Cardiometabolic Care: Assessing Patients with Diabetes Mellitus with No Overt Cardiovascular Disease in the Light of Heart Failure Development Risk
Abstract
:1. Introduction
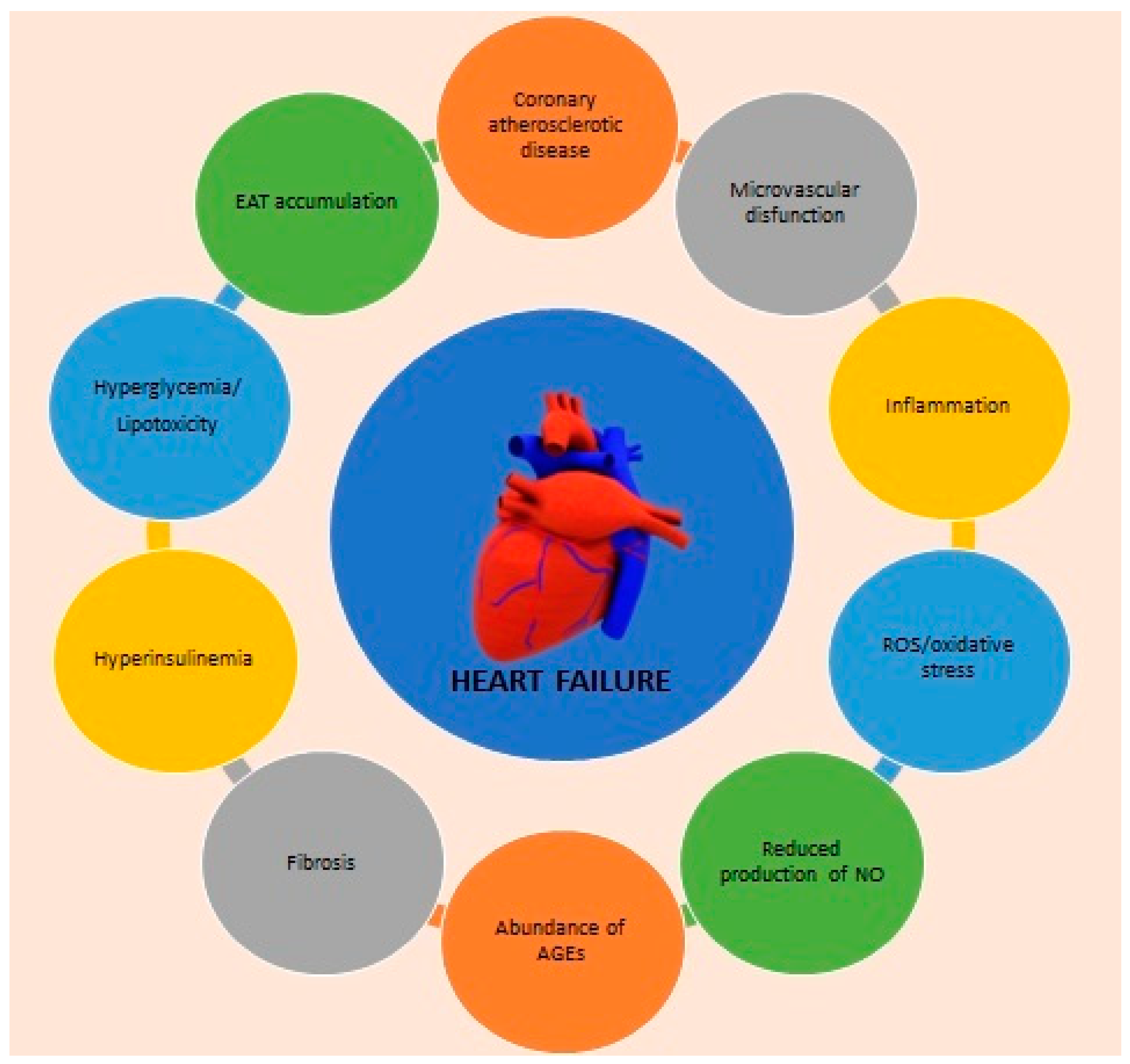
2. Major Interconnections between DM and HF
2.1. Structural Cardiac Alterations
2.2. Energetic Impairment
2.3. Other Contributors of HF Development in DM
3. Diabetic Cardiomyopathy as a Standalone Disease
4. Arrhythmogenic Considerations in DM
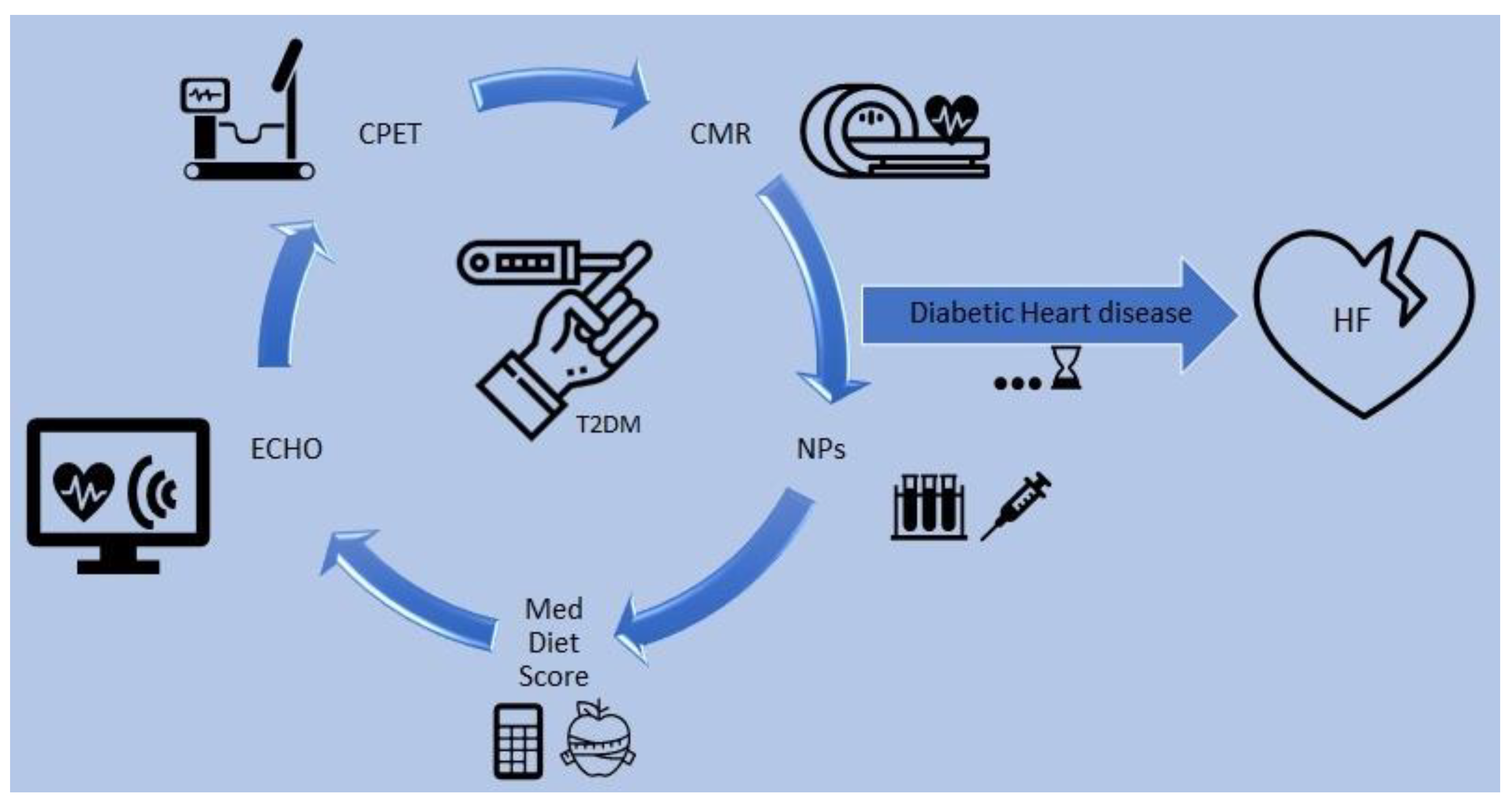
5. How to Follow up the Progression of Diabetic Cardiomyopathy
6. The Role of N-Terminal-Pro Hormone BNP (NT-proBNP)
7. Lifestyle Interventions
7.1. Weight Management
7.2. Physical Activity
7.3. Diet
7.4. Smoking
8. The Role of Medical Therapy—What Is an Effective Treatment?
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| Adenosine triphosphate | ATP |
| Advanced glycation end products | AGEs |
| Atrial natriuretic peptide | ANP |
| Brain natriuretic peptide | BNP |
| Cardiac magnetic resonance | CMR |
| Cardiovascular disease | CVD |
| Cardiovascular health | CV |
| Coronary artery disease | CAD |
| Coronary heart disease | CHD |
| Cyclic guanosine monophosphate | cGMP |
| Diabetes mellitus | DM |
| Dilated cardiomyopathy | DCM |
| Ejection fraction | EF |
| Epicardial adipose tissue | EAT |
| General practitioner | GP |
| HbA1c: hemoglobin | A1c |
| Heart failure | HF |
| Heart failure with mid-range ejection fraction | HFmEF |
| Heart failure with preserved ejection fraction | HFpEF |
| Interleukin | IL |
| Left ventricular | LV |
| Left ventricular ejection fraction | LVEF |
| Magnetic resonance imaging | MRI |
| Monocyte chemoattractant protein-1 | MCP-1 |
| Natriuretic peptides | NP |
| Nitric oxide | NO |
| N-terminal-pro hormone BNP | NT-proBNP |
| Plasminogen activator inhibitor-1 | PAI-1 |
| Protein kinase G | PKG |
| Quality of life | QoL |
| Reactive oxygen species | ROS |
| Sodium/glucose cotransporter 2 inhibitors | SGLT2i |
| Tumor necrosis factor alpha | TNF-α |
| Type 2 diabetes mellitus | T2DM |
| Ventricular Tachycardia | VT |
References
- Cosentino, F.; Grant, P.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V. The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients. Eur. Heart J. 2006, 27, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ai, C.; Bai, M.; Niu, J.; Zhang, Z. NLRP3 Inflammasome/Pyroptosis: A Key Driving Force in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 10632. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Liu, H.; Shi, M.; Wang, J.; Wang, Y. The Molecular Mechanisms of Defective Copper Metabolism in Diabetic Cardiomyopathy. Oxidative Med. Cell. Longev. 2022, 2022, 5418376. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Hear. J. 2021, 42, 4901. [Google Scholar]
- Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Li, T.Y.; Fung, T.T.; Li, S.; Willett, W.C.; Rimm, E.B.; Hu, F.B. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am. J. Clin. Nutr. 2014, 99, 172–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brener, M.I.; Borlaug, B.A.; Burkhoff, D. HF?EF: The Mysterious Relationship Between Heart Failure and Ejection Fraction Continues. Circulation 2022, 146, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Elsanhoury, A.; Nelki, V.; Kelle, S.; Van Linthout, S.; Tschöpe, C. Epicardial Fat Expansion in Diabetic and Obese Patients with Heart Failure and Preserved Ejection Fraction—A Specific HFpEF Phenotype. Front. Cardiovasc. Med. 2021, 8, 720690. [Google Scholar] [CrossRef]
- Wilson Tang, W.H.; Maroo, A.; Young, J.B. Ischemic heart disease and congestive heart failure in diabetic patients. Med. Clin. N. Am. 2004, 88, 1037–1061. [Google Scholar] [CrossRef]
- Crisafulli, A.; Pagliaro, P.; Roberto, S.; Cugusi, L.; Mercuro, G.; Lazou, A.; Beauloye, C.; Bertrand, L.; Hausenloy, D.J.; Aragno, M.; et al. Diabetic Cardiomyopathy and Ischemic Heart Disease: Prevention and Therapy by Exercise and Conditioning. Int. J. Mol. Sci. 2020, 21, 2896. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Scappaticcio, L.; Cirillo, P.; Maio, A.; Carotenuto, R.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Glycemic Control and the Heart: The Tale of Diabetic Cardiomyopathy Continues. Biomolecules 2022, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Chess, D.J.; Stanley, W.C. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc. Res. 2008, 79, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the spec. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Dhingra, R.; Vasan, R.S. Diabetes and the Risk of Heart Failure. Heart Fail. Clin. 2012, 8, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Paulus, W.J.; Tschöpe, C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Tschöpe, C.; Van Linthout, S. New Insights in (Inter)Cellular Mechanisms by Heart Failure with Preserved Ejection Fraction. Curr. Heart Fail. Rep. 2014, 11, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Iacobellis, G.; Bianco, A.C. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Verbrugge, F.H.; Omote, K.; Reddy, Y.N.V.; Sorimachi, H.; Obokata, M.; Borlaug, B.A. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur. Heart J. 2022, 43, 1941–1951. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Giamouzis, G.; Parissis, J.; Starling, R.C.; Boudoulas, H.; Skoularigis, J.; Butler, J.; Filippatos, G. Reframing the association and significance of co-morbidities in heart failure: Co-morbidities in heart failure. Eur. J. Heart Fail. 2016, 18, 744–758. [Google Scholar] [CrossRef]
