Soy Extract, Rich in Hydroxylated Isoflavones, Exhibits Antidiabetic Properties In Vitro and in Drosophila melanogaster In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Pre-Fermented and Hydroxy-Isoflavone (HI)-Enriched Post-Fermented Soybean Extract and Isoflavone Analysis Using HPLC
2.2. Antioxidant Capacity Assays
2.3. Enzymatic Assays
2.3.1. In Vitro α-Glucosidase Inhibition Assay
2.3.2. In Vitro α-Amylase Inhibition (Disc) Assay
2.3.3. In Vitro Dipeptidyl Peptidase-4 (DPP4) Inhibition Assay
2.4. Testing for Mycoplasma Contamination
2.5. Sodium-Dependent Glucose Transporter 1 (SGLT1) Assay Using Ussing Chambers in Caco-2/PD7 Cells
2.6. Induction of CRP in Hep 3B Cells and Measurement of CRP mRNA and Secreted Protein Level
2.7. Drosophila Melanogaster Feeding Assay Using a High-Starch Diet
2.8. Statistics
3. Results
3.1. Post-Fermented Hydroxy-Isoflavone (HI)-Rich Soybean Extract Exhibited Significant Inhibitory Activity towards α-Glucosidase and DPP4 In Vitro, but Not towards α-Amylase
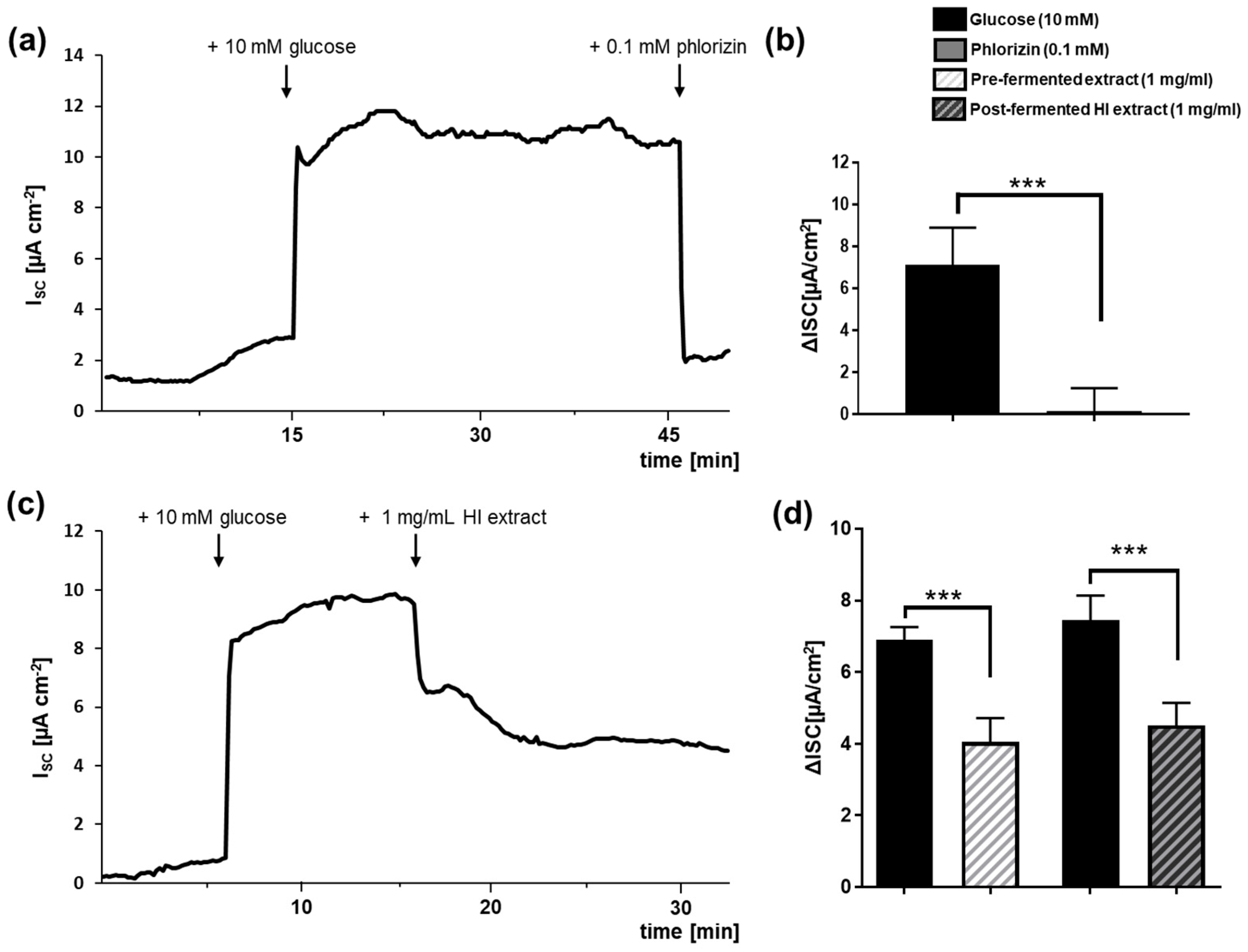
3.2. Pre- and Post-Fermented Soy Isoflavone Extracts Were Moderate Inhibitors of SGLT1-Mediated Glucose Transport
3.3. Expression of C-Reactive Protein (CRP)-Coding mRNA and CRP Protein Secretion Were Reduced in Hep 3B Cells by Pre- and HI-Enriched Post-Fermented Soy Extract
3.4. Post-Fermented HI-Rich Soy Extract Exhibited Higher Antioxidative Capacity Than Pre-Fermented Soy Extract
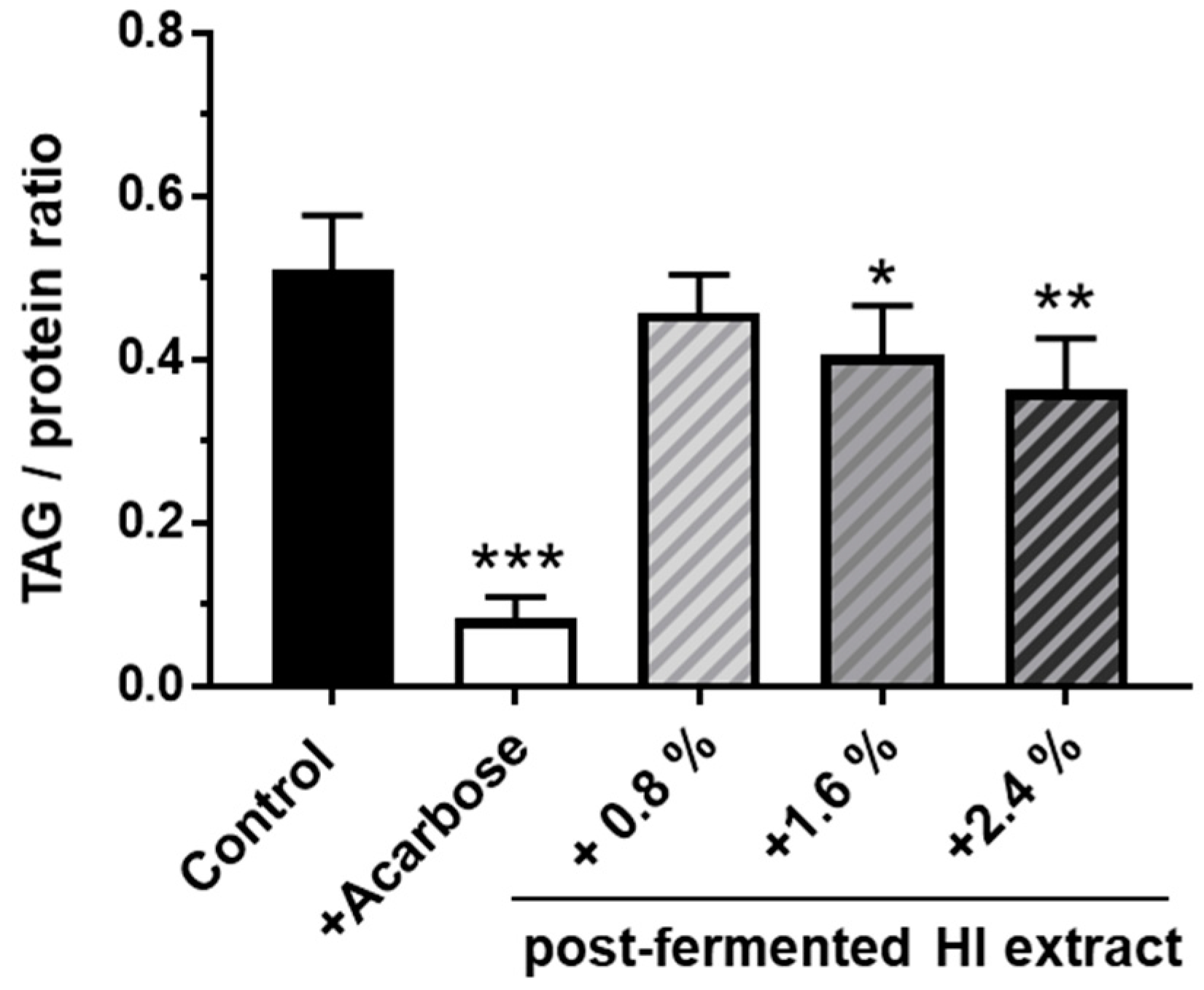
3.5. Supplementation of a High-Starch Drosophila Melanogaster Diet with Post-Fermented HI-Rich Extract Decreased the Triacylglyceride (TAG) Content of Female Fruit Flies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Francini, F.; Schinella, G.R.; Ríos, J.L. Activation of AMPK by Medicinal Plants and Natural Products: Its Role in Type 2 Diabetes Mellitus. Mini Rev. Med. Chem. 2019, 19, 880–901. [Google Scholar] [CrossRef]
- Chai, S.; Zhang, R.; Zhang, Y.; Carr, R.D.; Zheng, Y.; Rajpathak, S.; Ji, L. Effect of dipeptidyl peptidase-4 inhibitors on postprandial glucagon level in patients with type 2 diabetes mellitus: A systemic review and meta-analysis. Front. Endocrinol. 2022, 13, 994944. [Google Scholar] [CrossRef]
- Tyagi, N.K.; Kumar, A.; Goyal, P.; Pandey, D.; Siess, W.; Kinne, R.K. D-Glucose-recognition and phlorizin-binding sites in human sodium/D-glucose cotransporter 1 (hSGLT1): A tryptophan scanning study. Biochemistry 2007, 46, 13616–13628. [Google Scholar] [CrossRef]
