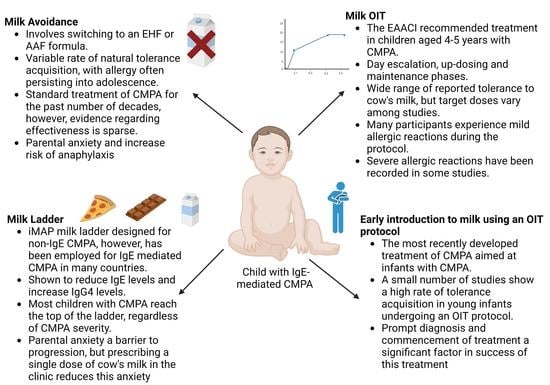
‘Early Introduction’ of Cow’s Milk for Children with IgE-Mediated Cow’s Milk Protein Allergy: A Review of Current and Emerging Approaches for CMPA Management
Abstract
1. Introduction
2. Avoidance of Cow’s Milk
2.1. The Acquisition of Natural Tolerance
2.2. Evidence of the Effectiveness of CM Avoidance
2.3. CM Avoidance: Evidence from Alternative Formula Studies
3. Home Introduction of Milk Using a Stepwise Strategy Milk Ladder
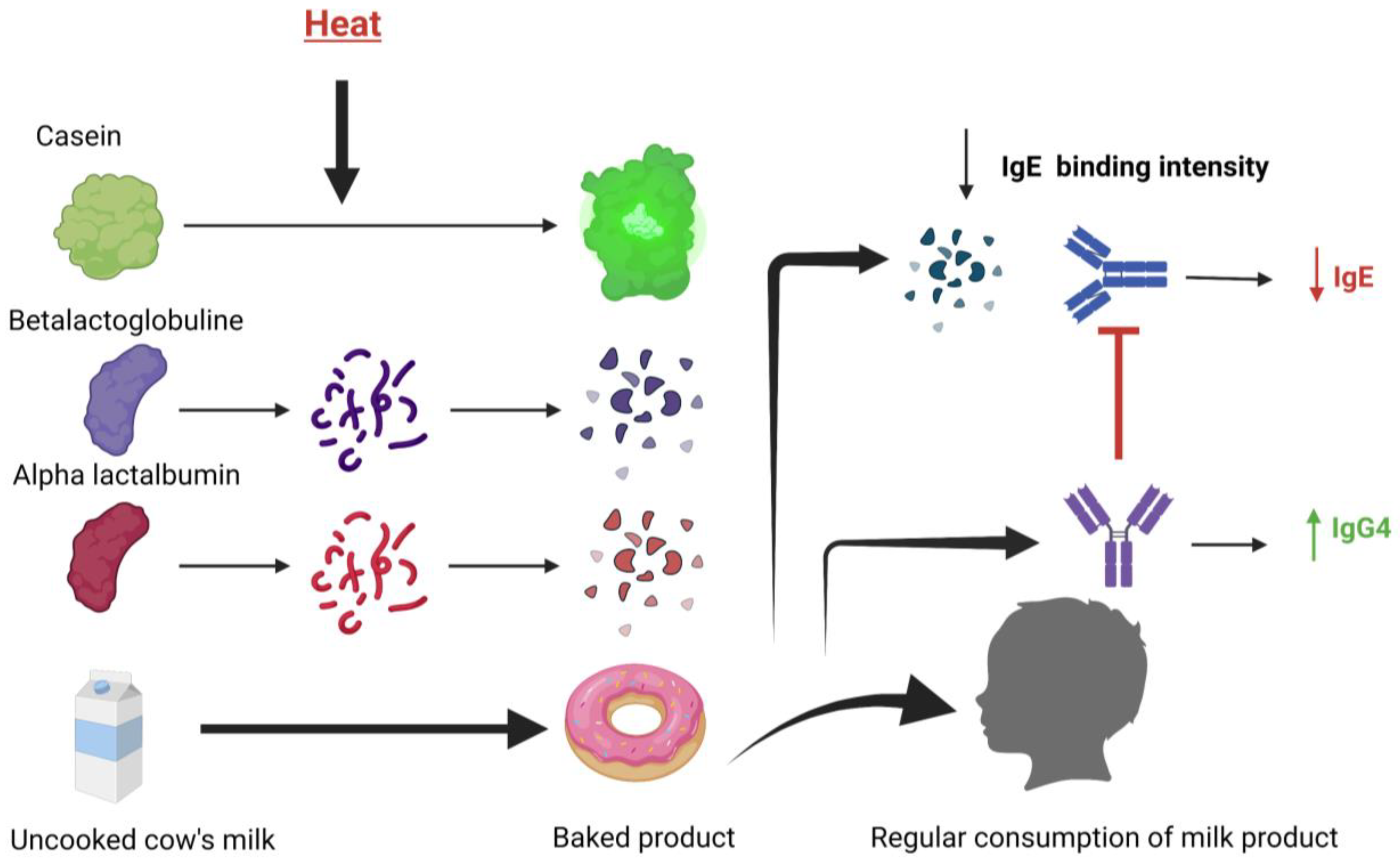
3.1. The Milk Ladder and Baked Milk
3.2. Exploring the Introduction to Milk Using Baked Milk
3.3. The Creation of the Milk Ladder
3.4. Investigating the Effectiveness of the Milk Ladder for IgE-Mediated CMPA
3.5. A Prospective Analysis of the Milk Ladder and Strategies of Improvement
4. Oral Immunotherapy
4.1. The Role of OIT and Its Implementation
4.2. Results of OIT Trials for CMPA Regarding CM Intake
| Author, Country, Sample Size | Population | Intervention | Outcome Reported | Outcome |
|---|---|---|---|---|
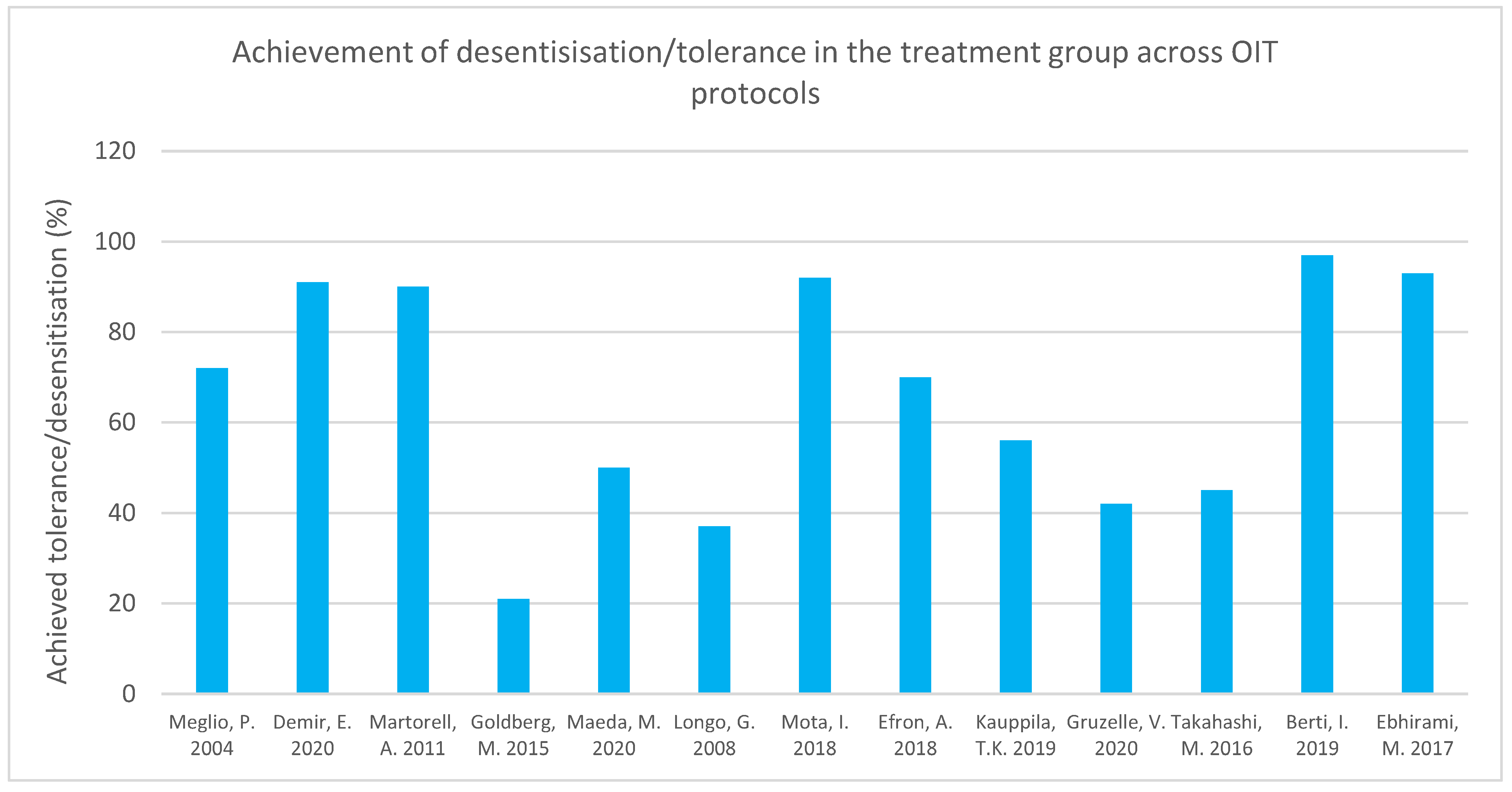
| Meglio P. et al., 2004 [68] Italy 21 | Children with severe IgE-mediated cow’s milk allergy, at least 6 years of age | Open-label, six-month protocol. Children administered increasing doses of fresh cow’s milk. Drops of milk diluted in a 1:25 ratio (initial dose = 1 drop). Until day 70, doses doubled every 7 days, and thereafter every 16 days, to achieve a total dose of 200 mL. | IgE measured at 3 months and at end of protocol. End point SPT. Monthly checkups for a minimum of 3 months post OIT. Target dose was achieving 200 mL CM. | A total of 71.4% (15/21) children were desensitized, achieving daily intake of 200 mL CM. A total of 14.3% (3/21) of the children tolerated 40–80 mL/day of undiluted CM, and 3/21 children were unable to follow the protocol due to symptoms arising after CM intake. |
| Goldberg M. et al., 2015 [77] Israel 15 | Patients > 4 years who had failed the milk OIT program were enrolled into the baked milk (BM) OIT | Baseline OFCs were performed, and escalating baked milk muffin doses were administered to determine each patient’s initial OIT dose. A dose below the eliciting dose was administered daily at home if tolerated. Doses were increased by 50% per month under medical guidance. OFCs with unheated milk conducted after 6 and 12 months of OIT. | Target dose was 1.3 g/day baked milk protein. Basophil reactivity and CM-specific IgE measured at 12 months. | A total of 3/14 (21%) were able to reach the target dose of 1.3 g/day baked milk protein. In patients continuing the protocol until 12 months, there was an increase in challenge threshold (p = 0.003). |
| Takahashi M. et al., 2016 [74] Japan 48 | Children with persistent CM allergy aged 5–18 years | Initial dose: 1/10th of patient’s threshold dose. Rush OIT phase: microwave-heated CM dose increased by 1.2-fold each time. Ingestion 2–4 times/day at 2 h intervals. Maintenance phase: ingestion of 200 mL daily at home. Rate of SU and desensitization measured 1 year after OIT in both groups. Longer follow up of OIT group. Untreated group on elimination diet post OFC. | OFC conducted after 1 year in the untreated group. Desensitization defined as daily consumption of 200 mL fresh CM without adverse reactions for 2 months. CM-OFC conducted after a week’s milk avoidance period for those achieving desensitization. Blood samples at 1 and 2 years of OIT. | Desensitization achieved in 14/31 (p value = 0.002) in OIT group. SU in 21% (7/31) of OIT group at 1 year follow up and by none in untreated group (p value = 0.036). Two years post protocol, rate of desensitization and SU (p = 0.025 and p = 0.008) in OIT group was significantly higher compared to rates one year post protocol. |
| Skripak et al., 2008 [69] USA 20 | Children aged 6–21 with history of milk allergy | Randomization into placebo and OIT group. OIT conducted in 3 phases: (i) build-up day (1st dose = 0.4 mg milk protein, maximum dose = 50 mg); (ii) home dosing with highest tolerated dose and dose increases every 7–14 days; (iii) maintenance dose of 500 mg for 13 weeks. OIT patients tolerating < 2540 mg at last DBPCFC put on avoidance diet. | DBPC, specific IgE, IgG, IgG4, and SPT conducted after OIT. | Post OIT, the median cumulative dose causing a reaction in OIT group was 5140 mg. In the placebo group, all patients had a reaction with 40 mg (p value = 0.0003). Median change in milk dose threshold in OIT group post OIT was 5100 mg (p value = 0.002). Six of seven patients choosing to undergo open-label OIT after this protocol increased their median threshold dose to 8140 mg from 40 mg (p value = 0.03). |
| Longo G. et al., 2008 [70] Italy 60 | Children aged 5–17 with severe allergic reactions and IgE levels > 85 kUA/L | SOTI 2 phase protocol: (i) Rush phase: patients admitted for 10 days and given daily dosing of increasing concentrations of fresh CM until concentration of the solution reached whole milk. Antihistamine given daily. (ii) Home dosing, 1 mL increase every 2nd day until 150 mL of whole milk in single dose reached. Antihistamines used until this point and then tapered off across 4 weeks. Patients asked to continue dairy product intake thereafter. | After 1 year, avoidance group underwent DBPCFC. Specific serum IgE measured at 6 months and 12 months. Measured the number of children tolerating ≥150 mL CM in single dose, children tolerating ≥5 mL in single dose but <150 mL (partial tolerance). | All patients in avoidance group had positive DBPCFC 1 year post protocol. A total of 11/30 in OIT group achieved complete tolerance, many of whom could continue on unrestricted diet (p value < 0.001). |
| Martorell A. et al., 2011 [66] Spain 60 | Children 24–36 months old with IgE-CMPA | Diluted doses administered in hospital on day 1 and 2. Thereafter, home administration of fresh cow’s milk twice a day, and doses increased at the research unit once a week for 16 weeks. Diary log kept. No preventative medications used. | Total tolerance defined as ability to consume 200 mL CM, partial tolerance as 20–200 mL CM after 1 year. SPT and IgE measured in both groups at 1 year follow up. Repeat DBPCFC, IgE, and SPT done in those failing desensitization. | In OIT group, 27/30 (90%) achieved 200 mL tolerance and remained tolerant at 1 year follow up. In control group, 23/27 (76.7%) were still allergic at 1 year follow up. Those tolerant continued 200 mL milk intake daily with unrestricted diets. Number needed to treat found to be 1.45. |
| Amat F. et al., 2017 [76] France 41 | Children > 3 years with persistent IgE-CMPA | Patients randomized into (i) baked milk (low-risk) OIT and (ii) raw milk (high-risk) OIT. (i) Doses increased at home every 15 days. Decreased heating of baked milk preparation gradually. When tolerance threshold reached 1970 mg, raw milk used. Daily maintenance dose of 2720 mg. (ii) Increased dosing every 5 weeks in hospital. Highest tolerated dose administered at home daily. | “Responders” defined as tolerating daily intake of 2720 mg milk protein without symptoms at 5 months (for high-risk OIT) or 9 months (for low-risk OIT) follow up. Partial responders as tolerating 340 mg–2720 mg. Serum casein-IgE and IgG measured at end of follow up. Asteir score of the reactions was determined. | At follow up, 104 mg tolerated by sensitive patients, and 1802 mg by others (p value = 0.02). After follow up, 36.6% classified as responders, 26.8% partial responders. Average threshold gain was 697 mg. A total of 36.6% remained non-responders. |