- Lam, C.S. Diabetic cardiomyopathy: An expression of stage B heart failure with preserved ejection fraction. Diabetes Vasc. Dis. Res. 2015, 12, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Camelliti, P.; Borg, T.; Kohl, P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005, 65, 40–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutalas, E.; Kanoupakis, E.; Vardas, P. Sudden cardiac death in non-ischemic dilated cardiomyopathy: A critical appraisal of existing and potential risk stratification tools. Int. J. Cardiol. 2013, 167, 335–341. [Google Scholar] [CrossRef]
- Richardson, P.J. Assessment of myocardial damage in dilated cardiomyopathy. Eur. Heart J. 1996, 17, 489–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121. Available online: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.118.311371 (accessed on 8 November 2022). [CrossRef]
- Nesbitt, G.C.; Mankad, S. Strain and Strain Rate Imaging in Cardiomyopathy. Echocardiography 2009, 26, 337–344. [Google Scholar] [CrossRef]
- Pirat, B.; Khoury, D.S.; Hartley, C.J.; Tiller, L.; Rao, L.; Schulz, D.G.; Nagueh, S.F.; Zoghbi, W.A. A Novel Feature-Tracking Echocardiographic Method for the Quantitation of Regional Myocardial Function: Validation in an Animal Model of Ischemia-Reperfusion. J. Am. Coll. Cardiol. 2008, 51, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11 2 Pt 1, 260. Available online: https://www.sciencedirect.com/science/article/pii/S1936878X17310860 (accessed on 8 November 2022). [CrossRef] [PubMed]
- Faragli, A.; Alogna, A.; Bin Lee, C.; Zhu, M.; Ghorbani, N.; Muzio, F.P.L.; Schnackenburg, B.; Stehning, C.; Kuehne, T.; Post, H.; et al. Non-invasive CMR-Based Quantification of Myocardial Power and Efficiency under Stress and Ischemic Conditions in Landrace Pigs. Front. Cardiovasc. Med. 2021, 8, 689255. [Google Scholar] [CrossRef]
- Kosiuk, J.; Dinov, B.; Bollmann, A.; Koutalas, E.; Müssigbrodt, A.; Sommer, P.; Arya, A.; Richter, S.; Hindricks, G.; Breithardt, O.-A. Association between ventricular arrhythmias and myocardial mechanical dispersion assessed by strain analysis in patients with nonischemic cardiomyopathy. Clin. Res. Cardiol. 2015, 104, 1072–1077. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Goebel, B.; Dahlslett, T.; Meyer, K.; Jung, C.; Lauten, A.; Figulla, H.R.; Poerner, T.C.; Edvardsen, T. Risk Assessment of Ventricular Arrhythmias in Patients with Nonischemic Dilated Cardiomyopathy by Strain Echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Sharma, K.; Yanek, L.R.; Vaidya, D.; Schär, M.; Markl, M.; Subramanya, V.; Soleimani, S.; Ouyang, P.; Michos, E.D.; et al. Visceral adiposity, muscle composition, and exercise tolerance in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Huelsmann, M.; Neuhold, S.; Strunk, G.; Moertl, D.; Berger, R.; Prager, R.; Abrahamian, H.; Riedl, M.; Pacher, R.; Luger, A.; et