The Influence of Dietary Interventions on Arterial Stiffness in Overweight and Obese Subjects
Abstract
:1. Introduction
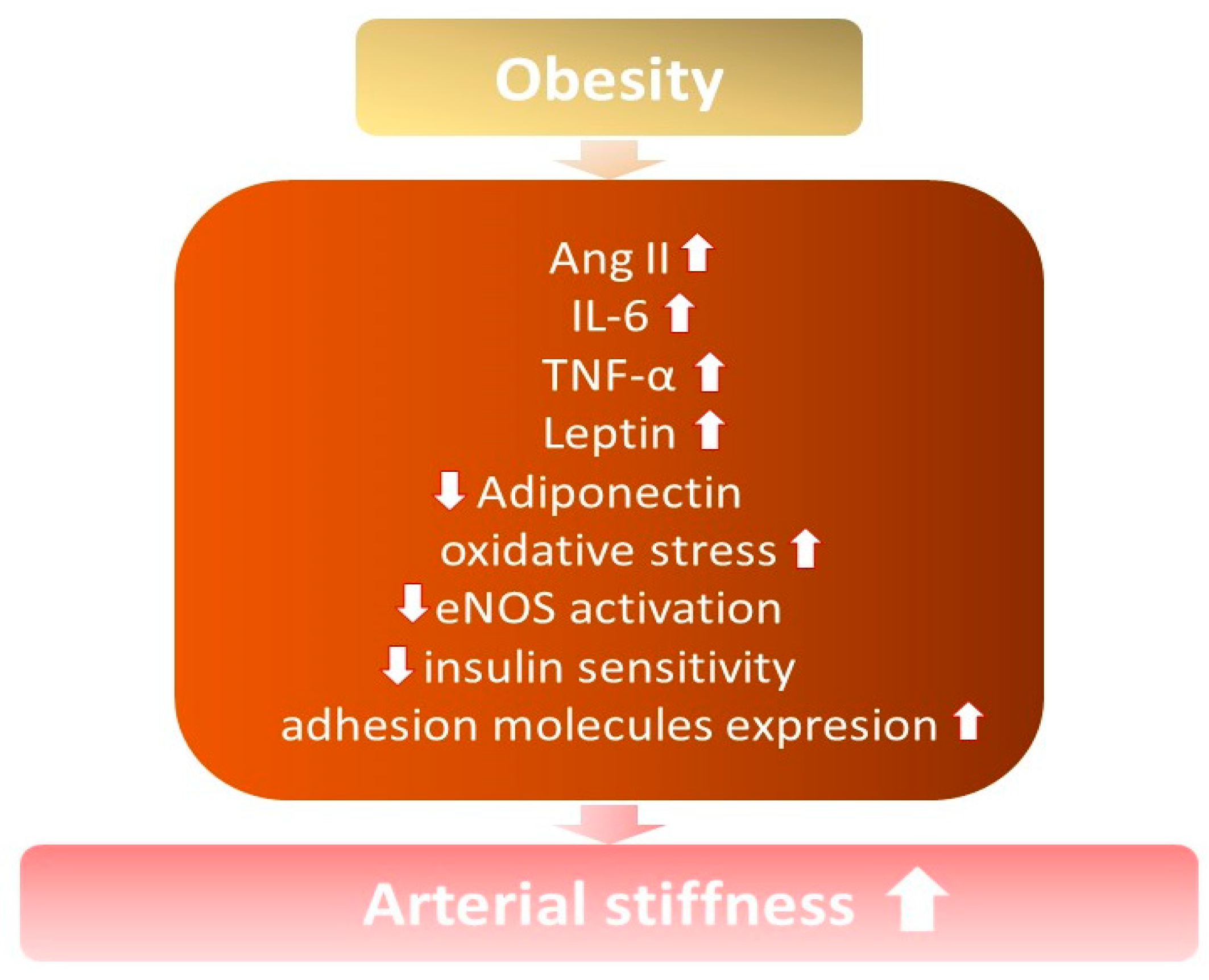
2. The relationship between Obesity, Oxidative Stress, and Inflammation
3. Arterial Stiffness among Overweight and Obese Subjects
3.1. Inflammatory Mediators
3.2. Hyperinsulinemia
3.3. Renin-Angiotensin-Aldosterone System Activation
3.4. Adipocyte-Derived Factors
4. Influence of Body Mass Reduction on Arterial Stiffness in Overweight and Obese Subjects
5. The Impact of Diet on Arterial Stiffness
5.1. Caloric Restriction
5.2. Fat and Fatty Acids Influence Arterial Stiffness
| Nutrients Influence Arterial Stiffness | |||
|---|---|---|---|
| Component | Dietary Modification | Health Effect/Influence on Arterial Stiffness | References |
| Fat | high-fat and high-sucrose diet (animal studies) | ↑ obesity ↑ arterial stiffness | [64] |
| ↑ SFA in the diet | ↑ arterial stiffness | [67] | |
| high SFA intake | ↑ baPWV at baseline and at 2 and 5 years (diabetic patients) | [74] | |
| substitution of 9.5–9.6% total energy dietary SFAs with either MUFA or n-6 PUFA | no significant effect on the flow-mediated dilatation beneficial effects on biomarkers of arterial stiffness: ↓E-selectin, ↓ fasting serum lipids (CH. LDL, and CH/HDL ratio) | [79] | |
| SFA replacement with MUFA and carbohydrates | ↓ arterial pulse pressure | [70] | |
| fish oils consumption with high EPA and DHA in diabetic hypertensive patients | ↓ blood pressure ↑ endothelium-dependent vasodilation | [70] | |
| a habitual large amount of fish consumption | ↓ arterial stiffness ↓ pulse wave velocity of the aorta ↓ intima-media thickness of the carotid artery ↓ atherosclerotic plaques | [84] | |
| the 25% energy deficit diet, including 4 g/d of EFA (46% EPA and 38% DHA) in obese patients | ↑ large and small artery elasticity (about 20% increase) | [86] | |
| Protein | vegetarian diet vs. omnivore control | ↓ PWV (−8%) in healthy male vegetarians vs. omnivore control sample (7.1 ± 0.8 and 7.7 ± 0.9 m/s, respectively) | [90] |
| vegetarian diet vs. omnivore control | a tendency to ↑ arterial stiffness in omnivores (mainly male) vs. vegetarians (PWV 7.0 ± 1.5 vs. 6.8 ± 1.1 m/s, respectively) limited effect in premenopausal women | [91] | |
| dairy products | inverse correlation with PWV | [92] | |
| milk consumption | ↓SBP (inverse dose-dependent effect) not associated with arterial stiffness | [93] | |
| dairy products (milk, cheese, cream excluding butter) cohort prospective study (22.8 years observation) | high consumption of dairy products ≥ ↓ 1.8% A. no detrimental to arterial stiffness and metabolic markers (insulin, TG, CH) | [93] | |
| casein-derived biologically active tripeptides in dairy products | ↓ angiotensin formation ≥ ↓ HA risk ≥ ↓ arterial stiffness | [94] | |
| dairy products containing ruminant-derived fatty acids (14:0, 15:0, 16:0, 17:0, and 17:1) | no influence on serum lipoproteins, PWV, central blood pressure, and AI. | [95] | |
| Carbohydrate | postprandial glucose in non-diabetic patients | ↑ postprandial glucose concentration ≥ ↑ arterial stiffness with age (measured by CAVIs) postprandial glycemia is an independent predictor of CAVI values in men and women over 50 years old | [96] |
| The high-sucrose diet in diabetic patients with kidney impairments (albuminuria) | postprandial hyperglycemia ≥ ↑ brachial PWV of intermediate-sized arteries (30 min before breakfast and up to 240 min after breakfast) | [94] | |
| low-carbohydrate diets | ↓ serum glucose and lipid level and ↓ aortic stiffness within a short time (four weeks) ≥ ↓ CVD and ↓ diabetes risk | [98,99,100] | |
| dietary carbohydrate restriction (about 645 kcal/day energy deficit) | ↓ body mass, glucose, and lipids ↓ arterial stiffness (↓PWV, significant only in women: from 7.2 ± 03 m/s to 6.3 ± 0.3 m/s, p = 0.028) | [100] | |
| low-GI diet (breakfast) in young, healthy adults compared to high-GI food | ↓ arterial stiffness compared to high-GI food high-GI products ≥ ↑ AI and heart rate (but stratifying data by gender, this interaction remained significant for AI only in males) | [101] | |
| high-GI diet | ↑ arterial stiffness immediately after food intake | [96] | |
| ~600 kcal breakfast including fiber with various GI (fiber amount~4 vs. 20 g and GI~44 vs. 70) | high-fiber diet with low-GI ≥ ↑ FMD four hours after meal ingestion | [102] | |
| A diet containing isomaltulose vs. sucrose glucose | 25 g of isomaltulose consumption ≥ stable baPWV in 30, 60, and 90 min after ingestion compared to the state before ingestion in healthy middle-aged and older adults 25 g sucrose intake ≥ ↑ baPWV 25 g glucose ≥ ↑ baPWV at 30, 60, and 90 min after ingestion and ↑ CAVI at 60 min after glucose intake | [103,104] | |
| Elements | sodium intake: low sodium <6 g, medium 6–10 g, and high >10 g sodium daily intake on arterial stiffness. | high sodium intake (>10 g/day) ≥ ↑ arterial stiffness (baPWV ≥1400 cm/s) | [105] |
| high sodium in normotensive subjects | AIx | [106] | |
| ↓ sodium intake | ↓ blood pressure + ↓ arterial stiffness (independently of antihypertensive effects) | [107] | |
| low-salt diet | was proved (meta-analysis) ≥ a reduction of 89.3 mmol/day in sodium intake in different populations is associated with a 2.84% reduction in PWV | [108] | |
| two weeks of dietary sodium restriction in older adults | ↓ arterial stiffness (rapid improvement of large elastic artery compliance and AIx) ≥ ↓ systolic hypertension | [109] | |
| Salt restriction for 6 weeks is untreated in patients with mildly raised blood pressure | ↓carotid-femoral PWV ↓ blood pressure ↓urinary albumin and ↓albumin/creatinine ratio | [110] | |
| diet with an additional 20 or 40 mmol K(+)/d from fruit and vegetables in a group with early-stage hypertension | no change in arterial stiffness and endothelial function | [111] | |
| potassium chloride and potassium bicarbonate supplementation | ↑brachial artery flow-mediated dilatation ↓carotid-femoral PWV | [112] | |
| low dietary potassium intake in healthy young adults | ↑ wave reflection and arterial stiffness ↑ potassium excretion ≥ ↓ aortic AIx + ↓ carotid-femoral PWV | [113] | |
| low sodium-to-potassium intake ratio | ↓ aortic AIx + ↓ PWV | [113] | |
| Vitamins | Vitamin D | ↓ central arterial stiffness (about 60.0% m/s) | [114,115,116] |
| not affect arterial stiffness short (2–12 months) vitamin D supplementation (1000 IU/day to 120,000 IU/month of cholecalciferol) ≥ no effect on aortic PWV and AIx | [117,118,119] | ||
| Vitamin C | improves flow-mediated dilation ↓ central blood pressure ↓ ADMA—an endogenous eNOS inhibitor ≥ ↑ vascular stiffness | [120,121] | |
