Effect of Maternal Diet on Maternal Milk and Breastfed Infant Gut Microbiomes: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Identifying the Research Question
2.3. Search Strategy and Eligibility Criteria
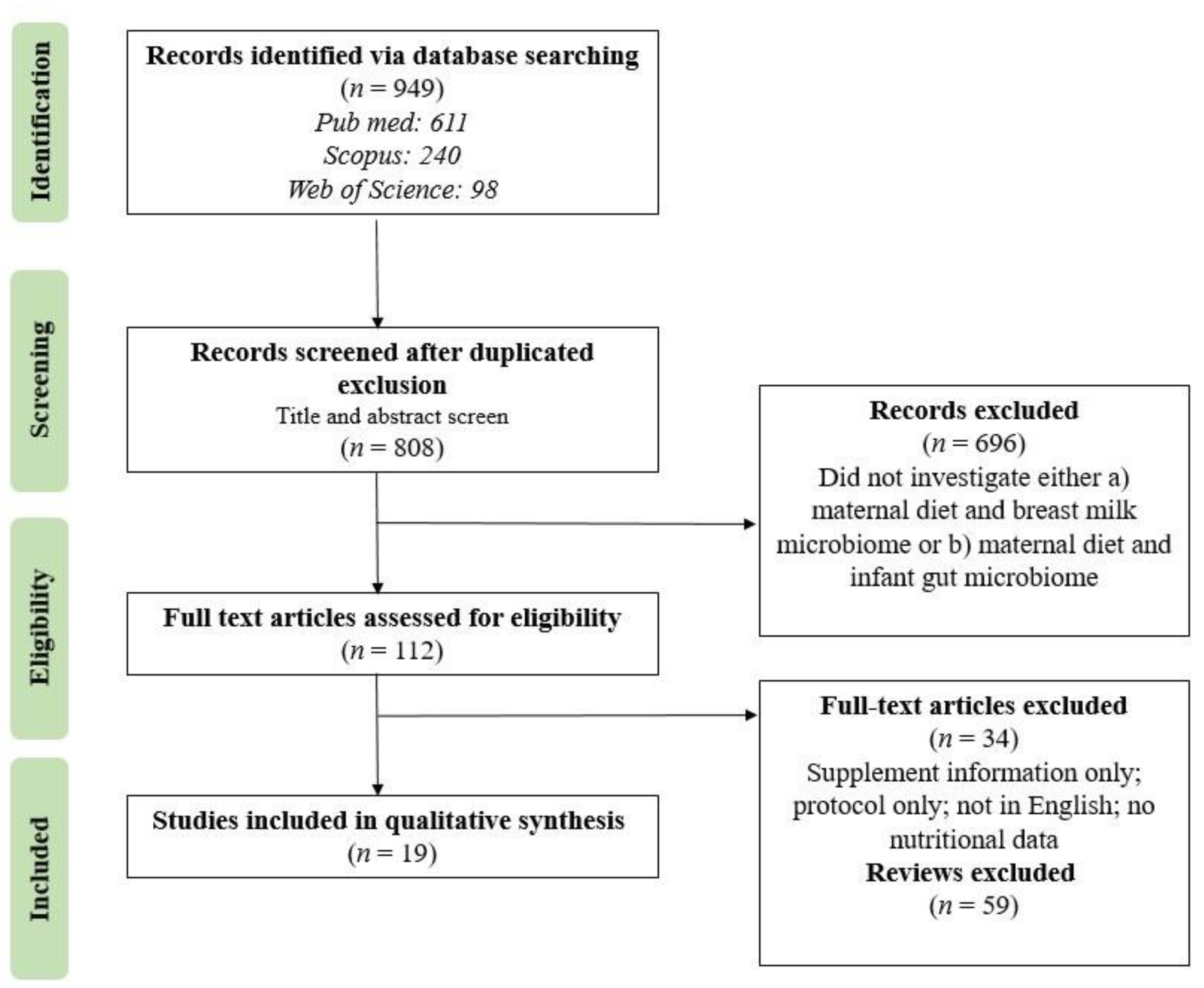
3. Results
3.1. Synthesis
3.2. Analysis of Methodologies
3.2.1. Sampling Procedures
3.2.2. Microbiome Analysis
3.2.3. Nutrition Assessment
3.2.4. Infant Feeding Assessment
4. Discussion
4.1. What Impact Does Maternal Diet Have on the Milk Microbiome?
4.2. What Impact Does Maternal Diet Have on the Breastfed Infant Gut Microbiome?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, K. The Composition of Human Milk and Infant Faecal Microbiota Over the First Three Months of Life: A Pilot Study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Järvinen, K.M.; Martin, H.; Oyoshi, M.K. Immunomodulatory effects of breast milk on food allergy. Ann. Allergy Asthma Immunol. 2019, 123, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Mantziari, A.; Rautava, S. Factors influencing the microbial composition of human milk. Semin. Perinatol. 2021, 45, 151507. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Babakobi, M.D.; Reshef, L.; Gihaz, S.; Belgorodsky, B.; Fishman, A.; Bujanover, Y.; Gophna, U. Effect of Maternal Diet and Milk Lipid Composition on the Infant Gut and Maternal Milk Microbiomes. Nutrients 2020, 12, 2539. [Google Scholar] [CrossRef]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Cortes-Macías, E.; Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; González, S.; Martínez-Costa, C.; Collado, M.C. Maternal Diet Shapes the Breast Milk Microbiota Composition and Diversity: Impact of Mode of Delivery and Antibiotic Exposure. J. Nutr. 2021, 151, 330–340. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; García-Villalba, R.; García-Mantrana, I.; Rodríguez-Varela, A.; Romo-Vaquero, M.; Collado, M.C.; Tomás-Barberán, F.A.; Espín, J.C.; Selma, M.V. Urolithins in Human Breast Milk after Walnut Intake and Kinetics of Gordonibacter Colonization in Newly Born: The Role of Mothers’ Urolithin Metabotypes. J. Agric. Food Chem. 2020, 68, 12606–12616. [Google Scholar] [CrossRef]
- Fan, H.; Tung, Y.; Yang, Y.; Hsu, J.; Lee, C.; Chang, T.; Su, E.; Hsieh, R.; Chen, Y. Maternal Vegetable and Fruit Consumption during Pregnancy and Its Effects on Infant Gut Microbiome. Nutrients 2021, 13, 1559. [Google Scholar] [CrossRef]
- LeMay-Nedjelski, L.; Asbury, M.; Butcher, J.; Ley, S.; Hanley, A.; Kiss, A.; Unger, S.; Copeland, J.; Wang, P.; Stintzi, A.; et al. Maternal Diet and Infant Feeding Practices Are Associated with Variation in the Human Milk Microbiota at 3 Months Postpartum in a Cohort of Women with High Rates of Gestational Glucose Intolerance. J. Nutr. 2021, 151, 320–329. [Google Scholar] [CrossRef]
- Lundgren, S.N.; Madan, J.C.; Emond, J.A.; Morrison, H.G.; Christensen, B.C.; Karagas, M.R.; Hoen, A.G. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilha, M.; Danneskiold-Samsoe, N.; Brejnrod, A.; Hoffmann, C.; Cabral, V.; Iaucci, J.; Sales, C.; Fisberg, R.; Cortez, R.; Brix, S.; et al. The Human Milk Microbiota is Modulated by Maternal Diet. Microorganisms 2019, 7, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quin, C.; Vicaretti, S.D.; Mohtarudin, N.A.; Garner, A.M.; Vollman, D.M.; Gibson, D.L.; Zandberg, W.F. Influence of sulfonated and diet-derived human milk oligosaccharides on the infant microbiome and immune markers. J. Biol. Chem. 2020, 295, 4035–4048. [Google Scholar] [CrossRef]