al. NT-proBNP has a high negative predictive value to rule-out short-term cardiovascular events in patients with diabetes mellitus. Eur. Heart J. 2008, 29, 2259–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhold, S.; Resl, M.; Huelsmann, M.; Strunk, G.; Adlbrecht, C.; Rath, C.; Prager, R.; Luger, A.; Clodi, M.; Pacher, R. Repeat measurements of glycated haemoglobin A1c and N-terminal pro-B-type natriuretic peptide: Divergent behaviour in diabetes mellitus: Repeat measurements of HbA1c and NT-proBNP. Eur. J. Clin. Investig. 2011, 41, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, J.; Chuck, L.; González-Ortiz, M.; Merton, K.; Craig, J.; Capuano, G.; Qiu, R. Initial Combination Therapy with Canagliflozin Plus Metformin Versus Each Component as Monotherapy for Drug-Naïve Type 2 Diabetes. Diabetes Care 2016, 39, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–1399. [Google Scholar] [CrossRef]
- Tarnow, L.; Gall, M.A.; Hansen, B.V.; Hovind, P.; Parving, H.H. Plasma N-terminal pro-B-type natriuretic peptide and mortality in type 2 diabetes. Diabetologia 2006, 49, 2256–2262. [Google Scholar] [CrossRef] [Green Version]
- Huelsmann, M.; Neuhold, S.; Resl, M.; Strunk, G.; Brath, H.; Francesconi, C.; Adlbrecht, C.; Prager, R.; Luger, A.; Pacher, R.; et al. PONTIAC (NT-proBNP Selected PreventiOn of cardiac eveNts in a populaTion of dIabetic patients without A history of Cardiac disease). J. Am. Coll. Cardiol. 2013, 62, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- Welsh, P.; Woodward, M.; Hillis, G.S.; Li, Q.; Marre, M.; Williams, B.; Poulter, N.; Ryan, L.; Harrap, S.; Patel, A.; et al. Do Cardiac Biomarkers NT-proBNP and hsTnT Predict Microvascular Events in Patients with Type 2 Diabetes? Results from the ADVANCE Trial. Diabetes Care 2014, 37, 2202–2210. [Google Scholar] [CrossRef] [Green Version]
- Jarolim, P.; White, W.B.; Cannon, C.P.; Gao, Q.; Morrow, D.A. Serial Measurement of Natriuretic Peptides and Cardiovascular Outcomes in Patients with Type 2 Diabetes in the EXAMINE Trial. Diabetes Care 2018, 41, 1510–1515. [Google Scholar] [CrossRef] [Green Version]
- Fralick, M.; Schneeweiss, S.; Redelmeier, D.A.; Razak, F.; Gomes, T.; Patorno, E. Comparative effectiveness and safety of sodium-glucose cotransporter-2 inhibitors versus metformin in patients with type 2 diabetes: An observational study using data from routine care. Diabetes Obes. Metab. 2021, 23, 2320–2328. [Google Scholar] [CrossRef]
- Ledwidge, M.; Gallagher, J.; Conlon, C.; Tallon, E.; O’Connell, E.; Dawkins, I.; Watson, C.; O’Hanlon, R.; Bermingham, M.; Patle, A.; et al. Natriuretic Peptide–Based Screening and Collaborative Care for Heart Failure: The STOP-HF Randomized Trial. JAMA 2013, 310, 66. [Google Scholar] [CrossRef]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic Durability of Rosiglitazone, Metformin, or Glyburide Monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef] [Green Version]