| B vitamins | insufficiency ≥ ↑ homocysteine concentration ≥ endothelial dysfunction ↑ homocysteine + ↑ uric acid levels ≥ ↑ baPWV | [122,123] | |
| phytochemicals | Flavonoids (cranberry juice consumption) | ↓ carotid-femoral PWV (immediate but no chronic vasodilatory effect | [124] |
| Soy isoflavones | ↓ PWV and improving arterial compliance | [125,126,127,128] | |
| no effect on PWV and AIx | [129,130] | ||
| carotenoids (lycopene intake) | ↓ brachial-ankle PWV healthy women ↓ PWV in Korean men | [131,134] | |
| no effect on arterial stiffness in healthy overweight volunteers | [133] | ||
5.3. Protein Intake and Arterial Stiffness
5.4. Carbohydrates and Arterial Stiffness
5.5. The Role of Sodium and Potassium in Arterial Stiffness
5.6. Vitamin Intake and Arterial Stiffness
5.7. Phytochemicals and Arterial Stiffness
6. Dietary Recommendations to Prevent Arterial Stiffness
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Obesity Day 2022-Accelerating Action to Stop Obesity. Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 9 January 2023).
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 55217. [Google Scholar] [CrossRef] [PubMed]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef] [PubMed]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. Oxidative Stress Markers among Obstructive Sleep Apnea Patients. Oxid. Med. Cell Longev. 2021, 2021, 9681595. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Stanek, A.; Hooshmand, A.; Khamineh, Y.; Ahi, S.; Kazim, S.N.; Ahmad, F.; Muronetz, V.; Abousenna, M.S.; Zolghadri, S.; et al. Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients. J. Clin. Med. 2021, 10, 4802. [Google Scholar] [CrossRef]
- Safar, M.E.; Czernichow, S.; Blacher, J. Obesity, arterial stiffness, and cardiovascular risk. J. Am. Soc. Nephrol. 2006, 17, S109–S111. [Google Scholar] [CrossRef] [Green Version]
- Femia, R.; Kozakova, M.; Nannipieri, M.; Gonzales-Villalpando, C.; Stern, M.P.; Haffner, S.M. Carotid intima-media thickness in confirmed prehypertensive subjects: Predictors and progression. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2244–2249. [Google Scholar] [CrossRef]
- Kim, H.L.; Ahn, D.W.; Kim, S.H.; Lee, D.S.; Yoon, S.H.; Zo, J.H.; Kim, M.A.; Jeong, J.B. Association between body fat parameters and arterial stiffness. Sci. Rep. 2021, 11, 20536. [Google Scholar] [CrossRef]
- Melo E Silva, F.V.; Almonfrey, F.B.; Freitas, C.M.N.; Fonte, F.K.; Sepulvida, M.B.C.; Almada-Filho, C.M.; Cendoroglo, M.S.; Quadrado, E.B.; Amodeo, C.; Povoa, R.; et al. Association of Body Composition with Arterial Stiffness in Long-lived People. Arq. Bras. Cardiol. 2021, 117, 457–462. [Google Scholar] [CrossRef]
- Peto, R.; Whitlock, G.; Jha, P. Effects of obesity and smoking on US life expectancy. N. Engl. J. Med. 2010, 362, 855–856. [Google Scholar] [CrossRef] [Green Version]
- Fernhall, B.; Agiovlasitis, S. Arterial function in youth: Window into cardiovascular risk. J. Appl. Physiol. 1985, 105, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Starzak, M.; Stanek, A.; Jakubiak, G.K.; Cholewka, A.; Cieślar, G. Arterial Stiffness Assessment by Pulse Wave Velocity in Patients with Metabolic Syndrome and Its Components: Is It a Useful Tool in Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 10368. [Google Scholar] [CrossRef]
- Urbina, E.M.; Kimball, T.R.; Khoury, P.R.; Daniels, S.R.; Dolan, L.M. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J. Hypertens. 2010, 28, 1692–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Farmer, J. Arterisal Stiffness as a Risk Factor for Coronary Artery Disease. Curr. Atheroscler. Rep. 2014, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; Lastra, G.; Ramirez-Perez, F.I.; Haertling, D.; DeMarco, V.G.; Aroor, A.R.; Jia, G.; Chen, D.; Barron, B.J.; Garro, M.; et al. Endothelial estrogen receptor-α does not protect against vascular stiffness induced by Western diet in female mice. Endocrinology 2016, 157, 1590–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amore, L.; Alghisi, F.; Pancaldi, E.; Pascariello, G.; Cersosimo, A.; Cimino, G.; Bernardi, N.; Calvi, E.; Lombardi, C.M.; Sciatti, E.; et al. Study of endothelial function and vascular stiffness in patients affected by dilated cardiomyopathy on treatment with sacubitril/valsartan. Am. J. Cardiovasc. Dis. 2022, 12, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Dengo, A.L.; Dennis, E.A.; Orr, J.S.; Marinik, E.L.; Ehrlich, E.; Davy, B.M.; Davy, K.P. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 2010, 55, 855–8561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroor, A.R.; Jia, G.; Sowers, J.R. Cellular mechanisms underlying obesity-induced arterial stiffness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R387–R398. [Google Scholar] [CrossRef]
- Bagheri, S.; Zolghadri, S.; Stanek, A. Beneficial Effects of Anti-Inflammatory Diet in Modulating Gut Microbiota and Controlling Obesity. Nutrients 2022, 14, 3985. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Zolghadri, S.; Cholewka, A.; Myśliński, W. The Role of Intermittent Energy Restriction Diet on Metabolic Profile and Weight Loss among Obese Adults. Nutrients 2022, 14, 1509. [Google Scholar] [CrossRef]
- Recio-Rodriguez, J.I.; Gomez-Marcos, M.A.; Patino-Alonso, M.C.; Agudo-Conde, C.; Rodriguez-Sanchez, E.; Garcia-Ortiz, L. Vasorisk group. Abdominal Obesity vs General Obesity for Identifying Arterial Stiffness, Subclinical Atherosclerosis and Wave Reflection in Healthy, Diabetics and Hypertensive. BMC Cardiovasc. Disord. 2012, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Luo, L.; Ye, P.; Liu, Y.; Zhu, B.; Zheng, J.; Bai, Y.; Bai, J. Overall and Abdominal Obesity Indicators Had Different Association with Central Arterial Stiffness and Hemodynamics Independent of Age, Sex, Blood Pressure, Glucose, and Lipids in Chinese Community-Dwelling Adults. Clin. Interv. Aging 2013, 8, 1579–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cildir, G.; Akıncılar, S.C.; Tergaonkar, V. Chronic Adipose Tissue Inflammation: All Immune Cells on the Stage. Trends. Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.F.; Braga, V.D.A.; Silva, M.E.S.D.F.; Cruz, J.D.C.; Santos, S.H.S.; Monteiro, M.M.D.O.; Balarini, C.D.M. Adipokines, Diabetes and Atherosclerosis: An Inflammatory Association. Front. Physiol. 2015, 6, 304. [Google Scholar] [CrossRef]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Wierzbicki, Z.; Chmura, A.; Pruszczyk, P.; Puzianowska-Kuznicka, M. Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity Is Associated with Their Increased Content in Visceral Fat Depots. Int. J. Mol. Sci. 2015, 16, 25817–25830. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Ikeda, U. Matrix Metalloproteinases and Atherosclerosis. Curr. Atheroscler. Rep. 2004, 6, 112–120. [Google Scholar] [CrossRef]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Shankar-Hari, M.; Vale, C.L.; Godolphin, P.J.; Fisher, D.; Higgins, J.P.T.; Spiga, F.; Savovic, J.; Tierney, J.; Baron, G.; et al. Association between Administration of IL-6 Antagonists and Mortality among Patients Hospitalized for COVID-19: A Meta-Analysis. JAMA 2021, 326, 499–518. [Google Scholar] [CrossRef]