- Sakwinska, O.; Moine, D.; Delley, M.; Combremont, S.; Rezzonico, E.; Descombes, P.; Vinyes-Pares, G.; Zhang, Y.; Wang, P.; Thakkar, S.K. Microbiota in Breast Milk of Chinese Lactating Mothers. PLoS ONE 2016, 11, e0160856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenker, N.; Perdones-Montero, A.; Burke, A.; Stickland, S.; McDonald, J.; Alexander-Hardiman, K.; Flanagan, J.; Takats, Z.; Cameron, S. Metabolomic and Metataxonomic Fingerprinting of Human Milk Suggests Compositional Stability over a Natural Term of Breastfeeding to 24 Months. Nutrients 2020, 12, 3450. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, T.; Wu, Y.; Liu, Y.; Zou, Z.; Bai, J. Impacts of maternal diet and alcohol consumption during pregnancy on maternal and infant gut microbiota. Biomolecules 2021, 11, 369. [Google Scholar] [CrossRef]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; et al. Human Milk Microbial Community Structure Is Relatively Stable and Related to Variations in Macronutrient and Micronutrient Intakes in Healthy Lactating Women. J. Nutr. 2017, 147, 1739–1748. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Enos, M.K.; PrayGod, G.; Seney, S.; Macklaim, J.M.; Chilton, S.; Willner, D.; Knight, R.; Fusch, C.; Fusch, G.; et al. Microbiota at Multiple Body Sites during Pregnancy in a Rural Tanzanian Population and Effects of Moringa-Supplemented Probiotic Yogurt. Appl. Environ. Microbiol. 2015, 81, 4965–4975. [Google Scholar] [CrossRef] [Green Version]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.E.; Lange, N.E.; Zhou, Y.; O’Connor, G.T.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. Diet during Pregnancy and Infancy and the Infant Intestinal Microbiome. J. Pediatr. 2018, 203, 47–54.e4. [Google Scholar] [CrossRef]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020, 10, 22092. [Google Scholar] [CrossRef] [PubMed]
- Urwin, H.J.; Miles, E.A.; Noakes, P.S.; Kremmyda, L.-S.; Vlachava, M.; Diaper, N.D.; Godfrey, K.M.; Calder, P.C.; Vulevic, J.; Yaqoob, P. Effect of salmon consumption during pregnancy on maternal and infant faecal microbiota, secretory IgA and calprotectin. Br. J. Nutr. 2014, 111, 773–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laforest-Lapointe, I.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; Sears, M.R.; Subbarao, P.; Sycuro, L.K.; Azad, M.B.; Arrieta, M.-C. Maternal consumption of artificially sweetened beverages during pregnancy is associated with infant gut microbiota and metabolic modifications and increased infant body mass index. Gut Microbes 2021, 13, 1857513. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Mantrana, I.; Alcántara, C.; Selma-Royo, M.; Boix-Amorós, A.; Dzidic, M.; Gimeno-Alcañiz, J.; Úbeda-Sansano, I.; Sorribes-Monrabal, I.; Escuriet, R.; Gil-Raga, F.; et al. MAMI: A birth cohort focused on maternal-infant microbiota during early life. BMC Pediatr. 2019, 19, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Database | Document Type Included |
|---|---|
| Web of Science | Abstract, Article, Case report, Correction, Early Access, Retraction, Review |
| Scopus | Article, Review, Short survey, Erratum |
| PubMed | Case Reports, Classical Article, Clinical Study, Clinical Trial Protocol, Clinical Trial, Phase I, II, III, IV, Controlled Clinical Trial, Corrected and Republished article, Journal Article, Letter, Multicenter Study, Preprint, Published Erratum Research support, Research Support, NIH, Extramural Research Support, NIH, Intramural Research Support, Non-US Gov’t Research Support, US Gov’t, Non-PHS Research Support, US Gov’t, PHS Research Support, US Gov’t Retracted Publication Retraction of Publication, Technical Report, Twin study, Validation Study |