| Maeda M et al., 2020 [71] Japan 28 | Patients with CMPA aged 3–12 years | Randomization into OIT and control group. Two-week rush OIT in hospital for OIT group. Gradual daily dose increase. Thereafter, CM intake once a day at home, with dose increase every 14 days by 10–20%. When 100 mL CM reached, this dose was taken daily. Epinastine hydrochloride taken daily. One year of monitoring as outpatients once a month, some with longer follow up. Control group avoided CM for 1 year. | OFC with 100 mL CM and DBCFC conducted after 1 year. SPT, eosinophil count, IgE, IgG4 levels measured. Transcriptome analysis. | Fifty percent of OIT group were desensitized after 1 year. After 1 year, 7/14 in OIT group and 0/14 in control group had negative OFC (p value < 0.01). Post protocol, greater CM intake threshold and percentage change in intake threshold in OIT vs. control group (p value < 0.01). Seven out of eight followed up 2 years post protocol able to consume >100 mL CM without reactions. |
| Mota I. et al., 2018 [75] Portugal | Patients aged 2–18 years with persistent moderate-severe IgE CM allergy | Prospective uncontrolled study. Patients who had undergone CM OIT were given 200 mL maintenance dose daily. OIT induction: Doses of unheated and undiluted CM administered, and dose increased at 14–28-day intervals. Minimum follow up of 36 months after maintenance dose reached. | Subjects followed in clinic to check maintenance of 200 mL CM for at least 36 months post reaching the maintenance phase. | Total of 92% of patients able to maintain diet without restrictions and daily ingestion of 200 mL CM. |
| Efron A. et al., 2018 [67] Israel 110 | Children aged 1–4 years passing baked milk challenge started OIT. Control group had patients previously diagnosed with CMPA, and they were followed at different clinics. | Retrospective case-control study comparing children on milk avoidance for 4 years with those treated with extensively heated and baked milk therapy. Home-based 3-month, 4-phase OIT. Daily consumption of a milk product deemed safe at each OFC, e.g., cookie, pizza, etc. Avoidance of milk products other than ones deemed safe. Final OFC: 150 mL raw milk. | OFC with 250 mL unheated CM to determine tolerance. | At follow up, 70% of OIT group able to consume all types of dairy and milk products, but not raw milk. Milk allergy resolution seen in both groups with age. Median resolution age in OIT group = 34 months and in control group = 57 months (p value = 0.006). OIT decreased resolution time of CM allergic patients who only had skin symptoms and those with history of anaphylactic reaction to milk. Total of 86% of treatment group achieved tolerance (250 mL of unheated CM) compared to 56% of control group (p = 0.003). |
| Kauppila T.K. et al., 2019 [72] Finland 296 | Children ≥ 5 years with IgE-mediated CMPA | Three groups: high dose, low dose, and avoidance. Build-up phase: increasing doses of fresh milk protein administered across 4 months until 6.4 g maintenance dose reached. Antihistamine used in build-up phase. Long-term follow up conducted. | Long-term follow up conducted to measure continuance of daily intake of ≥2 dL CM. Long-term follow-up questionnaire to measure adrenaline use in protocol. | Total of 56% of subjects maintained daily milk dosage of ≥2 dL at follow up (median follow-up duration = 6.5 years). |
| Demir E. et al., 2020 [73] Turkey 42 | Total of 47 patients, 3–13 years old with solely CM IgE allergy selected between 2009–2014 | Retrospective cohort study. OFC was considered the initial escalation phase. On 2nd day, a dose 3 doses behind tolerated dose given at home for a week. Build-up phase with fresh CM dose increases in hospital and daily home intake until 200 mL target dose reached (16 weeks). Antihistamines in build-up phase. Dose modified according to reaction. | OFC at 6-month and 1-year intervals to determine tolerance. CM-SPT performed 1 year post OIT. CM s-IgE measured at 6 months, 1, 2, and 3 years of maintenance phase. | Total of 91.3% (42/47) successfully reached target daily dose of 200 mL. Two percent achieved partial desensitization (tolerating 45 mL). |
| Gruzelle V. et al., 2020 [79] France 63 | Children <18 years with CMPA and high casein-specific IgE | Retrospective chart review using baked milk OIT. Initial dose was 1 mL CM. Home increases in dosage until 5 shortbreads reached. This dose taken until 2nd OFC. If positive, the OIT dosing was changed, and another OFC done 1 year later. | Between 1–3 OFCs conducted. Allergic reactions at OFC were graded. sIgE measured. | Desensitization achieved by 42.2% patients in an average duration of 521 days. Increased dose at which patients reacted in last OFC compared to 1st OFC. |
| Berti I. et al., 2019 [65] | Children < 12 months between 2015–2017 who were admitted to Institute for Maternal and Child Health IRCCS Burlo Garofolo due to hypersensitivity reactions to CM were enrolled in the study. | Initial OFC conducted. Thereafter, OIT was started with home dosing of milk with the highest dose that was tolerated in hospital. Evaluation every 3–4 weeks, and if previous dose was tolerated, dose was doubled and then continued at home. Parents advised to dilute milk into foods commonly consumed by infant. Once higher doses tolerated, dosing increments/frequency were increased. Target dose = 150 mL CM or dairy products with the same amount. | IgE and CM-IgG4 measured at 2 months and then post protocol completion. Target dose of protocol was 150 mL CM/equivalent dairy product. Number of reactions recorded. | Target dose achieved by 66 patients (97%). |
| Calvo et al., 2020 [64] Spain 335 | Children < 1 year with IgE-CM allergy whose parents agreed to OIT underwent this therapy. | Retrospective analysis. Initial oral challenge test conducted on a group of patients between 2007–2011, before starting OIT. From 2011–2018, another set of patients was given OIT without oral food test but with a fixed starting dose of 0.5 mL. OIT: For 1 week, doses given at home twice a day. Infant formula doses mixed with food. Increase in dosage at 7-day intervals in hospital and adapted according to patient. Target dose was 150–200 mL infant formula. | SPT and IgE measured post protocol. | Successful OIT completion in 98.5% of OCT group and 98.1% of FSD group. These patients were able to regularly consume dairy products after this. Median OIT duration in OCT group: 106 days vs. 77 days in FSD group (p value = 0.001). |
| Ebrahimi M. et al., 2017 [80] Iran 14 | Children > 3 years old who were supervised at the Allergy and Immunology Clinic Of Children’s Medical Center, Iran for more than 6 months, with a history of CM allergy | DPBCFC conducted. Three-phase fresh milk OIT protocol: rush, build-up, and maintenance phase. (i) Rush phase (1 day): increasing doses of milk administered at 30 min intervals. (ii) Build-up phase: weekly increases in daily milk dose. First dose of the week given as inpatient and the remainder as outpatient. (iii) Maintenance phase (90 days): 200–250 mL CM doses administered per day. Each patient’s doses were given according to severity of their allergy. Patient’s caregivers required to keep a daily log during protocol. | IgE and SPT measured at the end of protocol. Adverse effects recorded following each dose. | Total of 13/14 (92.9%) of subjects completed the protocol and were desensitized to CM. |