- Protogerou, A.D.; Zampeli, E.; Fragiadaki, K.; Stamatelopoulos, K.; Papamichael, C.; Sfikakis, P.P. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis 2011, 219, 734–736. [Google Scholar] [CrossRef]
- Nishimoto, N.; Kanakura, Y.; Aozasa, K.; Johkoh, T.; Nakamura, M.; Nakano, S.; Nakano, N.; Ikeda, Y.; Sasaki, T.; Nishioka, K.; et al. Humanized Anti-Interleukin-6 Receptor Antibody Treatment of Multicentric Castleman Disease. Blood 2005, 106, 2627–2632. [Google Scholar] [CrossRef]
- Urschel, K.; Cicha, I. TNF-alpha; in the Cardiovascular System: From Physiology to Therapy. Int. J. Interf. Cytokine Mediat. Res. 2015, 7, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; Desimone, C.; Song, X.; Diehl, A.M. Probiotics and Antibodies to TNF Inhibit Inflammatory Activity and Improve Nonalcoholic Fatty Liver Disease. Hepatology 2003, 37, 343–350. [Google Scholar] [CrossRef]
- Wascher, T.C.; Lindeman, J.H.; Sourij, H.; Kooistra, T.; Pacini, G.; Roden, M. Chronic TNF-α Neutralization Does Not Improve Insulin Resistance or Endothelial Function in “Healthy” Men with Metabolic Syndrome. Mol. Med. 2011, 17, 189–193. [Google Scholar] [CrossRef]
- Sudhakar, M.; Silambanan, S.; Chandran, A.S.; Prabhakaran, A.A.; Ramakrishnan, R. C-Reactive Protein (CRP) and Leptin Receptor in Obesity: Binding of Monomeric CRP to Leptin Receptor. Front. Immunol. 2018, 9, 1167. [Google Scholar] [CrossRef] [Green Version]
- Gnacińska, M.; Małgorzewicz, S.; Guzek, M.; Lysiak-Szydłowska, W.; Sworczak, K. Adipose Tissue Activity in Relation to Overweight or Obesity. Endokrynol. Polska 2010, 61, 160–168. [Google Scholar]
- Jia, G.; Aroor, A.R.; Sowers, J.R. Arterial Stiffness: A Nexus between Cardiac and Renal Disease. Cardiorenal. Med. 2014, 4, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroor, A.R.; Jia, G.; Sowers, J.R. The Role of Tissue Renin-Angiotensin-Aldosterone System in the Development of Endothelial Dysfunction and Arterial Stiffness. Front. Endocrinol. 2013, 4, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredt, D.S.; Snyder, S.H. Isolation of Nitric Oxide Synthetase, a Calmodulin-Requiring Enzyme. Proc. Natl. Acad. Sci. USA 1990, 87, 682–685. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Jang, H.J.; Martinez-Lemus, L.A.; Sowers, J.R. Activation of MTOR/P70S6 Kinase by ANG II Inhibits Insulin-Stimulated Endothelial Nitric Oxide Synthase and Vasodilation. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E201–E208. [Google Scholar] [CrossRef] [Green Version]
- Kane, M.O.; Etienne-Selloum, N.; Madeira, S.V.F.; Sarr, M.; Walter, A.; Dal-Ros, S.; Schott, C.; Chataigneau, T.; Schini-Kerth, V.B. Endothelium-Derived Contracting Factors Mediate the Ang II-Induced Endothelial Dysfunction in the Rat Aorta: Preventive Effect of Red Wine Polyphenols. Pflugers Arch.-Eur. J. Physiol. 2010, 459, 671–679. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nogami, T.; Taguchi, K.; Matsumoto, T.; Kamata, K. Diabetic State, High Plasma Insulin and Angiotensin II Combine to Augment Endothelin-1-Induced Vasoconstriction via ETA Receptors and ERK. Br. J. Pharmacol. 2008, 155, 974–983. [Google Scholar] [CrossRef] [Green Version]
- Kushibiki, M.; Yamada, M.; Oikawa, K.; Tomita, H.; Osanai, T.; Okumura, K. Aldosterone Causes Vasoconstriction in Coronary Arterioles of Rats via Angiotensin II Type-1 Receptor: Influence of Hypertension. Eur. J. Pharmacol. 2007, 572, 182–188. [Google Scholar] [CrossRef]
- Aghamohammadzadeh, R.; Unwin, R.D.; Greenstein, A.S.; Heagerty, A.M. Effects of Obesity on Perivascular Adipose Tissue. Vasorelaxant Function: Nitric Oxide, Inflammation and Elevated Systemic Blood Pressure. J. Vasc. Res. 2016, 52, 299–305. [Google Scholar] [CrossRef]
- Schroeter, M.R.; Eschholz, N.; Herzberg, S.; Jerchel, I.; Leifheit-Nestler, M.; Czepluch, F.S.; Chalikias, G.; Konstantinides, S.; Schäfer, K. Leptin-Dependent and Leptin-Independent Paracrine Effects of Perivascular Adipose Tissue on Neointima Formation. Arter. Thromb. Vasc. Biol. 2013, 33, 980–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almabrouk, T.A.M.; White, A.D.; Ugusman, A.B.; Skiba, D.S.; Katwan, O.J.; Alganga, H.; Guzik, T.J.; Touyz, R.M.; Salt, I.P.; Kennedy, S. High Fat Diet Attenuates the Anticontractile Activity of Aortic PVAT via a Mechanism Involving AMPK and Reduced Adiponectin Secretion. Front. Physiol. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmud, A.; Feely, J. Adiponectin and Arterial Stiffness. Am. J. Hypertens. 2005, 18, 1543–1548. [Google Scholar] [CrossRef] [Green Version]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPAR. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Korda, M.; Kubant, R.; Patton, S.; Malinski, T. Leptin-Induced Endothelial Dysfunction in Obesity. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1514–H1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opatrilova, R.; Caprnda, M.; Kubatka, P.; Valentova, V.; Uramova, S.; Nosal, V.; Gaspar, L.; Zachar, L.; Mozos, I.; Petrovic, D.; et al. Adipokines in Neurovascular Diseases. Biomed. Pharmacother. 2018, 98, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gairolla, J.; Kler, R.; Modi, M.; Khurana, D. Leptin and Adiponectin: Pathophysiological Role and Possible Therapeutic Target of Inflammation in Ischemic Stroke. Rev. Neurosci. 2017, 28, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L. Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [Green Version]
- Horvath, K.; Jeitler, K.; Siering, U.; Stich, A.K.; Skipka, G.; Gratzer, T.W.; Siebenhofer, A. Long-term effects of weight-reducing interventions in hypertensive patients: Systematic review and meta-analysis. Arch. Intern. Med. 2008, 168, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, P.D.; Stefanick, M.L.; Williams, P.T.; Haskell, W.L. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N. Engl. J. Med. 1991, 325, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Vega-López, S.; Venn, B.J.; Slavin, J.L. Relevance of the glycemic index and glycemic load for body weight, diabetes, and cardiovascular disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef] [Green Version]