| Reference | Exposure Variables | Study Design | Population & Sample Number | Maternal Nutritional Assessment | Milk | Stool Samples | Microbiome Analysis | Main Relevant Finding |
|---|---|---|---|---|---|---|---|---|
| Babakobi et al., 2020 [5] | Maternal nutrition during pregnancy and 3 months postpartum | cohort study | n = 22 mother– infant dyads | food frequency questionnaire (FFQ) | 1 week 1 month 3 months postpartum (pp) | Infant: 1 week 1 month 3 months | Power Soil DNA Kit (MoBio) 16S rRNA regions V3 and V4 (341F/806R) MiSeq (Illumina) PEAR database | Negative correlation between maternal unsaturated fat consumption and Streptococcus in milk at one month. No significant associations between maternal diet and infant gut microbiome. |
| Bisanz et al., 2015 [19] | Probiotic yogurt: Lactobacillus rhamnosus, plus ground Moringa olifera | randomized clinical trial (RCT) | n = 56 mother– infant dyads | Dietary recalls | 3 days pp and 1 week to 1 month after first sample | Infant & Maternal: 3 days pp and 1 week to 1 month after first sample | Power Soil DNA Kit (Mobio) 16S rRNA V4 (515F/806R) MiSeq (Illumina) Greengenes | Maternal yogurt consumption did not alter milk microbiome but was associated with changes in the infant gut microbiome: increase in Bifidobacterium and decrease in Enterobacteriaceae. |
| Chu et al., 2016 [6] | Maternal high-fat diet during gestation and lactation | cohort study | n = 163 mother– infant dyads | rapid dietary questionnaire | No | Infant: meconium and stool at 4–6 weeks of age | Power Soil DNA Kit (MoBio) 16S rRNA, V3-V5 Pyrosequencing with 454-FLX Titanium QIIME | Maternal consumption of a high-fat diet during gestation was associated with a depletion of Bacteriodes in meconium that persisted at 6 weeks. |
| Cortes-Macías et al., 2021 [7] | Maternal diet, mode of delivery, and antibiotic exposure | cohort study | n = 120 lactating women | FFQ | 7–15 days pp | No | MasterPure DNA Extraction Kit (Epicentre) 16S rRNA regions V3-V4 SILVA database | Identified significant associations between dietary nutrients and specific breast milk microbial genera (2 clusters). |
| Cortés-Martín et al., 2020 [8] | Walnut intake (30g daily for 3 days) | cohort study | pilot study: n = 11 full study: n = 30 mother– infant dyads | 30 g walnuts/day dietary intervention | pilot: 2 weeks to 24 months pp full study: within 1 year pp | Infant: within 1 year of birth | Milk:MasterPure Complete DNA & RNA Purification Kit; Infant stool: Nucleospin tissue DNA purification kit 16S rRNA qPCR for Gordonibacter; ABI 7500 real-time PCR System | Colonization of the infant gut with Gordonibacter occurred during the first year and was not dependent on breastfeeding but was influenced by maternal urolithin metabotype |
| Fan et al., 2021 [9] | High or low fruit and vegetable gestational intake | cohort study | n = 39 mother– infant dyads | 3-day dietary record | No | Infant: 2 months of age | Qiagen DNA Mini kit 16S rRNA V3–V4 (341F/805R) MiSeq 2000 SILVA database | Infant gut microbiome clustered differently for high & low maternal fruit and vegetable consumption. Higher maternal intake of fructose, dietary fiber, folic acid, and ascorbic acid negatively associated with unhealthy infant gut microbiome. |