- McKie, P.M.; Cataliotti, A.; Lahr, B.D.; Martin, F.L.; Redfield, M.M.; Bailey, K.R.; Rodeheffer, R.J.; Burnett, J.C. The Prognostic Value of N-Terminal Pro–B-Type Natriuretic Peptide for Death and Cardiovascular Events in Healthy Normal and Stage A/B Heart Failure Subjects. J. Am. Coll. Cardiol. 2010, 55, 2140–2147. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Song, Y.; Wang, Q.; Kralik, P.M.; Epstein, P.N. Causes and Characteristics of Diabetic Cardiomyopathy. Rev. Diabet. Stud. 2006, 3, 108. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Jia, Y.; Yu, J.; Liu, Y.; Li, F.; Liu, Y.; Wu, Q.; Liao, X.; Zeng, Z.; Wan, Z.; et al. Adherence to a Healthy Lifestyle and the Risk of All-Cause Mortality and Cardiovascular Events in Individuals with Diabetes: The ARIC Study. Front. Nutr. 2021, 8, 698608. [Google Scholar] [CrossRef]
- Galaviz, K.I.; Weber, M.B.; Straus, A.; Haw, J.S.; Narayan, K.M.V.; Ali, M.K. Global Diabetes Prevention Interventions: A Systematic Review and Network Meta-analysis of the Real-World Impact on Incidence, Weight, and Glucose. Diabetes Care 2018, 41, 1526–1534. [Google Scholar] [CrossRef] [Green Version]
- Sikand, G.; Severson, T. Top 10 dietary strategies for atherosclerotic cardiovascular risk reduction. Am. J. Prev. Cardiol. 2020, 4, 100106. [Google Scholar] [CrossRef]
- Anand, V.; Garg, S.; Garg, J.; Bano, S.; Pritzker, M. Impact of Exercise Training on Cardiac Function among Patients with Type 2 Diabetes: A Systematic Review and meta-analysis. J. Cardiopulm. Rehabil. Prev. 2018, 38, 358–365. [Google Scholar] [CrossRef]
- Gusso, S.; Pinto, T.; Baldi, J.C.; Derraik, J.G.; Cutfield, W.S.; Hornung, T.; Hofman, P.L. Exercise Training Improves but Does Not Normalize Left Ventricular Systolic and Diastolic Function in Adolescents with Type 1 Diabetes. Diabetes Care 2017, 40, 1264–1272. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Khan, F.; Fonarow, G.C.; Sreenivasan, J.; Greene, S.J.; Khan, S.U.; Usman, M.S.; Vaduganathan, M.; Fudim, M.; Anker, S.D.; et al. Dietary interventions and nutritional supplements for heart failure: A systematic appraisal and evidence map. Eur. J. Hear. Fail. 2021, 23, 1468–1476. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut Microbiota 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Ahonen, L.; Jäntti, S.; Suvitaival, T.; Theilade, S.; Risz, C.; Kostiainen, R.; Rossing, P.; Orešič, M.; Hyötyläinen, T. Targeted Clinical Metabolite Profiling Platform for the Stratification of Diabetic Patients. Metabolites 2019, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; Kuo, H.-C.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R.; Kong, A.-N. Gut Microbiota, Dietary Phytochemicals, and Benefits to Human Health. Curr. Pharmacol. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef]