- Sjöström, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.G.; Cotter, J.; Oliveira, P.; Vila, I.; Boutouyrie, P.; Laurent, S.; Nilsson, P.M.; Scuteri, A.; Sousa, N. Pulse wave velocity distribution in a cohort study: From arterial stiffness to early vascular aging. J. Hypertens. 2015, 33, 1438–1445. [Google Scholar] [CrossRef] [Green Version]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortal- ity with arterial stiffness: A systematic review and meta-analysis. J. Hypertens. 2015, 33, 1438–1445. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef]
- Ahmet, I.; Tae, H.J.; de Cabo, R.; Lakatta, E.G.; Talan, M.I. Effects of calorie restriction on cardioprotection and cardiovascular health. J. Mol. Cell. Cardiol. 2011, 51, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Fornieri, C.; Taparelli, F.; Quaglino, D., Jr.; Contri, M.B.; Davidson, J.M.; Algeri, S.; Ronchetti, I.P. The effect of s restriction on the aortic tissue of aging rats. Connect. Tissue Res. 1999, 40, 131–143. [Google Scholar] [CrossRef]
- Donato, A.J.; Walker, A.E.; Magerko, K.A.; Bramwell, R.C.; Black, A.D.; Henson, G.D.; Lawson, B.R.; Lesniewski, L.A.; Seals, D.R. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 2013, 12, 772–783. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, I.B.; Qasem, A.; McEniery, C.M.; Webb, D.J.; Avolio, A.P.; Cockcroft, J.R. Nitric oxide regulates local arterial distensibility in vivo. Circulation 2002, 105, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisbrod, R.M.; Shiang, T.; Al Sayah, L.; Fry, J.L.; Bajpai, S.; Reinhart-King, C.A.; Lob, H.E.; Santhanam, L.; Mitchell, G.; Cohen, R.A.; et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 2013, 2, 1105–11010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.S.; Blanch, N.; Keogh, J.B.; Clifton, P.M. Effect of weight loss on pulse wave velocity: Systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, A.; Vicil, F.; Sanchez-Gonzalez, M.A.; Wong, A.; Ormsbee, M.J.; Hooshmand, S.; Daggy, B. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am. J. Hypertens. 2013, 26, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferre, M.; Babio, N.; Martinez-Gonzalez, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2015, 6, CD011737. [Google Scholar] [CrossRef]
- Blekkenhorst, L.C.; Prince, R.L.; Hodgson, J.M.; Lim, W.H.; Zhu, K.; Devine, A.; Thompson, P.L.; Lewis, J.R. Dietary saturated fat intake and atherosclerotic vascular disease mortality in elderly women: A prospective cohort study. Am. J. Clin. Nutr. 2015, 101, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, J.A.; Huffman, F.G. Monounsaturated fatty acid, carbohydrate intake, and diabetes status are associated with arterial pulse pressure. Nutr. J. 2011, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Hall, W.L. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr. Res. Rev. 2009, 22, 18–38. [Google Scholar] [CrossRef] [Green Version]
- FAO; WHO. Fats and fatty acids in human nutrition. Proceedings of the Joint FAO/WHO Expert Consultation, 10–14 November 2008, Geneva, Switzerland. Ann. Nutr. Metab. 2009, 55, 5–300. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. American Heart Association. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Someya, Y.; Osonoi, Y.; Osonoi, T.; Saito, M.; Nakayama, S.; Ishida, H.; Sato, H.; Gosho, M.; Watada, H. Lower intake of saturated fatty acids is associated with persistently higher arterial stiffness in patients with type 2 diabetes. J. Diabetes Investig. 2021, 12, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Willett, W.C. Optimal diets for prevention of coronary heart disease. JAMA 2002, 288, 2569–2578. [Google Scholar] [CrossRef]
- Alonso, A.; Ruiz-Gutierrez, V.; Martínez-González, M.A. Monounsaturated fatty acids, olive oil and blood pressure: Epidemiological, clinical and experimental evidence. Public Health Nutr. 2006, 9, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Baylin, A.; Kabagambe, E.K.; Ascherio, A.; Spiegelman, D.; Campos, H. Adipose tissue alpha-linolenic acid and nonfatal acute myocardial infarction in Costa Rica. Circulation 2003, 107, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Lemaitre, R.N.; King, I.; Mozaffarian, D.; Kuller, L.H.; Tracy, R.P.; Siscovick, D.S. Plasma phospholipid n-3 polyunsaturated PUFAs, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2003, 77, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Vafeiadou, K.; Weech, M.; Altowaijri, H.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am. J. Clin. Nutr. 2015, 102, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Jayedi, A.; Zargar, M.S.; Shab-Bidar, S. Fish consumption and risk of myocardial infarction: A systematic review and dose-response meta-analysis suggests a regional difference. Nutr. Res. 2019, 62, 1–12. [Google Scholar] [CrossRef]
- Yang, B.; Shi, M.Q.; Li, Z.H.; Yang, J.J.; Li, D. Fish, Long-Chain n-3 PUFA and Incidence of Elevated Blood Pressure: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2016, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Xiong, K.; Cai, J.; Ma, A. Fish Consumption and Coronary Heart Disease: A Meta-Analysis. Nutrients 2020, 12, 2278. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Kobayashi, M.; Ishihara, J.; Sasaki, S.; Okada, K.; Kita, Y.; Kokubo, Y.; Tsugane, S. JPHC Study Group. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: The Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation 2006, 113, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, T.; Strong, J.P.; Ishii, T.; Ueno, T.; Koyama, M.; Wagayama, H.; Shimizu, A.; Sakai, T.; Malcom, G.T.; Guzman, M.A. Atherosclerosis and omega-3 fatty acids in the populations of a fishing village and a farming village in Japan. Atherosclerosis 2000, 153, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.D.; Feehan, R.P.; Blaha, C.; McLaughlin, D.J. Effect of omega-3 polyunsaturated fatty acid supplementation on central arterial stiffness and arterial wave reflections in young and older healthy adults. Physiol. Rep. 2015, 3, e12438. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.T.; Chan, D.C.; Barrett, P.H.; Adams, L.A.; Watts, G.F. Supplementation with n3 fatty acid ethyl esters increases large and small artery elasticity in obese adults on a weight loss diet. J. Nutr. 2013, 143, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvadó, J.; Becerra-Tomás, N.; García-Gavilán, J.F.; Bulló, M.; Barrubés, L. Mediterranean Diet and Cardiovascular Disease Prevention: What Do We Know? Prog. Cardiovasc. Dis. 2018, 61, 62–67. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P. Healthy traditional Mediterranean diet: An expression of culture, history, and lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Navarro, J.; Antoniazzi, L.; Oki, A.; Bonfim, M.C.; Hong, V.; Acosta-Cardenas, P.; Strunz, C.; Brunoro, E.; Miname, M.H.; Filho, W.S.; et al. Reduced subclinical carotid vascular disease and arterial stiffness in vegetarian men: The CARVOS Study. Int. J. Cardiol. 2017, 230, 562–566. [Google Scholar] [CrossRef]