- Stearns, A.T.; Balakrishnan, A.; Rhoads, D.B.; Tavakkolizadeh, A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann. Surg. 2010, 251, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Dyer, J.; Wood, I.S.; Palejwala, A.; Ellis, A.; Shirazi-Beechey, S.P. Expression of monosaccharide transporters in intestine of diabetic humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G241–G248. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Tao, S.; Peng, J.; Zhao, J.; Li, S.; Wu, N.; Wen, Y.; Xue, Q.; Yang, C.X.; Pan, X.F. High-sensitivity C-reactive protein and risk of type 2 diabetes: A nationwide cohort study and updated meta-analysis. Diabetes/Metab. Res. Rev. 2021, 37, e3446. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Serban, C.; Sahebkar, A.; Antal, D.; Ursoniu, S.; Banach, M. Effects of supplementation with green tea catechins on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2015, 31, 1061–1071. [Google Scholar] [CrossRef]
- Bajerska, J.; Łagowska, K.; Mori, M.; Reguła, J.; Skoczek-Rubińska, A.; Toda, T.; Mizuno, N.; Yamori, Y. A Meta-Analysis of Randomized Controlled Trials of the Effects of Soy Intake on Inflammatory Markers in Postmenopausal Women. J. Nutr. 2022, 152, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Rüfer, C.E.; Maul, R.; Donauer, E.; Fabian, E.J.; Kulling, S.E. In vitro and in vivo metabolism of the soy isoflavone glycitein. Mol. Nutr. Food Res. 2007, 51, 813–823. [Google Scholar] [CrossRef]
- Chang, T.S. Isolation, bioactivity, and production of ortho-hydroxydaidzein and ortho-hydroxygenistein. Int. J. Mol. Sci. 2014, 15, 5699–5716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Kim, B.G.; Ahn, J.H. Production of bioactive hydroxyflavones by using monooxygenase from Saccharothrix espanaensis. J. Biotechnol. 2014, 176, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef]
- Kumar, S.; Awana, M.; Rani, K.; Kumari, S.; Sasi, M.; Dahuja, A. Soybean (Glycine max) isoflavone conjugate hydrolysing β-glucosidase (GmICHG): A promising candidate for soy isoflavone bioavailability enhancement. 3 Biotech 2023, 13, 52. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2022, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Rimbach, G.; Boesch-Saadatmandi, C.; Frank, J.; Fuchs, D.; Wenzel, U.; Daniel, H.; Hall, W.L.; Weinberg, P.D. Dietary isoflavones in the prevention of cardiovascular disease—A molecular perspective. Food Chem. Toxicol. 2008, 46, 1308–1319. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H. Soybean isoflavones ameliorate ischemic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Food Funct. 2017, 8, 2935–2944. [Google Scholar] [CrossRef]