- Kouvari, M.; Panagiotakos, D.B.; Chrysohoou, C.; Georgousopoulou, E.; Notara, V.; Tousoulis, D.; Pitsavos, C.; ATTICA & GREECS Studies Investigators. Gender-specific, Lifestyle-related Factors and 10-year Cardiovascular Disease Risk; the ATTICA and GREECS Cohort Studies. Curr. Vasc. Pharmacol. 2019, 17, 401–410. [Google Scholar] [CrossRef]
- Kleissl-Muir, S.; Rasmussen, B.; Owen, A.; Zinn, C.; Driscoll, A. Low Carbohydrate Diets for Diabetic Cardiomyopathy: A Hypothesis. Front. Nutr. 2022, 9, 865489. [Google Scholar] [CrossRef]
- Evangelista, L.S.; Jose, M.M.; Sallam, H.; Serag, H.; Golovko, G.; Khanipov, K.; Hamilton, M.A.; Fonarow, G.C. High-protein vs. standard-protein diets in overweight and obese patients with heart failure and diabetes mellitus: Findings of the Pro-HEART trial. ESC Heart Fail. 2021, 8, 1342–1348. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; Da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitsavos, C.; Panagiotakos, D.B.; Tzima, N.; Chrysohoou, C.; Economou, M.; Zampelas, A.; Stefanadis, C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2005, 82, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Turati, F.; Lagiou, P.; Trichopoulos, D.; Augustin, L.S.; La Vecchia, C.; Trichopoulou, A. Mediterranean diet and glycaemic load in relation to incidence of type 2 diabetes: Results from the Greek cohort of the population-based European Prospective Investigation into Cancer and Nutrition (EPIC). Diabetologia 2013, 56, 2405–2413. [Google Scholar] [CrossRef] [Green Version]
- Georgoulis, M.; Kontogianni, M.; Yiannakouris, N. Mediterranean Diet and Diabetes: Prevention and Treatment. Nutrients 2014, 6, 1406–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.A.; Guillén-Grima, F.; De Irala, J.; Ruíz-Canela, M.; Bes-Rastrollo, M.; Beunza, J.J.; López del Burgo, C.; Toledo, E.; Carlos, S.; Sánchez-Villegas, A. The Mediterranean Diet Is Associated with a Reduction in Premature Mortality among Middle-Aged Adults. J. Nutr. 2012, 142, 1672–1678. [Google Scholar] [CrossRef] [Green Version]
- Mtintsilana, A.; Micklesfield, L.K.; Chorell, E.; Olsson, T.; Shivappa, N.; Hebert, J.R.; Kengne, A.P.; Goedecke, J.H. Adiposity Mediates the Association between the Dietary Inflammatory Index and Markers of Type 2 Diabetes Risk in Middle-Aged Black South African Women. Nutrients 2019, 11, 1246. [Google Scholar] [CrossRef] [Green Version]
- Abdurahman, A.A.; Azadbakhat, L.; Rasouli, M.; Chamari, M.; Qorbani, M.; Dorosty, A.R. Association of dietary inflammatory index with metabolic profile in metabolically healthy and unhealthy obese people. Nutr. Diet. 2019, 76, 192–198. [Google Scholar] [CrossRef]