- Mayra, S.T.; Johnston, C.S. Arterial stiffness and cardiometabolic health in omnivores and vegetarians: A cross-sectional pilot study. BMC Res. Notes 2022, 15, 69. [Google Scholar] [CrossRef]
- Diez-Fernández, A.; Álvarez-Bueno, C.; Martínez-Vizcaíno, V.; Sotos-Prieto, M.; Recio-Rodríguez, J.I.; Cavero-Redondo, I. Total Dairy, Cheese and Milk Intake and Arterial Stiffness: A Systematic Review and Meta-Analysis of Cross-sectional Studies. Nutrients 2019, 11, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingstone, K.M.; Lovegrove, J.A.; Cockcroft, J.R.; Elwood, P.C.; Pickering, J.E.; Givens, D.I. Does dairy food intake predict arterial stiffness and blood pressure in men?: Evidence from the Caerphilly Prospective Study. Hypertension 2013, 61, 42–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauhiainen, T.; Rönnback, M.; Vapaatalo, H.; Wuolle, K.; Kautiainen, H.; Groop, P.H.; Korpela, R. Long-term intervention with Lactobacillus helveticus fermented milk reduces augmentation index in hypertensive subjects. Eur. J. Clin. Nutr. 2010, 64, 424–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.S.; Keogh, J.B.; Meikle, P.J.; Garg, M.L.; Clifton, P.M. Dietary predictors of arterial stiffness in a cohort with type 1 and type 2 diabetes. Atherosclerosis 2015, 238, 175–1781. [Google Scholar] [CrossRef]
- Tsuboi, A.; Ito, C.; Fujikawa, R.; Yamamoto, H.; Kihara, Y. Association between the Postprandial Glucose Levels and Arterial Stiffness Measured According to the Cardio-ankle Vascular Index in Non-diabetic Subjects. Intern. Med. 2015, 54, 1961–1969. [Google Scholar] [CrossRef] [Green Version]
- Gordin, D.; Saraheimo, M.; Tuomikangas, J.; Soro-Paavonen, A.; Forsblom, I.; Paavonen, K.; Steckel-Hamann, B.; Vandenhende, F.; Nicolaou, L.; Pavo, I.; et al. Influence of Postprandial Hyperglycemic Conditions on Arterial Stiffness in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1134–1143. [Google Scholar] [CrossRef] [Green Version]
- Stern, L.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, M.; Gracely, E.J.; Samaha, F.F. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann. Int. Med. 2004, 140, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Wing, R.R. Look AHEAD Research Group.Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch. Int. Med. 2010, 170, 1566–1575. [Google Scholar] [CrossRef] [Green Version]
- Syed-Abdul, M.M.; Hu, Q.; Jacome-Sosa, M.; Padilla, J.; Manrique-Acevedo, C.; Heimowitz, C.; Parks, E.J. Effect of carbohydrate restriction-induced weight loss on aortic pulse wave velocity in overweight men and women. Appl. Physiol. Nutr. Metab. 2018, 43, 1247–1256. [Google Scholar] [CrossRef]
- Sanchez-Aguadero, N.; Patino-Alonso, M.C.; Mora-Simon, S.; Gomez-Marcos, M.A.; Alonso-Dominguez, R.; Sanchez-Salgado, B.; Recio-Rodriguez, J.I.; Garcia-Ortiz, L. Postprandial Effects of Breakfast Glycemic Index on Vascular Function among Young Healthy Adults: A Crossover Clinical Trial. Nutrients 2017, 9, 712. [Google Scholar] [CrossRef] [Green Version]
- Gaesser, G.A.; Rodriguez, J.; Patrie, J.T.; Whisner, C.M.; Angadi, S.S. Effects of Glycemic Index and Cereal Fiber on Postprandial Endothelial Function, Glycemia, and Insulinemia in Healthy Adults. Nutrients 2019, 11, 2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, R.; Sakazaki, M.; Nagai, Y.; Asaki, K.; Hashiguchi, T.; Negoro, H. Effects of Different Types of Carbohydrates on Arterial Stiffness: A Comparison of Isomaltulose and Sucrose. Nutrients 2021, 13, 4493. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Sato, K.; Sakazaki, M.; Nagai, Y.; Iwanuma, S.; Ohashi, N.; Hashiguchi, T. Acute effects of difference in glucose intake on arterial stiffness in healthy subjects. Cardiol. J. 2021, 28, 446–452. [Google Scholar] [CrossRef]
- Jin, M.; Miao, C.; An, L.; Guo, L.; Yang, X.; Zheng, M.; Hong, J.; Wu, S.; Su, Q. Association between Perceived Salt Intake and Arterial Stiffness. Biomed. Res. Int. 2022, 2022, 9072082. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Clifton, P.M.; Burrell, L.M.; Barrett, P.H.; Keogh, J.B. Postprandial effects of a high salt meal on serum sodium, arterial stiffness, markers of nitric oxide production and markers of endothelial function. Atherosclerosis 2014, 232, 211–216. [Google Scholar] [CrossRef]
- Safar, M.E.; Thuilliez, C.; Richard, V.; Benetos, A. Pressure-independent contribution of sodium to large artery structure and function in hypertension. Cardiovasc. Res. 2000, 46, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Elia, L.; Rossi, G.; di Cola, M.S.; Savino, I.; Galletti, F.; Strazzullo, P. Meta-Analysis of the Effect of Dietary Sodium Restriction with or without Concomitant Renin-Angiotensin-Aldosterone System-Inhibiting Treatment on Albuminuria. Clin. J. Am. Soc. Nephrol. 2015, 10, 1542–1552. [Google Scholar] [CrossRef] [Green Version]
- Gates, P.E.; Tanaka, H.; Hiatt, W.R.; Seals, D.R. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 2004, 44, 35–41. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; Marciniak, M.; Visagie, E.; Markandu, N.D.; Anand, V.; Dalton, R.N.; MacGregor, G.A. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 2009, 54, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Berry, S.E.; Mulla, U.Z.; Chowienczyk, P.J.; Sanders, T.A. Increased potassium intake from fruit and vegetables or supplements does not lower blood pressure or improve vascular function in UK men and women with early hypertension: A randomised controlled trial. Br. J. Nutr. 2010, 104, 1839–1847. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; Marciniak, M.; Carney, C.; Markandu, N.D.; Anand, V.; Fraser, W.D.; Dalton, R.N.; Kaski, J.C.; MacGregor, G.A. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension 2010, 55, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ortiz, L.; Recio-Rodriguez, J.I.; Rodriguez-Sanchez, E.; Patino-Alonso, M.C.; Agudo-Conde, C.; Rodriguez-Martin, C.; Castano-Sanchez, C.; Runkle, I.; Gomez-Marcos, M.A. Sodium and potassium intake present a J-shaped relationship with arterial stiffness and carotid intima-media thickness. Atherosclerosis 2012, 225, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Saz-Lara, A.; Cavero-Redondo, I.; Martínez-Vizcaíno, V.; Martínez-Ortega, I.A.; Notario-Pacheco, B.; Pascual-Morena, C. The Comparative Effects of Different Types of Oral Vitamin Supplements on Arterial Stiffness: A Network Meta-Analysis. Nutrients 2022, 14, 1009. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.A.; Désormeaux, A.; Labelle, A.; Soulez, M.; Soulez, G.; Langelier, Y.; Pshezhetsky, A.V.; Hébert, M.J. Endothelial stress induces the release of vitamin D-binding protein, a novel growth factor. Biochem. Biophys. Res. Commun. 2005, 338, 1374–1382. [Google Scholar] [CrossRef]