4.3. Factors Potentially Influencing OIT Results
5. Early Introduction of Milk Using an OIT Strategy in Young Infants
5.1. Early Studies in Early Introduction
5.2. Establishing Early Introduction in Young Infants as a Management Strategy for CMPA
5.3. Early Introduction or the Natural Development of Tolerance?
6. Discussion
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and Burden of Food Allergy. Curr. Allergy Asthma Rep. 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- d’Art, Y.M.; Forristal, L.; Byrne, A.M.; Fitzsimons, J.; van Ree, R.; DunnGalvin, A.; Hourihane, J.O.B. Single Dose Oral Food Challenge at Diagnosis Accelerates Cows Milk Allergic Infants’ Acquisition of Oral Tolerance to Milk, But Progress is Influenced by Maternal Anxiety; SSRN: Rochester, NY, USA, 2021. [Google Scholar]
- Feng, C.; Kim, J.H. Beyond Avoidance: The Psychosocial Impact of Food Allergies. Clin. Rev. Allergy Immunol. 2019, 57, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Flom, J.D.; Sicherer, S.H. Epidemiology of Cow’s Milk Allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.W.; Younus, M.A. Cow’s Milk Allergy; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307.e5. [Google Scholar] [CrossRef]
- Toro Monjaraz, E.M.; Ramírez Mayans, J.A.; Cervantes Bustamante, R.; Gómez Morales, E.; Molina Rosales, A.; Montijo Barrios, E.; Zárate Mondragón, F.; Cadena León, J.; Cazares Méndez, M.; López-Ugalde, M.; et al. Factores perinatales asociados al desarrollo de alergia a las proteínas de la leche de vaca. Rev. Gastroenterol. Mex. 2015, 80, 27–31. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhou, S.M.; Wang, S.H.; Sui, F.X.; Gao, W.H.; Liu, Q.; Cai, H.B.; Jiang, H.Y.; Li, W.Y.; Wang, L.T.; et al. Risk factors for cow’s milk protein allergy in infants: A multicenter survey. Chin. J. Contemp. Pediatr. 2020, 22, 42–46. [Google Scholar]
- Calvani, M.; Bianchi, A.; Reginelli, C.; Peresso, M.; Testa, A. Oral Food Challenge. Medicina 2019, 55, 651. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Ulfman, L.; Tsuang, A.; Sprikkelman, A.B.; Goh, A.; van Neerven, R.J.J. Relevance of Early Introduction of Cow’s Milk Proteins for Prevention of Cow’s Milk Allergy. Nutrients 2022, 14, 2659. [Google Scholar] [CrossRef]
- Giannetti, A.; Toschi Vespasiani, G.; Ricci, G.; Miniaci, A.; di Palmo, E.; Pession, A. Cow’s milk protein allergy as a model of food allergies. Nutrients 2021, 13, 1525. [Google Scholar] [CrossRef]
- Shanahan, L.; Zucker, N.; Copeland, W.E.; Costello, E.J.; Angold, A. Are children and adolescents with food allergies at increased risk for psychopathology? J. Psychosom. Res. 2014, 77, 468–473. [Google Scholar] [CrossRef]
- Sekerel, B.E.; Seyhun, O. Expert panel on practice patterns in the management of cow’s milk protein allergy and associated economic burden of disease on health service in Turkey. J. Med. Econ. 2017, 20, 923–930. [Google Scholar] [CrossRef]
- Ferrari, R. Writing narrative style literature reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Knol, E.F.; de Jong, N.W.; Ulfman, L.H.; Tiemessen, M.M. Management of Cow’s Milk Allergy from an Immunological Perspective: What Are the Options? Nutrients 2019, 11, 2734. [Google Scholar] [CrossRef]
- Vila, L.; Beyer, K.; Järvinen, K.M.; Chatchatee, P.; Bardina, L.; Sampson, H.A. Role of conformational and linear epitopes in the achievement of tolerance in cow’s milk allergy. Clin. Exp. Allergy 2001, 31, 1599–1606. [Google Scholar] [CrossRef]
- Høst, A.; Halken, S.; Jacobsen, H.P.; Christensen, A.E.; Herskind, A.M.; Plesner, K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr. Allergy Immunol. 2002, 13, 23–28. [Google Scholar] [CrossRef]
- Host, A.; Halken, S. Hypoallergenic formulas—When, to whom and how long: After more than 15 years we know the right indication! Allergy 2004, 59, 45–52. [Google Scholar] [CrossRef]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World allergy organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. Pediatr. Allergy Immunol. 2010, 3, 57–161. [Google Scholar] [CrossRef]
- Vanto, T.; Helppilä, S.; Juntunen-Backman, K.; Kalimo, K.; Klemola, T.; Korpela, R.; Koskinen, P. Prediction of the development of tolerance to milk in children with cow’s milk hypersensitivity. J. Pediatr. 2004, 144, 218–222. [Google Scholar] [CrossRef]
- Garcia-Ara, M.C.; Boyano-Martinez, M.T.; Diaz-Pena, J.M.; Martin-Munoz, M.F.; Martin-Esteban, M. Cow’s milk-specific immunoglobulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clin. Exp. Allergy 2004, 34, 866–870. [Google Scholar] [CrossRef]
- Saarinen, K.M.; Pelkonen, A.S.; Mäkelä, M.J.; Savilahti, E. Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific IgE status. J. Allergy Clin. Immunol. 2005, 116, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Skripak, J.M.; Matsui, E.C.; Mudd, K.; Wood, R.A. The natural history of IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2007, 120, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Terracciano, L.; Bouygue, G.R.; Veglia, F.; Sarratud, T.; Martelli, A.; Restani, P. Incremental prognostic factors associated with cow’s milk allergy outcomes in infant and child referrals: The Milan Cow’s Milk Allergy Cohort study. Ann. Allergy Asthma Immunol. 2008, 101, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Martorell, A.; García Ara, M.C.; Plaza, A.M.; Boné, J.; Nevot, S.; Echeverria, L.; Alonso, E.; Garde, J. The predictive value of specific immunoglobulin E levels in serum for the outcome of the development of tolerance in cow’s milk allergy. Allergol. Immunopathol. 2008, 36, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Dias, A.; Pinheiro, J.A. Predictive factors for the persistence of cow’s milk allergy. Pediatr. Allergy Immunol. 2010, 21, 1127–1134. [Google Scholar] [CrossRef]
- Ahrens, B.; Lopes de Oliveira, L.C.; Grabenhenrich, L.; Schulz, G.; Niggemann, B.; Wahn, U.; Garde, J. Individual cow’s milk allergens as prognostic markers for tolerance development? Clin. Exp. Allergy 2012, 42, 1630–1637. [Google Scholar] [CrossRef]
- Elizur, A.; Rajuan, N.; Goldberg, M.R.; Leshno, M.; Cohen, A.; Katz, Y. Natural Course and Risk Factors for Persistence of IgE-Mediated Cow’s Milk Allergy. J. Pediatr. 2012, 161, 482–487.e1. [Google Scholar] [CrossRef]
- Wood, R.A.; Sicherer, S.H.; Vickery, B.P.; Jones, S.M.; Liu, A.H.; Fleischer, D.M.; Henning, A.K.; Mayer, L.; Burks, A.W.; Grishin, A.; et al. The natural history of milk allergy in an observational cohort. J. Allergy Clin. Immunol. 2013, 131, 805–812.e4. [Google Scholar] [CrossRef]
- Yavuz, S.T.; Buyuktiryaki, B.; Sahiner, U.M.; Birben, E.; Tuncer, A.; Yakarisik, S.; Karabulut, E.; Kalayci, O.; Sackesen, C. Factors that predict the clinical reactivity and tolerance in children with cow’s milk allergy. Ann. Allergy Asthma Immunol. 2013, 110, 284–289. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children-EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Yang, H.; kyoung Won, H.J.; Kim, K.; Kim, J.; Ahn, K. The Natural Course of Immediate-Type Cow’s Milk and Egg Allergies in Children. Int. Arch. Allergy Immunol. 2020, 181, 103–110. [Google Scholar] [CrossRef]
- IgE-mediated cow’s milk protein allergy in Singaporean children. Asian Pac. J. Allergy Immunol. 2022, 40, 65–71.