- Voss, C.; Sepulveda-Boza, S.; Zilliken, F.W. New isoflavonoids as inhibitors of porcine 5-lipoxygenase. Biochem. Pharmacol. 1992, 44, 157–162. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, X.; Yu, H.; Zhou, H. Genistein suppresses ox-LDL-elicited oxidative stress and senescence in HUVECs through the SIRT1-p66shc-Foxo3a pathways. J. Biochem. Mol. Toxicol. 2022, 36, e22939. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Rimbach, G.; Moini, H.; Weber, S.; Packer, L. ESR and cell culture studies on free radical-scavenging and antioxidant activities of isoflavonoids. Toxicology 2002, 179, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Onozaki, H.; Morimitsu, Y.; Kawakishi, S.; Osawa, T. Potent Antioxidative Isoflavones Isolated from Soybeans Fermented with Aspergillus saitoi. Biosci. Biotechnol. Biochem. 1998, 62, 740–746. [Google Scholar] [CrossRef]
- Turner, R.; Baron, T.; Wolffram, S.; Minihane, A.M.; Cassidy, A.; Rimbach, G.; Weinberg, P.D. Effect of circulating forms of soy isoflavones on the oxidation of low density lipoprotein. Free Radic. Res. 2004, 38, 209–216. [Google Scholar] [CrossRef]
- Hirota, A.; Taki, S.; Kawaii, S.; Yano, M.; Abe, N. 1,1-Diphenyl-2-picrylhydrazyl radical-scavenging compounds from soybean miso and antiproliferative activity of isoflavones from soybean miso toward the cancer cell lines. Biosci. Biotechnol. Biochem. 2000, 64, 1038–1040. [Google Scholar] [CrossRef] [Green Version]
- Fujita, T.; Funako, T.; Hayashi, H. 8-Hydroxydaidzein, an aldose reductase inhibitor from okara fermented with Aspergillus sp. HK-388. Biosci. Biotechnol. Biochem. 2004, 68, 1588–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staats, S.; Lüersen, K.; Wagner, A.E.; Rimbach, G. Drosophila melanogaster as a Versatile Model Organism in Food and Nutrition Research. J. Agric. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef] [PubMed]
- Nayak, N.; Mishra, M. Simple techniques to study multifaceted diabesity in the fly model. Toxicol. Mech. Methods 2019, 29, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Perrimon, N. What fuels the fly: Energy metabolism in Drosophila and its application to the study of obesity and diabetes. Sci. Adv. 2021, 7, eabg4336. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Wagner, A.E. Drosophila melanogaster as a Model Organism for Obesity and Type-2 Diabetes Mellitus by Applying High-Sugar and High-Fat Diets. Biomolecules 2022, 12, 307. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, R.; Wang, X.; Zhang, J.; Tang, W.; Zhang, Z.; Liu, Y.; Xu, Q. Drosophila melanogaster diabetes models and its usage in the research of anti-diabetes management with traditional Chinese medicines. Front. Med. 2022, 9, 953490. [Google Scholar] [CrossRef] [PubMed]
- Günther, I.; Rimbach, G.; Nevermann, S.; Neuhauser, C.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Ipharraguerre, I.R.; Weghuber, J.; Lüersen, K. Avens Root (Geum Urbanum L.) Extract Discovered by Target-Based Screening Exhibits Antidiabetic Activity in the Hen’s Egg Test Model and Drosophila melanogaster. Front. Pharmacol. 2021, 12, 794404. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Duckstein, N.; Hasler, M.; Klotz, L.O.; Rimbach, G. Flavonoids as Putative Inducers of the Transcription Factors Nrf2, FoxO, and PPARgamma. Oxidative Med. Cell. Longev. 2017, 2017, 4397340. [Google Scholar] [CrossRef] [Green Version]