- Wu-Wong, J.R.; Nakane, M.; Ma, J.; Ruan, X.; Kroeger, P.E. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis 2006, 186, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Karimi, E.; Rezaie, P.; Vatanparast, H. The impact of vitamin D supplement intake on vascular endothelial function; a systematic review and meta-analysis of randomized controlled trials. Food Nutr. Res. 2017, 61, 1273574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabrizi, R.; Vakili, S.; Lankarani, K.B.; Akbari, M.; Jamilian, M.; Mahdizadeh, Z.; Mirhosseini, N.; Asemi, Z. Effects of Vitamin D Supplementation on Markers Related to Endothelial Function Among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. A Systematic Review and Meta-Analysis of Clinical Trials. Horm. Metab. Res. 2018, 50, 587–596. [Google Scholar] [CrossRef]
- Upala, S.; Sanguankeo, A.; Congrete, S.; Jaruvongvanich, V. Effect of cholecalciferol supplementation on arterial stiffness: A systematic review and meta-analysis. Scand. Cardiovasc. J. 2016, 50, 230–235. [Google Scholar] [CrossRef]
- Gillis, K.; Stevens, K.K.; Bell, E.; Patel, R.K.; Jardine, A.G.; Morris, S.T.W.; Schneider, M.P.; Delles, C.; Mark, P.B. Ascorbic acid lowers central blood pressure and asymmetric dimethylarginine in chronic kidney disease. Clin. Kidney J. 2018, 11, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Kielstein, J.T.; Donnerstag, F.; Gasper, S.; Menne, J.; Kielstein, A.; Martens-Lobenhoffer, J.; Scalera, F.; Cooke, J.P.; Fliser, D.; Bode-Böger, S.M. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke 2006, 37, 2024–2029. [Google Scholar] [CrossRef] [Green Version]
- Esse, R.; Barroso, M.; Tavares de Almeida, I.; Castro, R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Zhang, H.; Li, Z.; Li, H.; Miao, X.; Pan, H.; Wang, J.; Liu, X.; Kang, X.; Li, X.; et al. Mutual effect of homocysteine and uric acid on arterial stiffness and cardiovascular risk in the context of predictive, preventive, and personalized medicine. EPMA J. 2022, 13, 581–595. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Pase, M.P.; Grima, N.A.; Sarris, J. The effects of dietary and nutrient interventions on arterial stiffness: A systematic review. Am. J. Clin. Nutr. 2011, 93, 446–454. [Google Scholar] [CrossRef] [Green Version]
- Teede, H.J.; McGrath, B.P.; DeSilva, L.; Cehun, M.; Fassoulakis, A.; Nestel, P.J. Isoflavones reduce arterial stiffness: A placebo-controlled study in men and postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1066–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazim, S.; Curtis, P.J.; Schär, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Schouw, Y.T.; Pijpe, A.; Lebrun, C.E.; Bots, M.L.; Peeters, P.H.; van Staveren, W.A.; Lamberts, S.W.; Grobbee, D.E. Higher usual dietary intake of phytoestrogens is associated with lower aortic stiffness in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1316–1322. [Google Scholar] [CrossRef] [Green Version]
- Hoshida, S.; Miki, T.; Nakagawa, T.; Shinoda, Y.; Inoshiro, N.; Terada, K.; Adachi, T. Different effects of isoflavones on vascular function in premenopausal and postmenopausal smokers and nonsmokers: NYMPH study. Heart Vessels 2011, 26, 590–595. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Fleming, J.A.; Link, C.J.; Mukherjea, R.; Krul, E.S.; Kris-Etherton, P.M. Effects of isoflavone-containing soya protein on ex vivo cholesterol efflux, vascular function and blood markers of CVD risk in adults with moderately elevated blood pressure: A dose-response randomised controlled trial. Br. J. Nutr. 2017, 117, 1403–1413. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.Y.; Yoe, H.Y.; Kim, H.J.; Park, J.Y.; Kim, J.Y.; Lee, S.H.; Lee, J.H.; Lee, K.P.; Jang, Y.; Lee, J.H. Independent inverse relationship between serum lycopene concentration and arterial stiffness. Atherosclerosis 2010, 208, 581–586. [Google Scholar] [CrossRef]
- Yeo, H.Y.; Kim, O.Y.; Lim, H.H.; Kim, J.Y.; Lee, J.H. Association of serum lycopene and brachial-ankle pulse wave velocity with metabolic syndrome. Metabolism 2011, 60, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavani, C.; Petracci, M.; Trocino, A.; Xiccato, G. Advances in Research on Poultry and Rabbit Meat Quality. Ital. J. Anim. Sci. 2009, 8 (Suppl. 2), 741–750. [Google Scholar] [CrossRef] [Green Version]
- Barroeta, A. Nutritive value of poultry meat: Relationship between vitamin E and PUFA. World’s Poult. Sci. J. 2007, 63, 277–284. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; Avezum, A.; et al. Prospective Urban Rural Epidemiology (PURE) study investigators. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2018, 392, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fox, C.S.; Troy, L.M.; Mckeown, N.M.; Jacques, P.F. Longitudinal association of dairy consumption with the changes in blood pressure and the risk of incident hypertension: The Framingham Heart Study. Br. J. Nutr. 2015, 114, 1887–1899. [Google Scholar] [CrossRef] [Green Version]
- Tognon, G.; Nilsson, L.M.; Shungin, D.; Lissner, L.; Jansson, J.H.; Renström, F.; Wennberg, M.; Winkvist, A.; Johansson, I. Nonfermented milk and other dairy products: Associations with all-cause mortality. Am. J. Clin. Nutr. 2017, 105, 1502–1511. [Google Scholar] [CrossRef] [Green Version]
- Drouin-Chartier, J.P.; Brassard, D.; Tessier-Grenier, M.; Côté, J.A.; Labonté, M.È.; Desroches, S.; Couture, P.; Lamarche, B. Systematic Review of the Association between Dairy Product Consumption and Risk of Cardiovascular-Related Clinical Outcomes. Adv. Nutr. 2016, 7, 1026–1040. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvadó, J.; Farrés, X.; Luque, X.; Narejos, S.; Borrell, M.; Basora, J.; Anguera, A.; Torres, F.; Bulló, M.; Balanza, R. and Group, Fiber in Obesity-Study. Effect of two doses of a mixture of soluble fibres on body weight and metabolic variables in overweight or obese patients: A randomised trial. Br. J. Nutr. 2008, 99, 1380–1387. [Google Scholar] [CrossRef]
- Theuwissen, E.; Mensink, R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008, 94, 85–92. [Google Scholar] [CrossRef]
- Kizirian, N.V.; Kong, Y.; Muirhead, R.; Brodie, S.; Garnett, S.P.; Petocz, P.; Sim, K.A.; Celermajer, D.S.; Louie, J.C.; Markovic, T.P.; et al. Effects of a low-glycemic index diet during pregnancy on offspring growth, body composition, and vascular health: A pilot randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Maresch, C.C.; Petry, S.F.; Theis, S.; Bosy-Westphal, A.; Linn, T. Low Glycemic Index Prototype Isomaltulose-Update of Clinical Trials. Nutrients 2017, 9, 381. [Google Scholar] [CrossRef] [Green Version]