- de Silva, D.; Geromi, M.; Panesar, S.S.; Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Cardona, V.; Dubois, A.E.J.; Halken, S.; et al. Acute and long-term management of food allergy: Systematic review. Allergy 2014, 69, 159–167. [Google Scholar] [CrossRef]
- Agata, H.; Kondo, N.; Fukutomi, O.; Shinoda, S.; Orii, T. Effect of elimination diets on food-specific IgE antibodies and lymphocyte proliferative responses to food antigens in atopic dermatitis patients exhibiting sensitivity to food allergens. J. Allergy Clin. Immunol. 1993, 91, 668–679. [Google Scholar] [CrossRef]
- de Boissieu, D.; Dupont, C. Allergy to extensively hydrolyzed cow’s milk proteins in infants: Safety and duration of amino acid–based formula. J. Pediatr. 2002, 141, 271–273. [Google Scholar] [CrossRef]
- Berni Canani, R.; di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; di Scala, C.; Granata, V.; Nocerino, R. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4. [Google Scholar] [CrossRef]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.W.; Sangsupawanich, P.; van Ampting, M.T.J.; Oude Nijhuis, M.M.; Harthoorn, L.F.; Langford, J.E.; et al. Tolerance development in cow’s milk–allergic infants receiving amino acid–based formula: A randomized controlled trial. J. Allergy Clin. Immunol. 2022, 149, 650–658.e5. [Google Scholar] [CrossRef]
- Kim, J.S.; Nowak-Węgrzyn, A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Sampson, H.A. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J. Allergy Clin. Immunol. 2011, 128, 125–131.e2. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Bloom, K.A.; Sicherer, S.c.o.t.t.H.; Shreffler, W.G.; Noone, S.; Wanich, N.; Sampson, H.A. Tolerance to extensively heated milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 342–347.e2. [Google Scholar] [CrossRef]
- Esmaeilzadeh, H.; Alyasin, S.; Haghighat, M.; Nabavizadeh, H.; Esmaeilzadeh, E.; Mosavat, F. The effect of baked milk on accelerating unheated cow’s milk tolerance: A control randomized clinical trial. Pediatr. Allergy Immunol. 2018, 29, 747–753. [Google Scholar] [CrossRef]
- Savilahti, E.M.; Rantanen, V.; Lin, J.S.; Karinen, S.; Saarinen, K.M.; Goldis, M.; Mäkelä, M.J.; Hautaniemi, S.; Savilahti, E.; Sampson, H.A. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J. Allergy Clin. Immunol. 2010, 125, 1315–1321.e9. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Węgrzyn, A.; Lawson, K.; Masilamani, M.; Kattan, J.; Bahnson, H.T.; Sampson, H.A. Increased Tolerance to Less Extensively Heat-Denatured (Baked) Milk Products in Milk-Allergic Children. J. Allergy Clin. Immunol. Pract. 2018, 6, 486–495.e5. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, J.H.; Keet, C.A.; Mudd, K.; Wood, R.A. Long-Term Follow-Up After Baked Milk Introduction. J. Allergy Clin. Immunol. Pract. 2018, 6, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Brown, T.; Shah, N.; Walsh, J.; Fox, A.T. Diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy—A UK primary care practical guide. Clin. Transl. Allergy 2013, 3, 23. [Google Scholar] [CrossRef]
- Venter, C.; Brown, T.; Meyer, R.; Walsh, J.; Shah, N.; Nowak-Węgrzyn, A.; Chen, T.; Fleischer, D.M.; Heine, R.G.; Levin, M.; et al. Better recognition, diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy: IMAP—An international interpretation of the MAP (Milk Allergy in Primary Care) guideline. Clin. Transl. Allergy 2017, 7, 26. [Google Scholar] [CrossRef]
- Athanasopoulou, P.; Deligianni, E.; Dean, T.; Dewey, A.; Venter, C. Use of baked milk challenges and milk ladders in clinical practice: A worldwide survey of healthcare professionals. Clin. Exp. Allergy 2017, 47, 430–434. [Google Scholar] [CrossRef]
- Chomyn, A.; Chan, E.S.; Yeung, J.; vander Leek, T.K.; Williams, B.A.; Soller, L.; Abrams, E.M.; Mak, R.; Wong, T. Canadian food ladders for dietary advancement in children with IgE-mediated allergy to milk and/or egg. Allergy Asthma Clin. Immunol. 2021, 17, 83. [Google Scholar] [CrossRef]
- Ball, H.B.; Luyt, D. Home-based cow’s milk reintroduction using a milk ladder in children less than 3 years old with IgE-mediated cow’s milk allergy. Clin. Exp. Allergy 2019, 49, 911–920. [Google Scholar] [CrossRef]
- d’Art, Y.M.; Forristal, L.; Byrne, A.M.; Fitzsimons, J.; van Ree, R.; DunnGalvin, A.; O'B Hourihane, J. Single low-dose exposure to cow’s milk at diagnosis accelerates cow’s milk allergic infants’ progress on a milk ladder programme. Allergy 2022, 77, 2760–2769. [Google Scholar] [CrossRef]
- Turner, P.J.; d’Art, Y.M.; Duca, B.; Chastell, S.A.; Marco-Martin, G.; Vera-Berrios, R.N.; Alvarez, O.; Bazire, R.; Rodríguez del Río, P.; Vazquez-Ortiz, M.; et al. Single-dose oral challenges to validate eliciting doses in children with cow’s milk allergy. Pediatr. Allergy Immunol. 2021, 32, 1056–1065. [Google Scholar] [CrossRef]
- Sampath, V.; Sindher, S.B.; Alvarez Pinzon, A.M.; Nadeau, K.C. Can food allergy be cured? What are the future prospects? Allergy 2020, 75, 1316–1326. [Google Scholar] [CrossRef]
- Karlsson, M.R.; Rugtveit, J.; Brandtzaeg, P. Allergen-responsive CD4+CD25+ Regulatory T Cells in Children who Have Outgrown Cow’s Milk Allergy. J. Exp. Med. 2004, 199, 1679–1688. [Google Scholar] [CrossRef]
- Shreffler, W.G.; Wanich, N.; Moloney, M.; Nowak-Wegrzyn, A.; Sampson, H.A. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J. Allergy Clin. Immunol. 2009, 123, 43–52.e7. [Google Scholar] [CrossRef]
- Taniuchi, S.; Takahashi, M.; Soejima, K.; Hatano, Y.; Minami, H. Immunotherapy for cow’s milk allergy. Hum. Vaccin. Immunother. 2017, 13, 2443–2451. [Google Scholar] [CrossRef]