- Park, Y.-M.M.; Choi, M.K.; Lee, S.-S.; Shivappa, N.; Han, K.; Steck, S.E.; Hébert, J.R.; Merchant, A.T.; Sandler, D.P. Dietary inflammatory potential and risk of mortality in metabolically healthy and unhealthy phenotypes among overweight and obese adults. Clin. Nutr. 2018, 38, 682–688. [Google Scholar] [CrossRef]
- Kouvari, M.; Damigou, E.; Florentin, M.; Kosti, R.I.; Chrysohoou, C.; Pitsavos, C.S.; Panagiotakos, D.B. Egg Consumption, Cardiovascular Disease and Cardiometabolic Risk Factors: The Interaction with Saturated Fatty Acids. Results from the ATTICA Cohort Study (2002–2012). Nutrients 2022, 14, 5291. [Google Scholar] [CrossRef]
- Kouvari, M.; Tsiampalis, T.; Kosti, R.I.; Naumovski, N.; Chrysohoou, C.; Skoumas, J.; Pitsavos, C.S.; Panagiotakos, D.B.; Mantzoros, C.S. Quality of plant-based diets is associated with liver steatosis, which predicts type 2 diabetes incidence ten years later: Results from the ATTICA prospective epidemiological study. Clin. Nutr. 2022, 41, 2094–2102. [Google Scholar] [CrossRef]
- Kosti, R.I.; Tsiampalis, T.; Kouvari, M.; Chrysohoou, C.; Georgousopoulou, E.; Pitsavos, C.S.; Panagiotakos, D.B. The association of specific types of vegetables consumption with 10-year type II diabetes risk: Findings from the ATTICA cohort study. J. Hum. Nutr. Diet. 2023, 36, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B. Relation of Smoking with Total Mortality and Cardiovascular Events among Patients with Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation 2015, 132, 1795–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadka, S.; Awasthi, M.; Lamichhane, R.R.; Ojha, C.; Mamudu, H.M.; Lavie, C.J.; Daggubati, R.; Paul, T.K. The Cardiovascular Effects of Electronic Cigarettes. Curr. Cardiol. Rep. 2021, 23, 40. [Google Scholar] [CrossRef]
- Castagno, D.; Baird-Gunning, J.; Jhund, P.S.; Biondi-Zoccai, G.; MacDonald, M.R.; Petrie, M.C.; Gaita, F.; McMurray, J.J. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: Evidence from a 37,229 patient meta-analysis. Am. Heart J. 2011, 162, 938–948.e. Available online: https://www.sciencedirect.com/science/article/pii/S000287031100634X (accessed on 8 November 2022). [CrossRef] [PubMed]
- Tzoulaki, I.; Molokhia, M.; Curcin, V.; Little, M.P.; Millett, C.J.; Ng, A.; Hughes, R.I.; Khunti, K.; Wilkins, M.R.; Majeed, A.; et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: Retrospective cohort study using UK general practice research database. BMJ 2009, 339, b4731. Available online: https://www.bmj.com/content/339/bmj.b4731 (accessed on 8 November 2022). [CrossRef] [PubMed] [Green Version]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1812389 (accessed on 8 November 2022). [CrossRef]