- Holub, I.; Gostner, A.; Theis, S.; Nosek, L.; Kudlich, T.; Melcher, R.; Scheppach, W. Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose). Br. J. Nutr. 2010, 103, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Starmans-Kool, M.J.; Stanton, A.V.; Xu, Y.Y.; Thom, S.A.M.; Parker, K.H.; Hughes, A.D. High dietary salt intake increases carotid blood pressure and wave reflection in normotensive healthy young men. J. Appl. Physiol. 2011, 110, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Muth, B.J.; Brian, M.S.; Chirinos, J.A.; Lennon, S.L.; Farquhar, W.B.; Edwards, D.G. Central systolic blood pressure and aortic stiffness response to dietary sodium in young and middle-aged adults. J. Am. Soc. Hypertens. 2017, 11, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Lennon-Edwards, S.; Allman, B.R.; Schellhardt, T.A.; Ferreira, C.R.; Farquhar, W.B.; Edwards, D.G. Lower potassium intake is associated with increased wave reflection in young healthy adults. Nutr. J. 2014, 13, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huggins, C.E.; O’Reilly, S.; Brinkman, M.; Hodge, A.; Giles, G.G.; English, D.R.; Nowson, C.A. Relationship of urinary sodium and sodium-to-potassium ratio to blood pressure in older adults in Australia. Med. J. Aust. 2011, 195, 128–132. [Google Scholar] [CrossRef]
- Schröder, H.; Schmelz, E.; Marrugat, J. Relationship between diet and blood pressure in a representative Mediterranean population. Eur. J. Nutr. 2002, 41, 161–167. [Google Scholar] [CrossRef]
- Del Giorno, R.; Ceresa, C.; Gabutti, S.; Troiani, C.; Gabutti, L. Arterial Stiffness and Central Hemodynamics are Associated with Low Diurnal Urinary Sodium Excretion. Diabetes Metab. Syndr. Obes. 2020, 13, 3289–3299. [Google Scholar] [CrossRef]
- Han, W.; Han, X.; Sun, N.; Chen, Y.; Jiang, S.; Li, M. Relationships between urinary electrolytes excretion and central hemodynamics, and arterial stiffness in hypertensive patients. Hypertens. Res. 2017, 40, 746–751. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.; Larson, M.G.; Dupuis, J.; Lunetta, K.L.; Lipinska, I.; Meigs, J.B.; Yin, X.; Rong, J.; Vita, J.A.; Newton-Cheh, C.; et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension 2008, 51, 1651–1657. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef] [Green Version]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef] [Green Version]
- Zoccali, C.; Benedetto, F.A.; Maas, R.; Mallamaci, F.; Tripepi, G. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J. Am. Soc. Nephrol. 2002, 13, 490–496. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yoon, Y.; Lee, K.-Y. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014, 28, 3197–3204. [Google Scholar] [CrossRef]
- Zharikov, S.; Krotova, K.; Hu, H.; Baylis, C.; Johnson, R.J.; Block, E.R.; Patel, J. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am. J. Physiol. Cell. Physiol. 2008, 295, C1183–C1190. [Google Scholar] [CrossRef] [Green Version]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef]
- Khosla, U.M.; Zharikov, S.; Finch, J.L.; Nakagawa, T.; Roncal, C.; Mu, W.; Krotova, K.; Block, E.R.; Prabhakar, S.; Johnson, R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005, 67, 1739–1742. [Google Scholar] [CrossRef] [Green Version]
- du Plooy, C.S.; Mels, C.M.; Huisman, H.W.; Kruger, R. The Association of Endothelin-1 with Markers of Arterial Stiffness in Black South African Women: The SABPA Study. J. Amino Acids. 2015, 2015, 481517. [Google Scholar] [CrossRef] [Green Version]
- Drüppel, V.; Kusche-Vihrog, K.; Grossmann, C.; Gekle, M.; Kasprzak, B.; Brand, E.; Pavenstädt, H.; Oberleithner, H.; Kliche, K. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013, 27, 3652–3659. [Google Scholar] [CrossRef]
- Izzo, J.L., Jr. Systolic hypertension, arterial stiffness, and vascular damage: Role of the renin-angiotensin system. Blood Press Monit. 2000, 5 (Suppl. 2), S7–S11. [Google Scholar] [CrossRef]
- Suzuki, T. Nitrosation of uric acid induced by nitric oxide under aerobic conditions. Nitric Oxide 2007, 16, 266–273. [Google Scholar] [CrossRef]
- McNulty, H.; Scott, J.M. Intake and status of folate and related B-vitamins: Considerations and challenges in achieving optimal status. Br. J. Nutr. 2008, 99, S48–S54. [Google Scholar] [CrossRef] [Green Version]
- Ganji, S.H.; Qin, S.; Zhang, L.; Kamanna, V.S.; Kashyap, M.L. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis 2009, 202, 68–75. [Google Scholar] [CrossRef]
- Hariri, M.; Darvishi, L.; Maghsoudi, Z.; Khorvash, F.; Aghaei, M.; Iraj, B.; Ghiasvand, R.; Askari, G. Intakes of Vegetables and Fruits are Negatively Correlated with Risk of Stroke in Iran. Int. J. Prev. Med. 2013, 4 (Suppl. 2), S300–S305. [Google Scholar]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef] [Green Version]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans fats-sources, health risks and alternative approach. J. Food. Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Prakash, B. Functional and Preservative Properties of Phytochemicals, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128196861. [Google Scholar]
- Spencer, J.P.E.; El Mohsen, M.M.A.; Minihane, A.-M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc. Ther. 2010, 28, e20–e32. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [Green Version]
- Hozawa, A.; Jacobs, D.R., Jr.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.H. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: The coronary artery risk development in young adults (CARDIA)/Young adult longitudinal trends in antioxidants (YALTA) study. Clin. Chem. 2007, 53, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Markovits, N.; Ben Amotz, A.; Levy, Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Isr. Med. Assoc. J. 2009, 11, 598–601. [Google Scholar]
- Sesso, H.D.; Buring, J.E.; Norkus, E.P.; Gaziano, J.M. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2004, 79, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Teede, H.J.; Giannopoulos, D.; Dalais, F.S.; Hodgson, J.; McGrath, B.P. Randomised, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J. Am. Coll. Nutr. 2006, 25, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
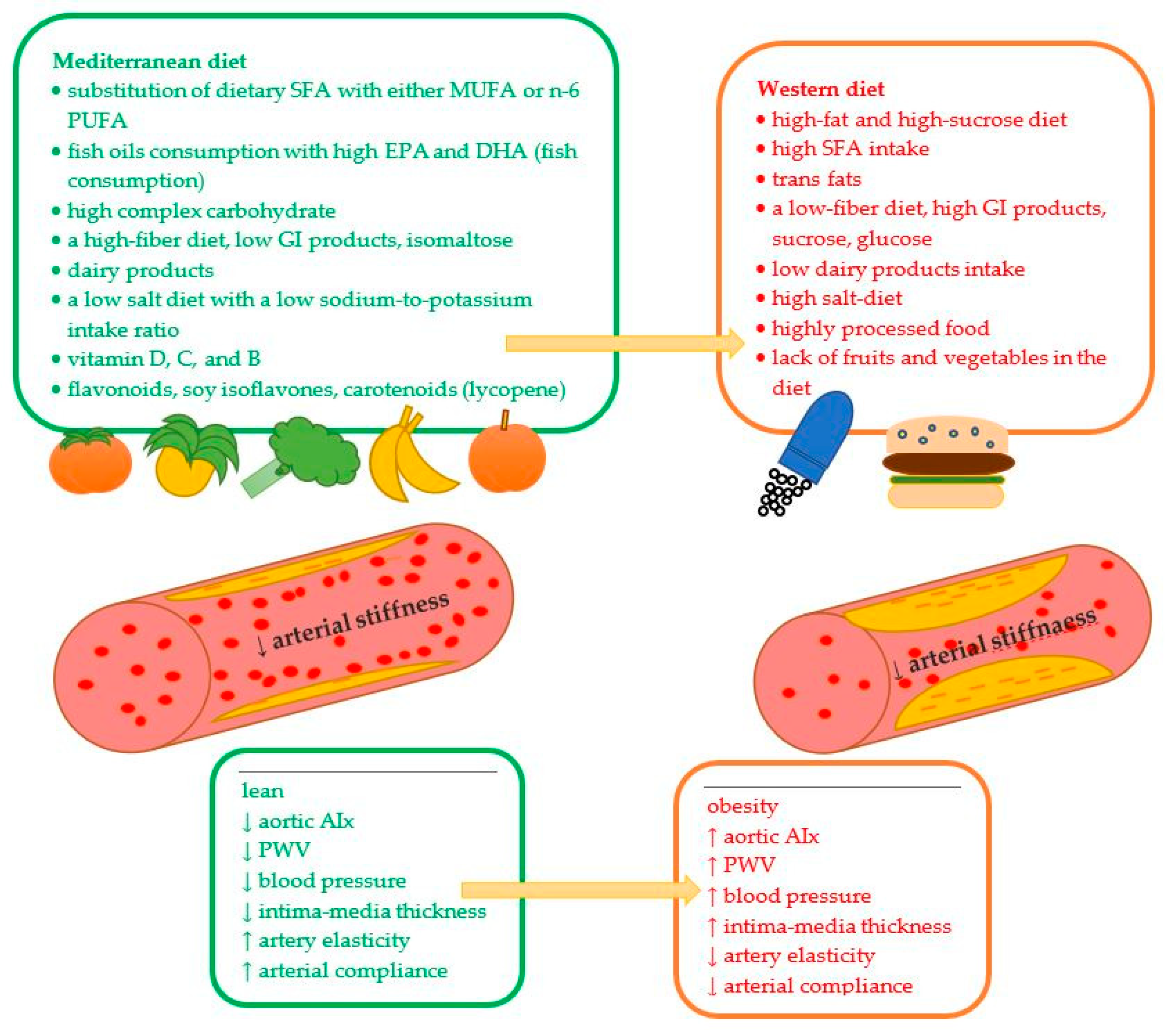
| Dietary Modification in the Prevention of CVD and High Arterial Stiffening | |||
|---|---|---|---|
| Modifications | Dietary Recommendations | Effect | Reference |
| Keep isoenergetic diet | Keep a balance of energy intake no additional meals and snacks | ↓ weight loss ↓ arterial stiffness | [63] |