- Burton, O.T.; Logsdon, S.L.; Zhou, J.S.; Medina-Tamayo, J.; Abdel-Gadir, A.; Noval Rivas, M.; Koleoglou, K.J.; Chatila, T.A.; Schneider, L.C.; Rachid, R.; et al. Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. J. Allergy Clin. Immunol. 2014, 134, 1310–1317.e6. [Google Scholar] [CrossRef]
- Shamji, M.H.; Durham, S.R. Mechanisms of immunotherapy to aeroallergens. Clin. Exp. Allergy 2011, 41, 1235–1246. [Google Scholar] [CrossRef]
- Keet, C.A.; Wood, R.A. Emerging therapies for food allergy. J. Clin. Investig. 2014, 124, 1880–1886. [Google Scholar] [CrossRef]
- Pajno, G.B.; Cox, L.; Caminiti, L.; Ramistella, V.; Crisafulli, G. Oral Immunotherapy for Treatment of Immunoglobulin E-Mediated Food Allergy: The Transition to Clinical Practice. Pediatr. Allergy Immunol. Pulmonol. 2014, 27, 42–50. [Google Scholar] [CrossRef]
- Mestecky, J.; Bienenstock, J.; McGhee, J.R.; Lamm, M.E.; Strober, W.; Cebra, J.J.; Mayer, L.; Ogra, P.L. Historical Aspects of Mucosal Immunology. In Mucosal Immunology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 33–58. [Google Scholar]
- Mori, F.; Barni, S.; Liccioli, G.; Novembre, E. Oral Immunotherapy (OIT): A Personalized Medicine. Medicina 2019, 55, 684. [Google Scholar] [CrossRef]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 799–815. [Google Scholar] [CrossRef]
- Boné Calvo, J.; Clavero Adell, M.; Guallar Abadía, I.; Laliena Aznar, S.; Sancho Rodríguez, M.L.; Claver Monzón, A.; Aliaga Mazas, Y. As soon as possible in IgE-cow’s milk allergy immunotherapy. Eur. J. Pediatr. 2021, 180, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Berti, I.; Badina, L.; Cozzi, G.; Giangreco, M.; Bibalo, C.; Ronfani, L.; Barbi, E.; Ventura, A.; Longo, G. Early oral immunotherapy in infants with cow’s milk protein allergy. Pediatr. Allergy Immunol. 2019, 30, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Martorell, A.; de la Hoz, B.; Ibáñez, M.D.; Bone, J.; Terrados, M.S.; Michavila, A.; Plaza, A.M.; Alonso, E.; Garde, J.; Nevot, S.; et al. Oral desensitization as a useful treatment in 2-year-old children with cow’s milk allergy. Clin. Exp. Allergy 2011, 41, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Efron, A.; Zeldin, Y.; Gotesdyner, L.; Stauber, T.; Maoz Segal, R.; Binson, I.; Dinkin, M.; Dinkowitz, L.; Shahar, D.; Deutch, M.; et al. A Structured Gradual Exposure Protocol to Baked and Heated Milk in the Treatment of Milk Allergy. J. Pediatr. 2018, 203, 204–209.e2. [Google Scholar] [CrossRef]
- Meglio, P.; Bartone, E.; Plantamura, M.; Arabito, E.; Giampietro, P.G. A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy 2004, 59, 980–987. [Google Scholar] [CrossRef]
- Skripak, J.M.; Nash, S.D.; Rowley, H.; Brereton, N.H.; Oh, S.; Hamilton, R.G.; Matsui, E.C.; Burks, A.W.; Wood, R.A. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 1154–1160. [Google Scholar] [CrossRef]
- Longo, G.; Barbi, E.; Berti, I.; Meneghetti, R.; Pittalis, A.; Ronfani, L.; Ventura, A. Specific oral tolerance induction in children with very severe cow’s milk–induced reactions. J. Allergy Clin. Immunol. 2008, 121, 343–347. [Google Scholar] [CrossRef]
- Maeda, M.; Imai, T.; Ishikawa, R.; Nakamura, T.; Kamiya, T.; Kimura, A.; Fujita, S.; Akashi, K.; Tada, H.; Morita, H.; et al. Effect of oral immunotherapy in children with milk allergy: The ORIMA study. Allergol. Int. 2021, 70, 223–228. [Google Scholar] [CrossRef]
- Kauppila, T.K.; Paassilta, M.; Kukkonen, A.K.; Kuitunen, M.; Pelkonen, A.S.; Makela, M.J. Outcome of oral immunotherapy for persistent cow’s milk allergy from 11 years of experience in Finland. Pediatr. Allergy Immunol. 2019, 30, 356–362. [Google Scholar] [CrossRef]
- Demir, E.; Ciğerci Günaydın, N.; Gülen, F.; Tanaç, R. Oral Immunotherapy with Cow’s Milk Allergy: Five Years’ Experience from a Single Center in Turkey. Balkan Med. J. 2020, 37, 316. [Google Scholar] [CrossRef]
- Takahashi, M.; Taniuchi, S.; Soejima, K.; Hatano, Y.; Yamanouchi, S.; Kaneko, K. Two-weeks-sustained unresponsiveness by oral immunotherapy using microwave heated cow’s milk for children with cow’s milk allergy. Allergy Asthma Clin. Immunol. 2016, 12, 44. [Google Scholar] [CrossRef]
- Mota, I.; Piedade, S.; Gaspar, Â.; Benito-Garcia, F.; Sampaio, G.; Borrego, L.M.; Morais-Almeida, M. Cow’s milk oral immunotherapy in real life: 8-year long-term follow-up study. Asia Pac. Allergy 2018, 8, e28. [Google Scholar] [CrossRef]
- Amat, F.; Kouche, C.; Gaspard, W.; Lemoine, A.; Guiddir, T.; Lambert, N.; Zakariya, M.; Ridray, C.; Nemni, A.; Saint-Pierre, P.; et al. Is a slow-progression baked milk protocol of oral immunotherapy always a safe option for children with cow’s milk allergy? A randomized controlled trial. Clin. Exp. Allergy 2017, 47, 1491–1496. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Nachshon, L.; Appel, M.Y.; Elizur, A.; Levy, M.B.; Eisenberg, E.; Sampson, H.A.; Katz, Y. Efficacy of baked milk oral immunotherapy in baked milk–reactive allergic patients. J. Allergy Clin. Immunol. 2015, 136, 1601–1606. [Google Scholar] [CrossRef]
- Gruzelle, V.; Juchet, A.; Martin-Blondel, A.; Michelet, M.; Chabbert-Broue, A.; Didier, A. Benefits of baked milk oral immunotherapy in French children with cow’s milk allergy. Pediatr. Allergy Immunol. 2020, 31, 364–370. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Gharagozlou, M.; Mohebbi, A.; Hafezi, N.; Azizi, G.; Movahedi, M. The Efficacy of Oral Immunotherapy in Patients with Cow’s Milk Allergy. Iran. J. Allergy Asthma Immunol. 2017, 16, 183–192. [Google Scholar]