| Laforest-Lapointe et al., 2021 [23] | Maternal consumption of artificially sweetened beverages (ASB) | case-control study | n = 100 mother– infant dyads | reported ASB consumption | No | Infant: 3 and 12 months of age | DNeasy Power Soil Kit (Qiagen) 16S rRNA V4 (F515/R806) MiSeq | Maternal ASB consumption was associated with higher infant BMI, and infant BMI was associated with the microbiome composition at 12 months, but not at 3 months of age. Estimated impact of ASB consumption on the infant microbiome was notably smaller than other known drivers (breastfeeding, birth mode, ethnicity, infant age, and antibiotics). |
| LeMay-Nedjelski et al., 2021 [10] | Maternal diet and infant feeding practices | cohort study | n = 93 lactating women | FFQ | 3 months postpartum | No | NucleoSpin Fod DNA Isolation kit (macherey-Nagel) 16S rRNA V4 (515F/805R) | Maternal intake of polyunsaturated fat and fiber was associated with increased alpha diversity in milk microbiota at 3 months pp. Infant feeding practices were associated with milk microbiome. |
| Lundgren et al., 2018 [11] | Maternal diet during pregnancy | cohort study | n = 145 mother– infant dyads | FFQ | No | Infant: 6 weeks of age | Zymo DNA Extraction kit 16S rRNA V4–V5 | Identified three clusters of the infant gut microbiome in vaginally delivered infants and caesarean delivered infants. Cluster 2 (high abundance of Streptococcus and Clostridium) was associated with maternal fruit consumption. |
| Moossavi et al., 2019 [12] | Maternal and infant early-life factors | cohort study | n = 393 mother– infant dyads | FFQ | 3–4 months pp | No | Quick-DNA Fungal/Bacterial extraction kit 16S rRNA V4 (515F/806R) MiSeq | Factor analysis showed that maternal diet influences BMI, which indirectly affects milk microbiota by altering factors in milk. Mode of breastfeeding was associated with breast milk microbiota composition. Maternal BMI and parity associated with milk microbiota in a sex-specific manner. |
| Padilha et al., 2019 [13] | Maternal diet during pregnancy and first month of lactation | cohort study | n = 94 lactating women | quantitative FFQ | ~30 days pp | No | QIAmp DNA Mini kit 1Nested PCR 16s rDNA Nested PCR 1st (341F/806R) 2nd (515F/806R) MiSeq | Vitamin C intake during pregnancy was correlated with the presence of Staphylococcus genus. Intake of PUFAs and linoleic acid during lactation were correlated with Bifidobacterium. |
| Quin et al., 2020 [14] | Maternal diet & diet-induced HMO alterations | prospective cohort study | n = 109 mother– infant dyads | 24-h dietary recalls | 5 months pp | Infant: 5 months of age | QIAMP DNA Stool Mini kit 16S rRNA V3–V4 (341F/805R) MiSeq | Maternal diet affected HMOs in breast milk. HMOs influenced the infant microbiome composition. Maternal secretory status also had a modest effect on infant microbiome. |
| Sakwinska et al., 2016 [15] | Lactation stage & aseptic vs. non-aseptic collection | cohort study | n = 90 lactating women | lifestyle questionnaire | 0–4 days 5–11 days 2 months postpartum | No | DNA Stool Mini kit (Qiagen) Or Fast DNA SPIN kit for soil (MoBio) 16S rRNA V4 | Confirmed the presence of the dominant species in breast milk such as Streptococci and Staphylococci & low abundance of Bifidobacteria and Lactobacilli. Results suggest that the microbiota of milk from Chinese lactating mothers is similar to that observed from other geographic locations. |