| ↓ SFA intake and cholesterol ↑ MUFA and PUFA in the diet | ↓ fat meat, eggs, sweat intake minimize the intake of red meat and replace it with poultry and fish) use Mediterranean diet | ↓ myocardial infarction ↓ hypertension ↓ CHD incidence and mortality ↓ risk of nonfatal coronary events ↓ CVD risk | [67,72,80,81,82,83,88,134,135,140] |
| SFA replaced by MUFA and PUFA | Replace fatty red meat with lean meat, olive oil, fish, dairy products, nuts, and seeds Mediterranean diet | ↓ SFA intake at least two years ≥ 17% CHD events reduction ↓ atherosclerotic vascular disease ↓ mortality (mainly in the elderly) SFA replacement by MUFA—no effect on ↓ CVD risk PUFA (vegetable oil) ≥ ↓ CVD by ≈ 30% (similar to the statin effect) | [68,69,73,170] |
| avoid trans fats or replace them with MUFA | Eliminate or minimize consumption of margarine, fried potatoes, potato chips, corn chips, popcorn, animal products, household shortening, cakes, cookies, crackers, candy | ↓ CVD risk | [67,171] |
| include dairy products | Intake of milk, cheese, and cream (particularly fermented products such as whey, buttermilk, natural yogurt, sweetened yogurt, and matured/semi-matured cheese) Non-fermented milk consumption is not necessary Avoid high-fat dairy such as butter Mediterranean diet | ↓prevent or delay the onset of hypertension (both SBP and DBP) ↓ major CVD events ↓ CVD mortality independently of ethnicity each additional serving of yogurt ≥ ↓ 6% risk of HA incident active tripeptides ≥ ↓ angiotensin formation ≥ ↓ HA risk ≥ ↓ arterial stiffness non-fermented milk (≥2.5 times/day vs. ≤1 time per week) can ↑ all-cause mortality fermented milk and cheese intakes ≥ ↓all-cause mortality butter intake ≥ ↑ higher all-cause mortality | [88,93,136,137,138,139] |
| eliminate simple carbohydrates | Avoid sweets (glucose, sucrose) | Simple sugars ↑ fasting and postprandial glucose concentration ↑ CAVI values with age, particularly > 50. | [96] |
| use a diet with a low GI | Implement in the diet complex carbohydrates and fibers (cereals and whole-meal pasta) | impact metabolic changes and modulate insulin production ↓ absorption of bile acids ≥ ↑ hepatic CH conversion into bile acids leading to ↑ LDL uptake by the liver | [140,141] |
| avoid sugary drinks | For sweating, use isomaltose Instead of sweets, use isomaltose-containing products, e.g., food and drink additives or clinical formula diets Mediterranean diet | isomaltose sustained glucose release inhibits an acute ↑ arterial stiffness compared to sucrose and glucose | [88,96,103,143,144] |
| eliminate or minimalize sodium intake | Decrease salt intake (even for a short time, e.g., 7 to 14 days) | High-salt intake ↑hypertension ≥ ↓ arterial elasticity + ↑ arterial stiffness ↑ incidences of CVD after 7 days of intake ↑ wave reflection and carotid blood pressure after 14 days of intake | [106,145,146] |
| ↑products rich in potassium | Dried fruits (raisins, apricots), beans, lentils, potatoes, pumpkin, spinach, broccoli, beet greens, avocado, bananas | ↓ blood pressure (even with high sodium consumption) | [147] |
| keep a low Na: K ratio in the diet | Avoid kitchen salt Eat products rich in potassium | high Na:K ratio ≥ higher ↑ SBP and DBP than either sodium or potassium alone | [148,149,150] |
| use DASH or the Mediterranean diet for hypertension | DASH diet: a low sodium diet rich in vegetables, fruits, and low-fat dairy products Mediterranean diet—rich in vegetables, seeds, legumes (e.g., lentils and beans), fruit, cereals, and whole grains (e.g., unprocessed maize, millet, oats, wheat, and brown rice), containing lean meat, fish, and olive oil | ↓ blood pressure in subjects with and without hypertension, regardless of race and gender | [88,153] |
| antioxidative vitamins (C, E, and β-carotene) | Fruits and vegetables (minimum five servings daily) | scavenge free radicals vitamin C protects membranes from peroxidation by regenerating α-tocopherol, scavenges radical species, prevents endothelial dysfunction, ↓ CVD risk | [153,155,156] |
| B vitamins | Lean meat, fish, milk, cheese, eggs, some fortified breakfast cereals | ↓homocysteine level folate prevents stroke, cancers, cognitive impairment, and osteoporosis Nicotinic acid (niacin) ≥ hypolipemic effect + ↓endothelial ROS synthesis ≥ ↓LDL oxidation ≥ ↓inflammatory cytokine synthesis in aortic endothelial cells | [122,123,169] |
| include products with polyphenols, tocopherols, and phytosterols | Eat fresh red grapes, apples, pears, cherries, and berries cereals, dry legumes, whole grains, cereals, peanuts low amount of red wine, tea, coffee, and chocolate Mediterranean diet | ↑ polyphenols, anti-inflammatory effect protect against CVD ↓ overall mortality | [73,89,172,173,174,175] |
| increase intake of flavonoids | Plant food, including apples, berries, grapes, and onions | inhibit lipid peroxidation ≥ promote vascular relaxation and prevent atherosclerosis Chronic consumption of cranberry juice (including a high dose of polyphenols) ≥ ↓ carotid-femoral PWV (immediate but without chronic vasodilatory effect) | [124,172,173,174,176,177] |
| implement soy isoflavones | Soy and soy products Other isoflavones: fruits, vegetables, cereals, beverages, legumes, chocolates, oilseeds | ↑ nitrite/nitrate levels ↓ ET-1 levels alleviated arterial stiffness in men and postmenopausal women | [172,173,178] |
| add carotenoids to the diet | Tomatoes, watermelon, papaya, red grapefruits, guava, carrots, parsley, orange and green leafy vegetables, chenopods, fenugreek, spinach, cabbage, radish, turnips | antiatherogenic properties ↓ inflammation and ROS formation within the arterial wall lycopene: high antioxidant properties,↓ CVD risk | [172,174,179,180,181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanek, A.; Grygiel-Górniak, B.; Brożyna-Tkaczyk, K.; Myśliński, W.; Cholewka, A.; Zolghadri, S. The Influence of Dietary Interventions on Arterial Stiffness in Overweight and Obese Subjects. Nutrients 2023, 15, 1440. https://doi.org/10.3390/nu15061440
Stanek A, Grygiel-Górniak B, Brożyna-Tkaczyk K, Myśliński W, Cholewka A, Zolghadri S. The Influence of Dietary Interventions on Arterial Stiffness in Overweight and Obese Subjects. Nutrients. 2023; 15(6):1440. https://doi.org/10.3390/nu15061440
Chicago/Turabian StyleStanek, Agata, Bogna Grygiel-Górniak, Klaudia Brożyna-Tkaczyk, Wojciech Myśliński, Armand Cholewka, and Samaneh Zolghadri. 2023. "The Influence of Dietary Interventions on Arterial Stiffness in Overweight and Obese Subjects" Nutrients 15, no. 6: 1440. https://doi.org/10.3390/nu15061440
APA StyleStanek, A., Grygiel-Górniak, B., Brożyna-Tkaczyk, K., Myśliński, W., Cholewka, A., & Zolghadri, S. (2023). The Influence of Dietary Interventions on Arterial Stiffness in Overweight and Obese Subjects. Nutrients, 15(6), 1440. https://doi.org/10.3390/nu15061440