- Reche, M.; Valbuena, T.; Fiandor, A.; Padial, A.; Cañete, A.; Pascual, C. Early Induction of Oral Tolerance Protocol (OTI) In Children With Cow’S Milk Allergy. J. Allergy Clin. Immunol. 2011, 127, AB24. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, W.S.; Kim, H.; Hahn, Y.S. Increased cow’s milk protein–specific IgG4 levels after oral desensitization in 7- to 12-month-old infants. Ann. Allergy Asthma Immunol. 2013, 111, 523–528. [Google Scholar] [CrossRef]
- Chatchatee, P.; Järvinen, K.M.; Bardina, L.; Vila, L.; Beyer, K.; Sampson, H.A. Identification of IgE and IgG binding epitopes on β- and κ-casein in cow’s milk allergic patients. Clin. Exp. Allergy 2001, 31, 1256–1262. [Google Scholar] [CrossRef]
- Chatchatee, P.; Järvinen, K.M.; Bardina, L.; Beyer, K.; Sampson, H.A. Identification of IgE- and IgG-binding epitopes on αs1-casein: Differences in patients with persistent and transient cow’s milk allergy. J. Allergy Clin. Immunol. 2001, 107, 379–383. [Google Scholar] [CrossRef]
- Järvinen, K.M.; Beyer, K.; Vila, L.; Chatchatee, P.; Busse, P.J.; Sampson, H.A. B-cell epitopes as a screening instrument for persistent cow’s milk allergy. J. Allergy Clin. Immunol. 2002, 110, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Lebrero, E.A.; Moya, L.F.; Somoza Alvarez, M.L. Alergia a leche y huevo en ninos. Alergol. Immunol. Clin. 2001, 16, 96–115. [Google Scholar]
- Sinai, T.; Goldberg, M.R.; Nachshon, L.; Amitzur-Levy, R.; Yichie, T.; Katz, Y.; Monsonego-Ornan, E.; Elizur, A. Reduced Final Height and Inadequate Nutritional Intake in Cow’s Milk-Allergic Young Adults. J. Allergy Clin. Immunol. Pract. 2019, 7, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.; Petrus, N.; Venema, A.; Posthuma, D.; Mannens, M.; Sprikkelman, A.; Henneman, P. Higher Polygenetic Predisposition for Asthma in Cow’s Milk Allergic Children. Nutrients 2018, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
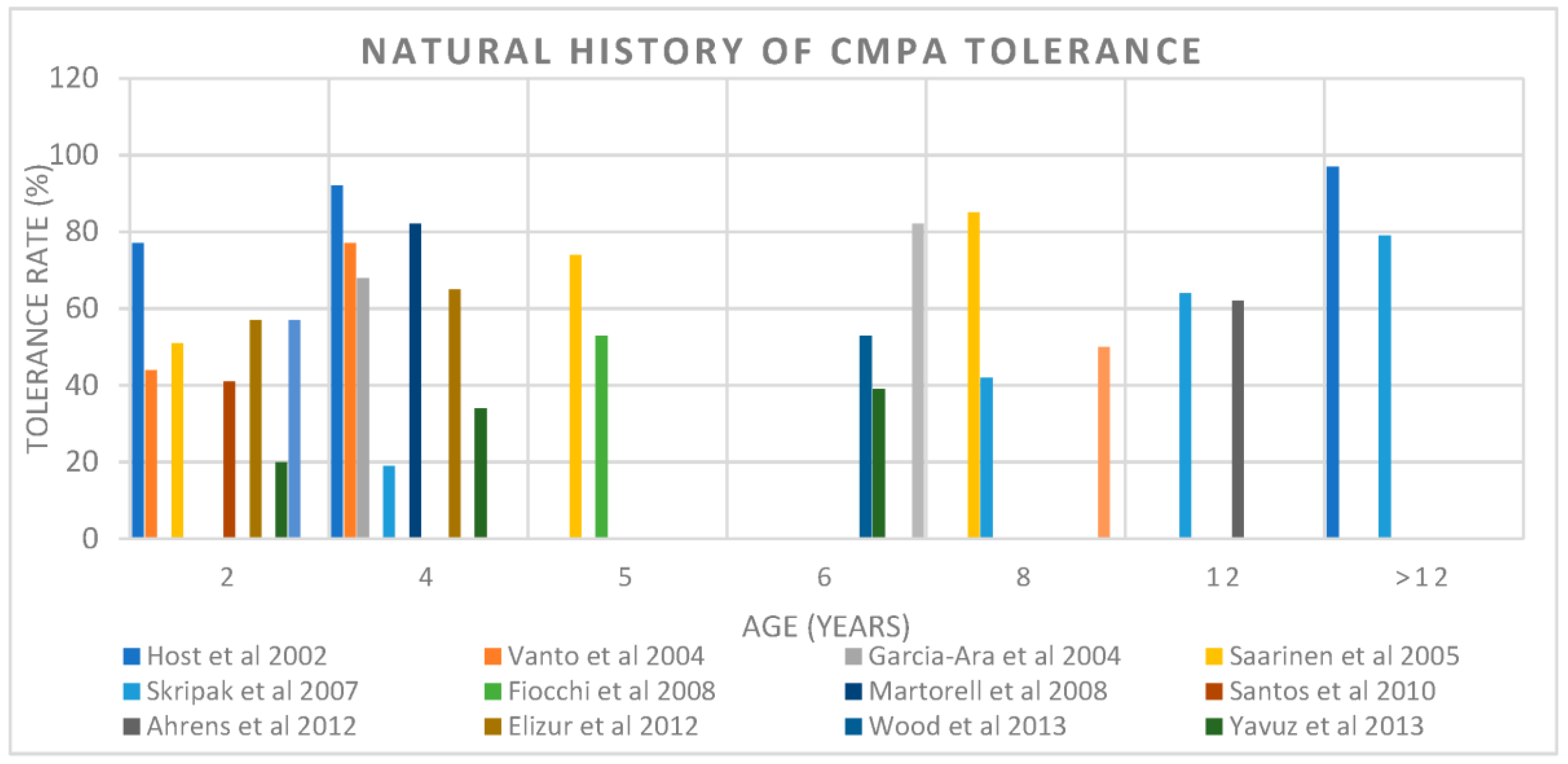
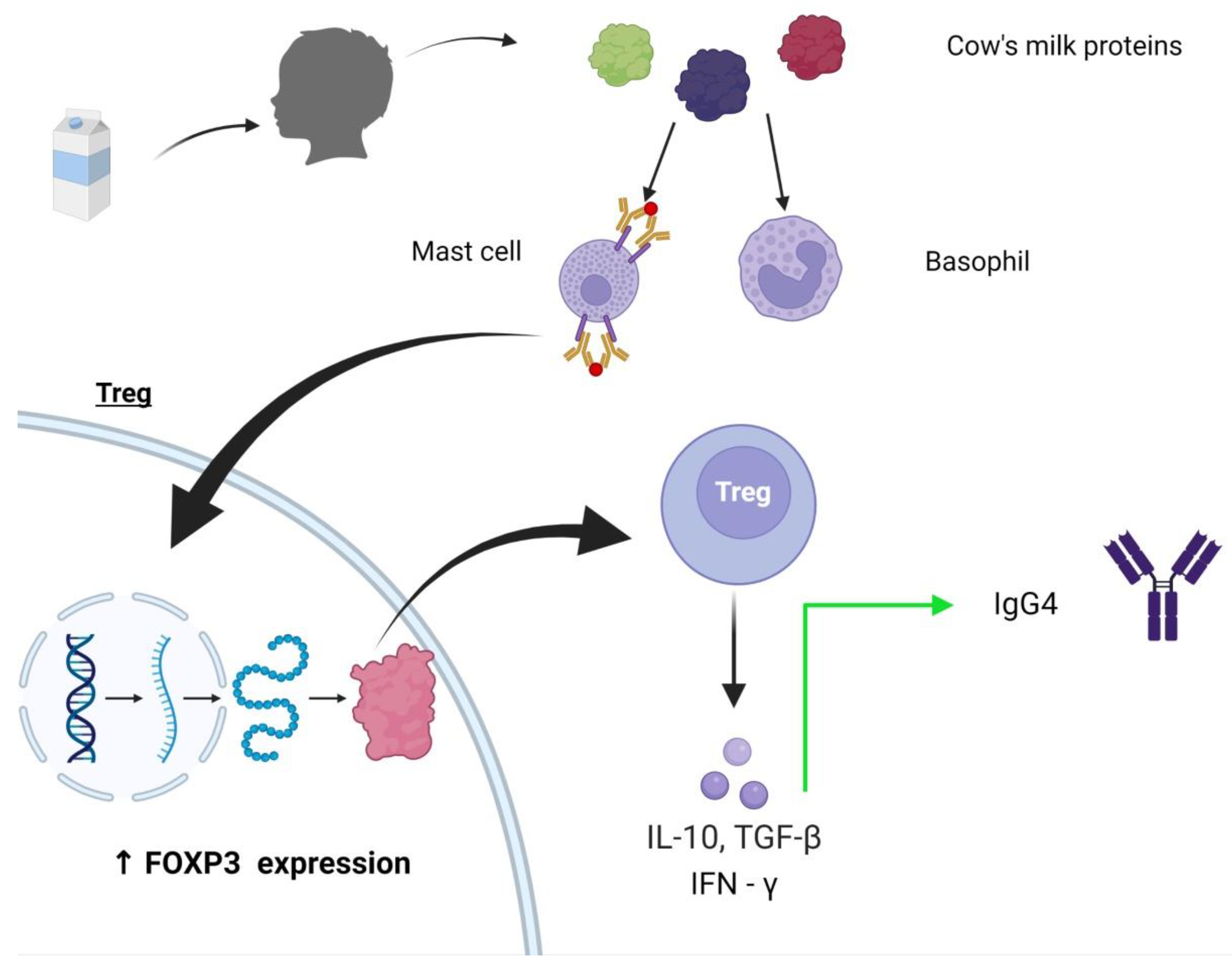
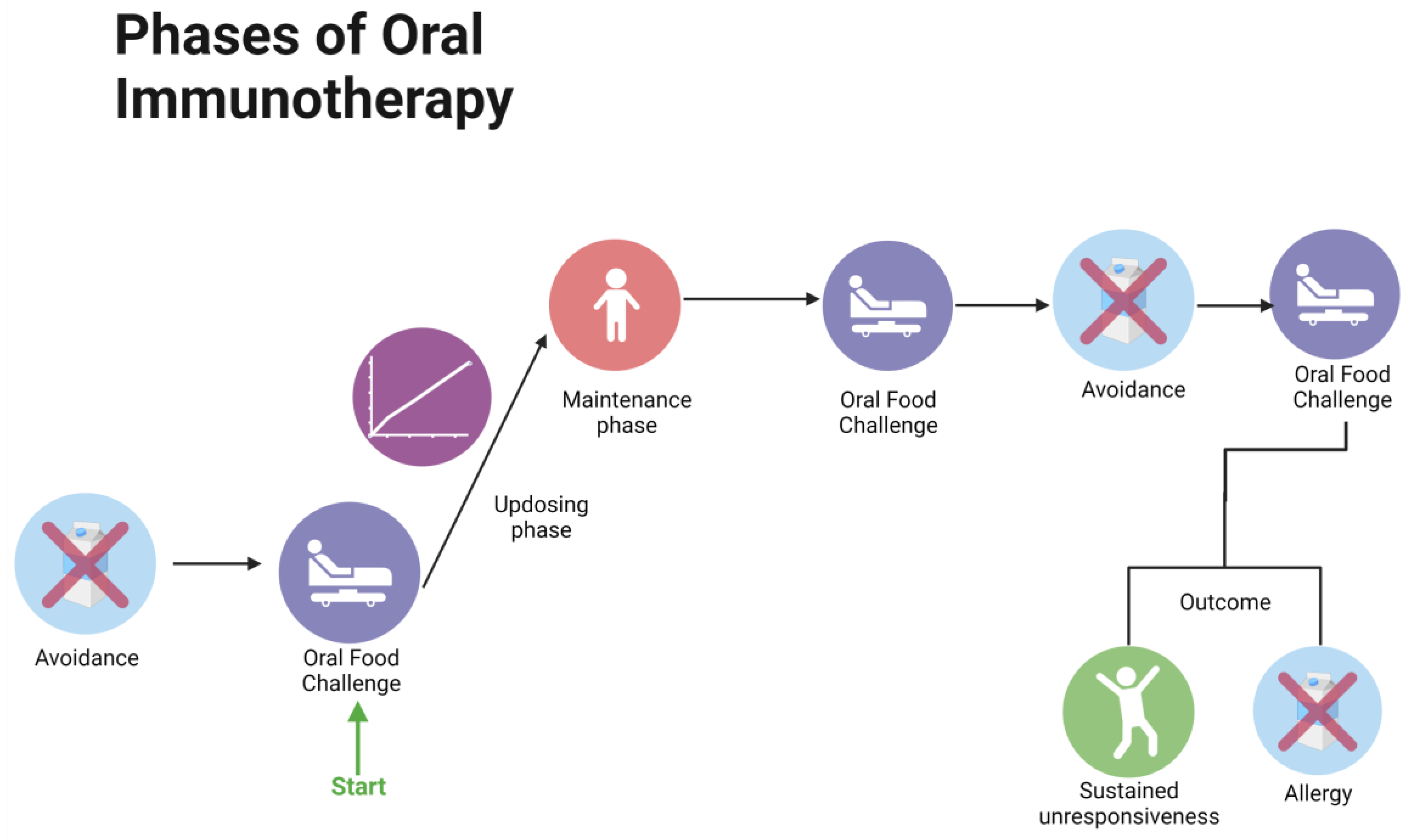
| Author, Year | Number of Subjects | Population/Study Design | Tolerance Rate | Age of Tolerance |
|---|---|---|---|---|
| Host et al., 2002 [18] | 39 (24 IgE mediated) | General prospective birth control | 56% | 1 |
| 77% | 2 | |||
| 87% | 3 | |||
| 92% | 5 | |||
| 97% | 15 | |||
| Vanto et al., 2004 [21] | 162 (95 IgE mediated) | Referral retrospective | 44% | 2 |
| 69% | 3 | |||
| 77% | 4 | |||
| Garcia-Ara et al., 2004 [22] | 66 IgE mediated | Referral retrospective | 68% | 4 |
| Saarinen et al., 2005 [23] | 118 (75 IgE mediated) | General prospective birth cohort | 51% | 2 |
| 74% | 5 | |||
| 85% | 8.6 | |||
| Skripak et al., 2007 [24] | 805 IgE mediated | Referral retrospective | 19% | 4 |
| 42% | 8 | |||
| 64% | 12 | |||
| 79% | 16 | |||
| Fiocchi et al., 2008 [25] | 112 IgE mediated | Referral retrospective | 52.7% | 5 |
| Martorell et al., 2008 [26] | 112 IgE mediated | Referral retrospective | 82% | 4 |
| Santos et al., 2010 [27] | 170 IgE mediated | Referral retrospective | 41% | 2 |
| Ahrens et al., 2012 [28] | 52 IgE mediated | Referral retrospective | 61.5% | 12 |
| Elizur et al., 2012 [29] | 54 IgE mediated | General prospective birth cohort | 57.4% | 2 |
| 65% | 4 | |||
| Wood F. et al., 2013 [30] | 293 IgE mediated | Prospective | 53% | 5.5 |
| Yavuz et al., 2013 [31] | 148 IgE mediated | Prospective | 20% | 2 |
| 34% | 4 | |||
| 39% | 6 | |||
| Schoemaker et al., 2015 [32] | 55 | EuroPrevall, European population-based prospective | 57% | 2 |
| Kim, M. et al., 2020 [33] | 189 IgE mediated | Retrospective | 50% | 8.7 |
| Chong, K. W. et al., 2022 [34] | 313 (IgE mediated) | Retrospective | 81.8% | 6 |
| Author, Country, Sample Size | Population | Intervention | Outcome Reported | Outcome |
|---|---|---|---|---|
| de Boissieu D. et al., 2002 [37] France 52 | Patients with CMPA allergy who do not respond to eHF, median age 5.3 months | Introduction of AAF. OFC to eHF every 6 months until 2 years of age. When the challenge indicated tolerance of eHF or in children above age 2 years, the challenge was performed with CMP once per year. | Growth (height and weight). OFC to eHF and CMP. | AAF was used for a mean duration of 11.4 ± 7.9 months (range, 3.5–41) and found to be safe in all cases. CMP tolerance occurred at <1 year of age in 8 children, 1 to 2 years in 22, 2 to 3 years in 8, and >3 years in 14. Eleven children did not tolerate CMP by the end of the survey period, the oldest being 5.5 years old. The median age of CMP tolerance was 20.5 months. |
| Canini, R.B. et al., 2017 [38] Italy 220 | Children with IgE-mediated CMA with a median age of 5.0 months (interquartile range, 3.0–8.0 months) | Children were randomly allocated to receive eHCF (n = 110) or eHCF containing the probiotic Lactobacillus rhamnosus GG (eHCF + LGG) (n = 110) groups and followed for 36 months. | Occurrence of at least 1 allergic manifestation (AM) (eczema, urticaria, asthma, or rhinoconjunctivitis). CM tolerance acquisition using an OFC at 12, 24, and 36 months. | The absolute risk difference for the occurrence of at least 1 AM over 36 months was 20.23 (95% CI, 20.36 to 20.10; p < 0.001). The absolute risk difference for the acquisition of cow’s milk tolerance was 0.20 (95% CI, 0.05–0.35; p < 0.01) at 12 months, 0.24 (95% CI, 0.08–0.41; p < 0.01) at 24 months, and 0.27 (95% CI, 0.11–0.43; p < 0.001) at 36 months. |