| Savage et al., 2018 [20] | Diet during pregnancy & infancy (breastfed versus formula) | RCT | n = 323 mother– infant dyads | FFQ | No | Infant: 3–6 months of age | 16S rRNA | No significant association between maternal diet during pregnancy and infant microbiome. Infant’s diet impacted infant gut microbiome. |
| Seferovic et al., 2020 [21] | Maternal carbohydrate (glucose/ galactose) & energy sources (high carb/high fat) | crossover study | n = 14 lactating women; 7 per intervention | Two crossover dietary interventions: Glu/Gal & Carb/Fat | 8–11 weeks pp | No | WGS sequencing | Short-term diet of 30–57 h altered HMOs, which in turn altered the microbial gene expression. |
| Shenker et al., 2020 [16] | Self-reported lifestyle and dietary factors | cohort study | n = 62 mother– infant dyads | self-reported intake | 3–48 months pp | No | 16S rRNA | Maternal intake of alcohol and soy were correlated with 11 and 12 taxonomic features, respectively. Intake of folic acid correlated Anaerococcus. Results suggest stability in human milk composition for up to 24 months. |
| Urwin et al., 2013 [22] | Maternal consumption of 150 g of salmon twice per week during pregnancy | RCT | n = 123 mother– infant dyads | diet intervention, two 150 g portions of salmon/week from week 20 gestation to delivery | No | Infant: 7, 14, 28, and 84 days of age Maternal: 38 weeks gestation | Five 16S rRNA probes to genus-specific microbes Fluorescence in situ hybridization | Increased salmon consumption during pregnancy had no significant effects on maternal microbiome. Salmon consumption during pregnancy was associated with lower abundance of the Atopobium cluster in stool of infants in the first 84 days postpartum, especially those who were not exclusively breastfed. |
| Wang et al., 2021 [17] | Maternal diet and alcohol consumption during pregnancy | cohort study | n = 29 mother– infant dyads | alcohol consumption questionnaire | No | Infant: within 48 h after birth; Maternal: late pregnancy | E.Z.N.A. soil DNA kit (Omega Bio-tek) 16S rRNA V3-V4 (338F/806R) MiSeq SILVA | Gut alpha diversity differed between alcohol & no alcohol groups. Pregnant mothers who consumed alcohol had higher diversity, and similar results were shown in newborns. Maternal dietary intake of meat, eggs, and soybean products during pregnancy were associated with the infant gut microbiota. |
| Williams et al., 2017 [18] | Maternal nutrient intake, time postpartum, delivery mode, and BMI (kg/m2) | cohort study | n = 21 lactating women | 24-h dietary recalls | 2 to 6 months pp | No | Enzymatic lysis & physical disruption, QIAmp DNA Mini Kit (Qiagen) 16S rRNA V1–V3 (F27/R534) MiSeq Ribosomal Database Project | Maternal consumption of thiamin, niacin, folate, and vit. B-6, and chromium was negatively associated with Lactobacillus in milk. Positive association between a nutrient-rich diet and maternal intake of various fatty acids with Proteobacteria in milk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, R.; Keane, D.; Borrego, P.; Arcaro, K. Effect of Maternal Diet on Maternal Milk and Breastfed Infant Gut Microbiomes: A Scoping Review. Nutrients 2023, 15, 1420. https://doi.org/10.3390/nu15061420
Taylor R, Keane D, Borrego P, Arcaro K. Effect of Maternal Diet on Maternal Milk and Breastfed Infant Gut Microbiomes: A Scoping Review. Nutrients. 2023; 15(6):1420. https://doi.org/10.3390/nu15061420
Chicago/Turabian StyleTaylor, Rachel, Deirdre Keane, Paulina Borrego, and Kathleen Arcaro. 2023. "Effect of Maternal Diet on Maternal Milk and Breastfed Infant Gut Microbiomes: A Scoping Review" Nutrients 15, no. 6: 1420. https://doi.org/10.3390/nu15061420