- Szekeres, Z.; Toth, K.; Szabados, E. The Effects of SGLT2 Inhibitors on Lipid Metabolism. Metabolites 2021, 11, 87. [Google Scholar] [CrossRef]
- Theofilis, P.; Oikonomou, E.; Tsioufis, K.; Tousoulis, D. Diabetes Mellitus and Heart Failure: Epidemiology, Pathophysiologic Mechanisms, and the Role of SGLT2 Inhibitors. Life 2023, 13, 497. [Google Scholar] [CrossRef]
- Paridari, P.; Jabermoradi, S.; Gholamzadeh, R.; Vazifekhah, S.; Vazirizadeh-Mahabadi, M.; Roshdi Dizaji, S.; Forouzannia, S.A.; Hosseini, M.; Yousefifard, M. Can metformin use reduce the risk of stroke in diabetic patients? A systematic review and meta-analysis. Diabetes Metab. Syndr. 2023, 17, 102721. [Google Scholar] [CrossRef]
- Melichova, J.; Sivco, P.; Rusnak, M.; Phuong Truc, P.; Majdan, M. International evidence-based guidelines on hypertension and type 2 diabetes mellitus: A systematic review. J. Public Health Res. 2023, 12, 22799036221146913. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Bell, A.; Girard, L.; McFarlane, P.; Moist, L.; Nessim, S.J.; Soroka, S.; Stafford, S.; Steele, A.; Tangri, N.; et al. Management of Type 2 Diabetic Kidney Disease in 2022: A Narrative Review for Specialists and Primary Care. Can. J. Kidney Health Dis. 2023, 10, 20543581221150556. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysohoou, C.; Fragoulis, C.; Leontsinis, I.; Gastouniotis, I.; Fragouli, D.; Georgopoulos, M.; Mantzouranis, E.; Noutsou, M.; Tsioufis, K.P. Cardiometabolic Care: Assessing Patients with Diabetes Mellitus with No Overt Cardiovascular Disease in the Light of Heart Failure Development Risk. Nutrients 2023, 15, 1384. https://doi.org/10.3390/nu15061384
Chrysohoou C, Fragoulis C, Leontsinis I, Gastouniotis I, Fragouli D, Georgopoulos M, Mantzouranis E, Noutsou M, Tsioufis KP. Cardiometabolic Care: Assessing Patients with Diabetes Mellitus with No Overt Cardiovascular Disease in the Light of Heart Failure Development Risk. Nutrients. 2023; 15(6):1384. https://doi.org/10.3390/nu15061384
Chicago/Turabian StyleChrysohoou, Christina, Christos Fragoulis, Ioannis Leontsinis, Ioannis Gastouniotis, Dimitra Fragouli, Maximos Georgopoulos, Emmanouil Mantzouranis, Marina Noutsou, and Konstantinos P. Tsioufis. 2023. "Cardiometabolic Care: Assessing Patients with Diabetes Mellitus with No Overt Cardiovascular Disease in the Light of Heart Failure Development Risk" Nutrients 15, no. 6: 1384. https://doi.org/10.3390/nu15061384
APA StyleChrysohoou, C., Fragoulis, C., Leontsinis, I., Gastouniotis, I., Fragouli, D., Georgopoulos, M., Mantzouranis, E., Noutsou, M., & Tsioufis, K. P. (2023). Cardiometabolic Care: Assessing Patients with Diabetes Mellitus with No Overt Cardiovascular Disease in the Light of Heart Failure Development Risk. Nutrients, 15(6), 1384. https://doi.org/10.3390/nu15061384