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Ozcelik, B.; Frank, J.; Rimbach, G. Comprehensive analysis of polyphenols in 55 extra virgin olive oils by HPLC-ECD and their correlation with antioxidant activities. Plant Foods Hum. Nutr. (Dordr. Neth.) 2012, 67, 326–336. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, S.L.; Rzewnicki, D.; Samols, D.; Kushner, I. The effect of interleukin-1 on C-reactive protein expression in Hep3B cells is exerted at the transcriptional level. Biochem. J. 1995, 310, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Yamagishi, S.; Nakamura, K.; Matsui, T.; Imaizumi, T.; Inoue, H.; Ueno, T.; Sata, M. Pigment epithelium-derived factor (PEDF) blocks the interleukin-6 signaling to C-reactive protein expression in Hep3B cells by suppressing Rac-1 activation. Life Sci. 2006, 79, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, G.; Pallauf, K.; Schulz, C.; Rimbach, G. Flavonoids as putative modulators of Delta4-, Delta5-, and Delta6-desaturases: Studies in cultured hepatocytes, myocytes, and adipocytes. BioFactors 2018, 44, 485–495. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Zhao, R.; Yi, M.; Wan, Q.; Du, L.; Zhou, Y. Soy and Isoflavone Consumption and Multiple Health Outcomes: Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies and Randomized Trials in Humans. Mol. Nutr. Food Res. 2020, 64, 1900751. [Google Scholar] [CrossRef]
- Yan, F.; Eshak, E.S.; Shirai, K.; Dong, J.Y.; Muraki, I.; Tamakoshi, A.; Iso, H. Soy Intake and Risk of Type 2 Diabetes Among Japanese Men and Women: JACC Study. Front. Nutr. 2021, 8, 813742. [Google Scholar] [CrossRef]
- Merino, B.; Fernández-Díaz, C.M.; Cózar-Castellano, I.; Perdomo, G. Intestinal fructose and glucose metabolism in health and disease. Nutrients 2019, 12, 94. [Google Scholar] [CrossRef] [Green Version]
- Schloesser, A.; Esatbeyoglu, T.; Schultheiss, G.; Vollert, H.; Luersen, K.; Fischer, A.; Rimbach, G. Antidiabetic Properties of an Apple/Kale Extract In Vitro, In Situ, and in Mice Fed a Western-Type Diet. J. Med. Food 2017, 20, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Nath, M.; Halder, N.; Velpandian, T. Evaluation of the possibility of selective modulation of retinal glucose transporters in diabetic complications: An experimental study. Indian J. Pharmacol. 2020, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharm. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Altenhofen, D.; da Luz, G.; Frederico, M.J.; Venzke, D.; Brich, M.; Vigil, S.; Fröde, T.S.; Linares, C.E.; Pizzolatti, M.G.; Silva, F.R. Bis-Pyrano Prenyl Isoflavone Improves Glucose Homeostasis by Inhibiting Dipeptidyl Peptidase-4 in Hyperglycemic Rats. J. Cell. Biochem. 2017, 118, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Rajput, M.S.; Sarkar, P.D.; Nirmal, N.P. Inhibition of DPP-4 Activity and Neuronal Atrophy with Genistein Attenuates Neurological Deficits Induced by Transient Global Cerebral Ischemia and Reperfusion in Streptozotocin-Induced Diabetic Mice. Inflammation 2017, 40, 623–635. [Google Scholar] [CrossRef]
- Musselman, L.P.; Fink, J.L.; Narzinski, K.; Ramachandran, P.V.; Hathiramani, S.S.; Cagan, R.L.; Baranski, T.J. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech. 2011, 4, 842–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrat, O.B.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. High amylose starch consumption induces obesity in Drosophila melanogaster and metformin partially prevents accumulation of storage lipids and shortens lifespan of the insects. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 215, 55–62. [Google Scholar] [CrossRef]
- Eickelberg, V.; Lüersen, K.; Staats, S.; Rimbach, G. Phenotyping of Drosophila Melanogaster-A Nutritional Perspective. Biomolecules 2022, 12, 221. [Google Scholar] [CrossRef]
- Rimbach, G.; Weinberg, P.D.; de Pascual-Teresa, S.; Alonso, M.G.; Ewins, B.A.; Turner, R.; Minihane, A.M.; Botting, N.; Fairley, B.; Matsugo, S.; et al. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim. Biophys. Acta 2004, 1670, 229–237. [Google Scholar] [CrossRef]
- Schrader, C.; Ernst, I.M.; Sinnecker, H.; Soukup, S.T.; Kulling, S.E.; Rimbach, G. Genistein as a potential inducer of the anti-atherogenic enzyme paraoxonase-1: Studies in cultured hepatocytes in vitro and in rat liver in vivo. J. Cell. Mol. Med. 2012, 16, 2331–2341. [Google Scholar] [CrossRef]
- Chen, Y.C.; Sugiyama, Y.; Abe, N.; Kuruto-Niwa, R.; Nozawa, R.; Hirota, A. DPPH radical-scavenging compounds from dou-chi, a soybean fermented food. Biosci. Biotechnol. Biochem. 2005, 69, 999–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozawa, D.; Matsuyama, A.; Furuya, T. Biocatalytic synthesis and evaluation of antioxidant and antibacterial activities of hydroxyequols. Bioorg. Med. Chem. Lett. 2022, 73, 128908. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; De Pascual-Teresa, S.; Ewins, B.A.; Matsugo, S.; Uchida, Y.; Minihane, A.M.; Turner, R.; VafeiAdou, K.; Weinberg, P.D. Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiot. Fate Foreign Compd. Biol. Syst. 2003, 33, 913–925. [Google Scholar] [CrossRef]