| Chatchatee, P. et al., 2022 [39] Germany, Italy, Singapore, Thailand, UK, USA 169 | Infants aged <13 months with confirmed IgE-mediated CMA | Subjects were randomized to receive AAF including synbiotics (AAF-S) (n = 80) or AAF (n = 89) for 12 months. | OFC to CM at 12 and 24 months. | At 12 and 24 months, respectively, 49% and 62% of subjects were CM tolerant (AAF-S 45% and 64%; AAF 52% and 59%), with no statistical difference between the groups. |
| Author, Country, Sample Size | Population | Intervention | Outcome Reported | Outcome |
|---|---|---|---|---|
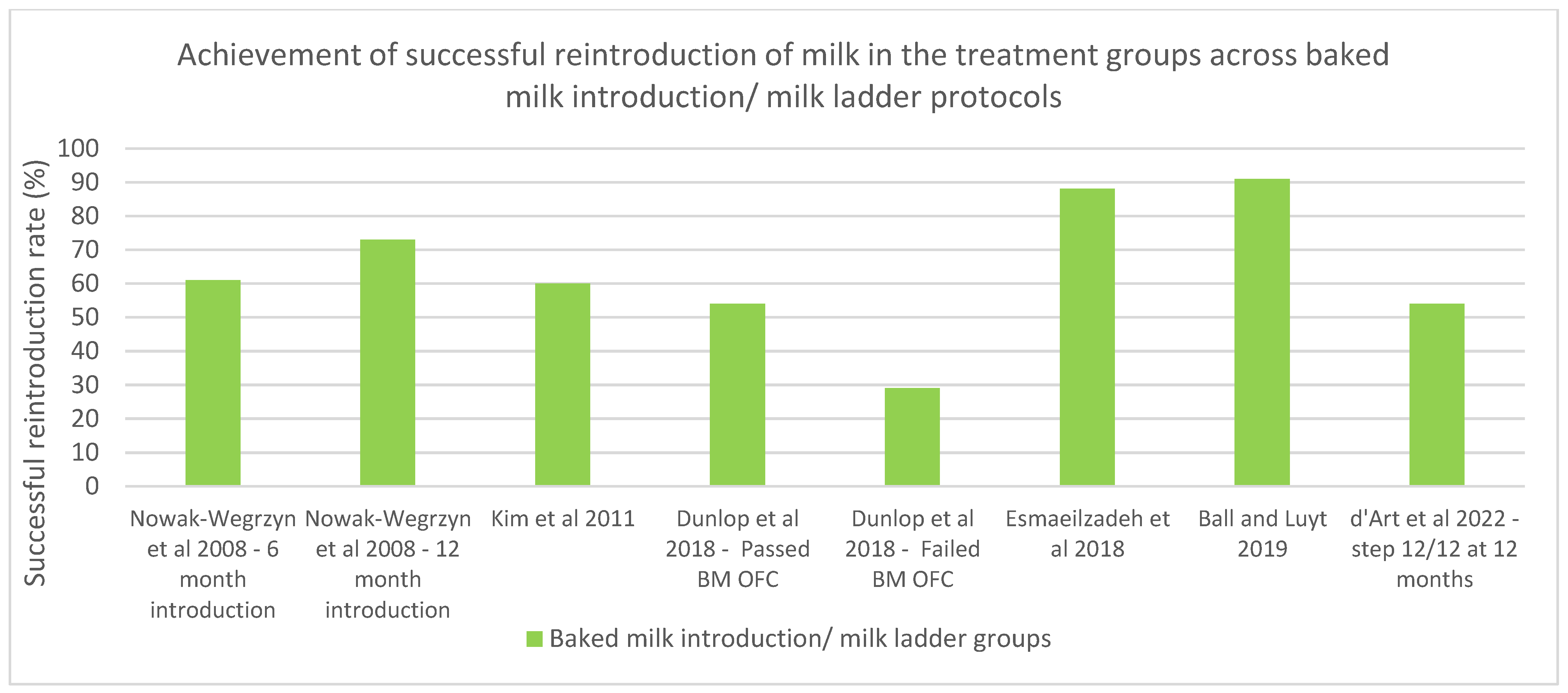
| Ball, H. and Luyt, D., 2019 [50] UK 86 | Children with IgE-mediated cow’s milk allergy presenting with skin and/or gastrointestinal symptoms and skin prick test < 8 mm | Home-based four-step milk ladder beginning with increasing doses of malt biscuits over 5 weeks. The following 3 steps can last from 4 to 6 months, with three formal clinical reviews occurring at 4–6-month intervals. | Tolerance was determined using a 7-scale scoring system based on the milk ladder (0 being no tolerance, 6 being normal diet). | At the final review, only eight patients of 86 were not tolerating almost all dairy products (≤level 4). |
| d’Art, Y. et al., 2022. [51] Ireland 60 | Infants less than 12 months old with suspected IgE-mediated cow’s milk allergy | Group 1: A single dose of fresh cow’s milk of elicited dose (ED05) (0.1 mL) prior to implementing the milk ladder. Group 2: Routine care. Both groups implemented graded exposure to CM (using the 12-step milk ladder) at home. | Milk ladder position (out of 12) at 6 months and 12 months post randomization. | Step 6 at 6 months: Group 1: 27/37 (73%) to Group 2; 10/20 (50%) (p = 0.048). Step 12 at 6 months: Group 1: 11/37 (30%) Group 2: 2/20 (10%) (p = 0.049). Step 12 at 12 months: Group 1: 24/37 (65%) Group 2: 7/20 (35%) (chi sq 4.7, p = 0.03) Cohort overall at 12 months: 47/57 (82%) on step 6 and 31/57 (54%) at step 12, at 12 months |
| Dunlop, J. et al., 2018 [45] USA 206 | Patients who underwent a baked milk OFC from 2009–2014 | Retrospective chart review of those who underwent a BM OFC and follow-up treatment. Those who passed the OFC were given instructions to begin a home-based introduction to BM to their diet or strict avoidance. Group 1: Passed BM OFC—BM introduction. Group 2: Failed BM OFC—BM introduction. Group 3: Failed BM OFC—strict avoidance. | Reported tolerance to baked milk (muffin), lesser baked milk (pancake or waffle), baked cheese, and direct milk at final follow-up. | Group 1: 54% were tolerating direct milk, and 19% were strictly avoiding milk. Group 2: 29% progressed to direct milk, and 38% were avoiding all milk products. Group 3: 10% progressed to direct milk, and 85% were strictly avoiding milk at final follow up. |
| Esmaeilzadeh, H. et al., 2018 [42] Iran 84 | Patients 6 months–3 years old with a history of IgE-mediated milk allergy who passed a BM OFC | Case group: Consumed baked milk in the form of muffin for 6 months and then consumed baked cheese in the form of pizza for another 6 months. Control group: Strict avoidance for 1 year. | After 1 year, both groups underwent unheated milk OFC to evaluate unheated milk tolerance at the end of the study. | Total of 88.1% (37/42) of the patients in the case group and 66.7% (28/42) of those in the control group had developed tolerance to unheated milk (p-value: 0.018). |
| Kim, J. et al., 2011. [40] USA 148 | Children aged 0.5–21 years with a diagnosed cow’s milk allergy: based on the initial baked milk oral challenge, subjects were categorized as baked milk reactive or baked milk tolerant. | Baked milk-reactive subjects avoided all forms of milk and were offered a repeat challenge ≥ 6 months from the initial challenge. Baked milk-tolerant subjects incorporated baked milk products daily into their diets and after ≥6 months were offered challenges to baked cheese products. | Baked milk-reactive subjects were offered a repeat baked milk challenge > 6 months from initial challenge. Baked milk-tolerant subjects were offered a challenge to baked cheese products > 6 months from initial challenge. Similarly, after ≥6 months, baked cheese-tolerant children were offered challenges to unheated milk. | Baked milk-tolerant subjects: 39 (60%) tolerated unheated milk, 18 (28%) tolerated baked milk/baked cheese, and 8 (12%) chose to avoid milk strictly. Baked milk-reactive subjects: 2 (9%) tolerated unheated milk, 3 (13%) tolerated baked milk/baked cheese, and the majority (78%) avoided milk strictly. Subjects who incorporated dietary baked milk were 16 times more likely than the comparison group to become unheated milk tolerant (p < 0.001). |
| Nowak-Wegrzyn, A. et al., 2008 [41] USA 91 | Individuals between the ages 0.5 and 21 years with diagnosed cow’s milk allergy | Baked milk-tolerant, unheated milk-reactive subjects ingested heated milk products for 3 months and were then re-evaluated. Unheated milk-tolerant subjects were instructed to add milk into the diet. Baked milk-reactive subjects were instructed strictly to avoid all forms of milk. | Follow-up SPT and serum-specific IgE and IgG4 to milk, casein, and b-lactoglobulin measured at baseline and at 3 months in baked milk-tolerant, unheated milk-reactive group. | Milk SPT: 8 mm (2.5–19) at baseline and 7 (2–10.5) at three months (p = 0.001). Casein IgG4 (mgA/L): 0.54 (0–8.1) at baseline and 1.02 (0.05–14.7) at 3 months (p = 0.005). |
