. https://doi.org/10.3390/nu15061340