- Hariyanto, I.; Hsieh, C.W.; Hsu, Y.H.; Chen, L.G.; Chu, C.; Weng, B.B. In Vitro and In Vivo Assessments of Anti-Hyperglycemic Properties of Soybean Residue Fermented with Rhizopus oligosporus and Lactiplantibacillus plantarum. Life 2022, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.E.; Twaddle, N.C.; Helferich, W.G.; Doerge, D.R. Absolute bioavailability of isoflavones from soy protein isolate-containing food in female BALB/c mice. J. Agric. Food Chem. 2010, 58, 4529–4536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, R.; Schwartz, B.; Peri, I.; Shimoni, E. Improving bioavailability and stability of genistein by complexation with high-amylose corn starch. J. Agric. Food Chem. 2011, 59, 7932–7938. [Google Scholar] [CrossRef]
- Zubik, L.; Meydani, M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am. J. Clin. Nutr. 2003, 77, 1459–1465. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Morató, J.; Farré, M.; Pérez-Mañá, C.; Papaseit, E.; Martínez-Riera, R.; de la Torre, R.; Pizarro, N. Pharmacokinetic Comparison of Soy Isoflavone Extracts in Human Plasma. J. Agric. Food Chem. 2015, 63, 6946–6953. [Google Scholar] [CrossRef] [Green Version]
- Esaki, H.; Shirasaki, T.; Yamashita, K.; Nakamura, Y.; Kawakishi, S.; Osawa, T. Absorption and excretion of the 8-hydroxydaidzein in rats after oral administration and its antioxidant effect. J. Nutr. Sci. Vitaminol. 2005, 51, 80–86. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lüersen, K.; Fischer, A.; Bauer, I.; Huebbe, P.; Uekaji, Y.; Chikamoto, K.; Nakata, D.; Hiramatsu, N.; Terao, K.; Rimbach, G. Soy Extract, Rich in Hydroxylated Isoflavones, Exhibits Antidiabetic Properties In Vitro and in Drosophila melanogaster In Vivo. Nutrients 2023, 15, 1392. https://doi.org/10.3390/nu15061392
Lüersen K, Fischer A, Bauer I, Huebbe P, Uekaji Y, Chikamoto K, Nakata D, Hiramatsu N, Terao K, Rimbach G. Soy Extract, Rich in Hydroxylated Isoflavones, Exhibits Antidiabetic Properties In Vitro and in Drosophila melanogaster In Vivo. Nutrients. 2023; 15(6):1392. https://doi.org/10.3390/nu15061392
Chicago/Turabian StyleLüersen, Kai, Alexandra Fischer, Ilka Bauer, Patricia Huebbe, Yukiko Uekaji, Keita Chikamoto, Daisuke Nakata, Naoto Hiramatsu, Keiji Terao, and Gerald Rimbach. 2023. "Soy Extract, Rich in Hydroxylated Isoflavones, Exhibits Antidiabetic Properties In Vitro and in Drosophila melanogaster In Vivo" Nutrients 15, no. 6: 1392. https://doi.org/10.3390/nu15061392
APA StyleLüersen, K., Fischer, A., Bauer, I., Huebbe, P., Uekaji, Y., Chikamoto, K., Nakata, D., Hiramatsu, N., Terao, K., & Rimbach, G. (2023). Soy Extract, Rich in Hydroxylated Isoflavones, Exhibits Antidiabetic Properties In Vitro and in Drosophila melanogaster In Vivo. Nutrients, 15(6), 1392. https://doi.org/10.3390/nu15061392