| Nowak-Wegrzyn, A. et al., 2018. [44] USA 170 | Children 4 to 10 years of age with diagnosis of cow’s milk allergy | Group 1: Those reactive to baked milk followed strict milk avoidance. Group 2: Those non-reactive to baked milk tried more allergenic (less heat-denatured) forms of milk (MAFM) food challenges with up dosing every 6 months or every 12 months. Group 3: Those non-reactive to non-baked milk tried unlimited milk and dairy in the diet. Group 4: Comparison with strict avoidance. | Challenges were repeated at 6- or 12-month intervals over 36 months. | Group 1: 20% developed tolerance to baked milk, 0% tolerated non-baked milk. Group 2: 61% children in the 6-month and 73% in the 12-month escalation groups tolerated MAFM. Overall, 41 (48%) children who ingested a baked milk diet became tolerant to non-baked milk; no difference was seen between 6 vs. 12 months’ escalations. |
| Author, Country, Sample Size | Population | Intervention | Outcome Reported | Outcome |
|---|---|---|---|---|
| Reche et al., 2011 [81] Spain 20 | Patients with mean age of 3 months diagnosed with IgE-mediated CMPA | Case group: a protocol of oral induction tolerance. Control group: strict avoidance of cow’s milk. | Tolerance to cow’s milk and specific IgE evaluated 1 year after diagnosis. | Case group: All children tolerant to milk at 1 year. Control group: 3/10 were tolerant to milk after 1 year (p = 0.003). OTI protocol was completed in a mean of 5.3 months. |
| Lee et al., 2013 [82] Korea 31 | Infants 7 to 12 months old with challenge-proved IgE-mediated CMPA | Case group: oral desensitization. Control group: strict avoidance. | Oral food challenge, specific IgE, and IgG4 at 6 months. | Case group: 14/16 could accept daily doses of 200 mL of CM. Control group: All but 3 dropout patients receiving the elimination diet still showed allergic symptoms at the follow-up food challenge. |
| Berti et al., 2019 [65] Italy 68 | Children < 12 months of age with a clinical history of IgE-mediated CMPA and SPT and IgE suggestive of CMPA | Home immunotherapy protocol | Number of children were able to take a dose of 150 mL of CM without reactions. | Sixty-six infants (97%) reached the target of the protocol. The target of the protocol was achieved in a median time of 5.5 months (IQR: 4.5–7, range: 3.5–16). |
| Calvo et al., 2020 [64] Spain 335 | Infants under 1 year of age diagnosed with CMA-IgE | Initial dose was administered at home at least twice a day for a week. An in-hospital dose increase was scheduled every 7 days. | Number of children who reached a dose of 150–200 mL of infant formula. | Total of 67 patients in the OCT group (98.5%) and 262 (98.1%) in the FSD group completed the treatment. Median duration of immunotherapy was 106 days (range 27–269) in the OCT group and 77 days (range 77–254) in the FSD group, (p value 0.001). |
| 1909 | First recorded use of OIT in animals. | [61] |
| 2001 | Allergenic IgE and IgG antibodies for b- and k-casein and alpha(s1)-casein epitopes were identified, with higher levels of these IgE antibodies associated with persistent CMPA. | [17,78,83,84] |
| 2002 | A significant number of infants who consume AAF achieve tolerance by 20.5 months. | [37] |
| 2004 | Children aged 6 years or more with severe CMPA found to be tolerating a daily intake of cow’s milk following 6-month OIT protocol. | [68] |
| 2008 | Significantly smaller SPT mean wheal diameters and significantly greater casein-IgG4 concentrations were shown in CMPA patients who ingested baked milk products for 3 months. | [41] |
| 2011 | Subjects who incorporated dietary baked milk were more likely to become unheated milk tolerant. | [40] |
| Infants with mean age of 3 months with CMPA who underwent an OIT protocol became tolerant to milk. | [81] | |
| 2013 | Publication of Milk Allergy Primary (MAP) guideline in 2013 for non-IgE-mediated cow’s milk allergy. | [46] |
| 2017 | International version of milk ladder (iMAP) published. | [47] |
| Consumption of Lactobacillus rhamnosus probiotic with eHCF promotes tolerance to cow’s milk. | [38] | |
| 2018 | Clinical trial evidence showing improved tolerance to fresh milk following baked milk introduction compared to strict avoidance. | [42,44,45] |
| 2019 | First study to assess the effectiveness of the milk ladder in IgE-mediated CMPA, with most children completing the ladder and tolerating almost all dairy products. | [50] |
| 2020 | Large trial in infants under 1 year showing increased tolerance to cow’s milk following OIT protocol. | [64] |
| 2022 | Parental anxiety correlates with progression through the milk ladder. | [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cronin, C.; Ramesh, Y.; De Pieri, C.; Velasco, R.; Trujillo, J. ‘Early Introduction’ of Cow’s Milk for Children with IgE-Mediated Cow’s Milk Protein Allergy: A Review of Current and Emerging Approaches for CMPA Management. Nutrients 2023, 15, 1397. https://doi.org/10.3390/nu15061397
Cronin C, Ramesh Y, De Pieri C, Velasco R, Trujillo J. ‘Early Introduction’ of Cow’s Milk for Children with IgE-Mediated Cow’s Milk Protein Allergy: A Review of Current and Emerging Approaches for CMPA Management. Nutrients. 2023; 15(6):1397. https://doi.org/10.3390/nu15061397
Chicago/Turabian StyleCronin, Caoimhe, Yukta Ramesh, Carlo De Pieri, Roberto Velasco, and Juan Trujillo. 2023. "‘Early Introduction’ of Cow’s Milk for Children with IgE-Mediated Cow’s Milk Protein Allergy: A Review of Current and Emerging Approaches for CMPA Management" Nutrients 15, no. 6: 1397. https://doi.org/10.3390/nu15061397
APA StyleCronin, C., Ramesh, Y., De Pieri, C., Velasco, R., & Trujillo, J. (2023). ‘Early Introduction’ of Cow’s Milk for Children with IgE-Mediated Cow’s Milk Protein Allergy: A Review of Current and Emerging Approaches for CMPA Management. Nutrients, 15(6), 1397. https://doi.org/10.3390/nu15061397