Phloretamide Prevent Hepatic and Pancreatic Damage in Diabetic Male Rats by Modulating Nrf2 and NF-κB
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Establishment of DM
2.3. Experimental Design
2.4. Serum Collection and Measurements
2.5. Tissue Collection
2.6. Analysis of the Serum and Plasma
2.7. Extraction of Lipid from Frozen Livers
2.8. Preparation of the Tissue Homogenates and Nuclear Extraction
2.9. Measurements of the Tissue Homogenates and Nuclear/Cytoplasmic Extracts
2.10. Real-Time PCR (qPCR)
2.11. Hematoxylin and Eosin (H&E) Staining
2.12. Statistical Analysis
3. Results
3.1. Changes in Rat Body Weights
3.2. Changes in the Markers of Glucose Homeostasis
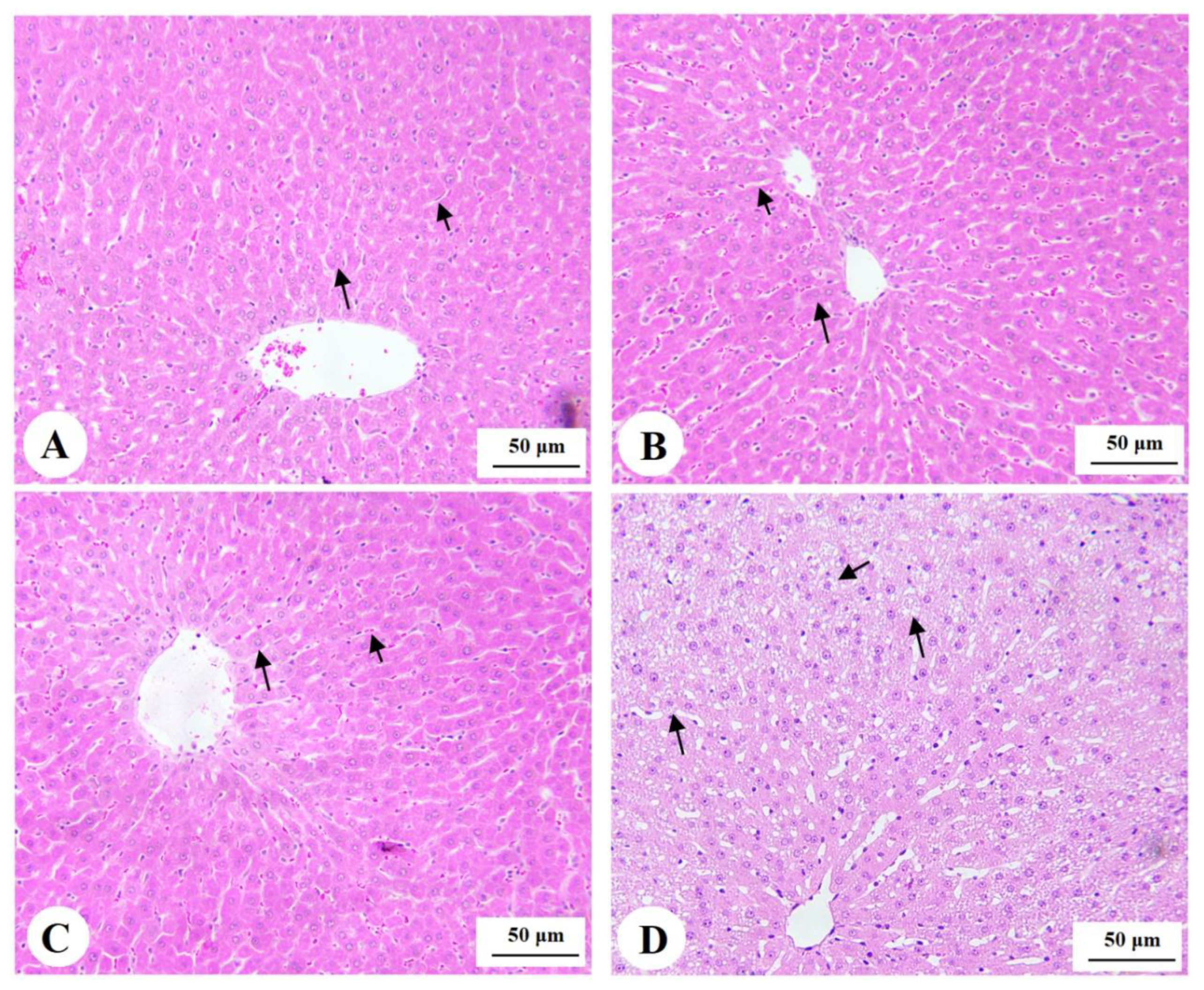
3.3. Histology of the Pancreas
3.4. Changes in the Serum and Hepatic Lipid Profiles
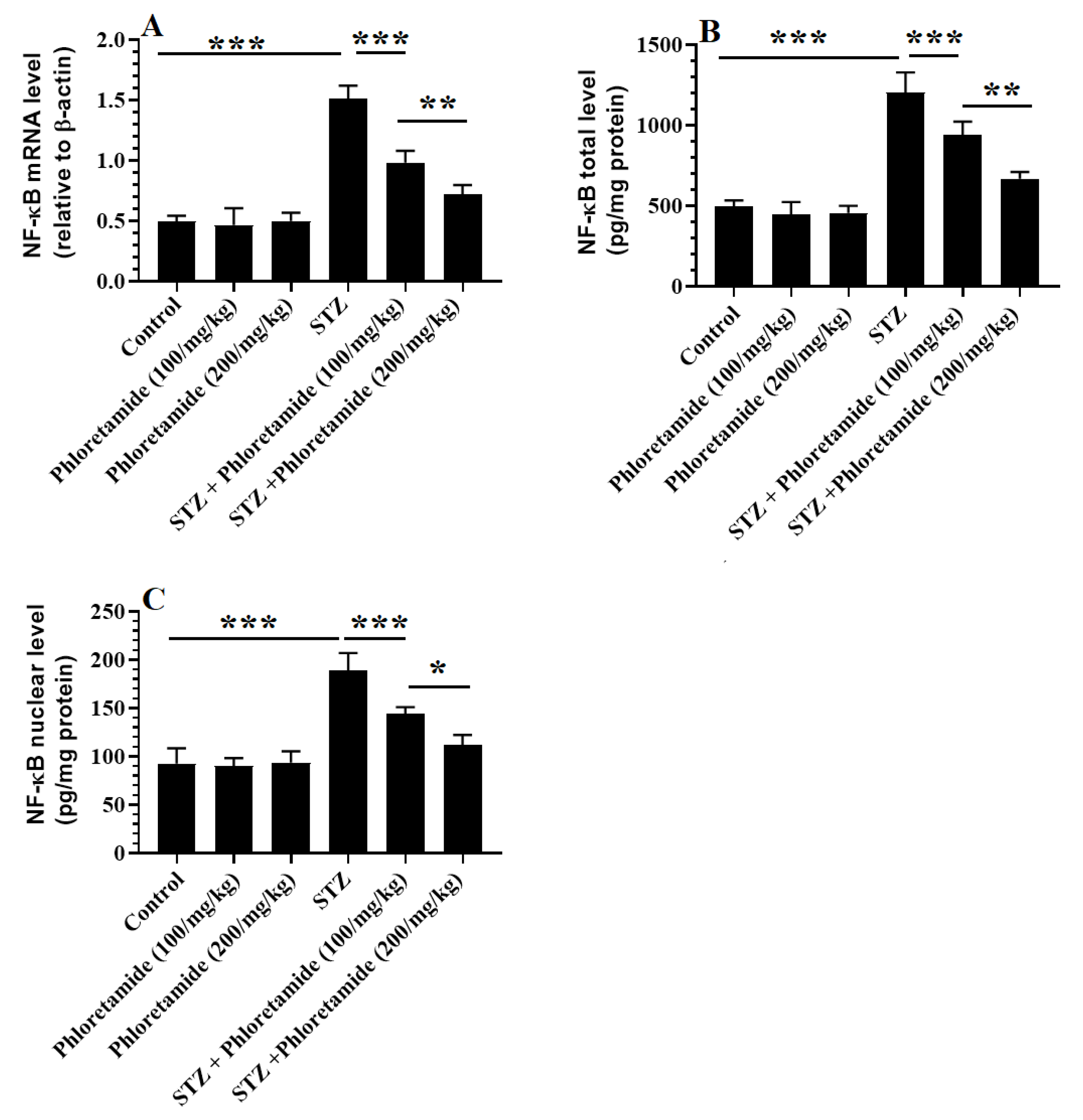
3.5. Changes in the Hepatic Markers of Oxidative Stress and Inflammation
3.6. Changes in the Hepatic Keap-1/Nrf2 Axis
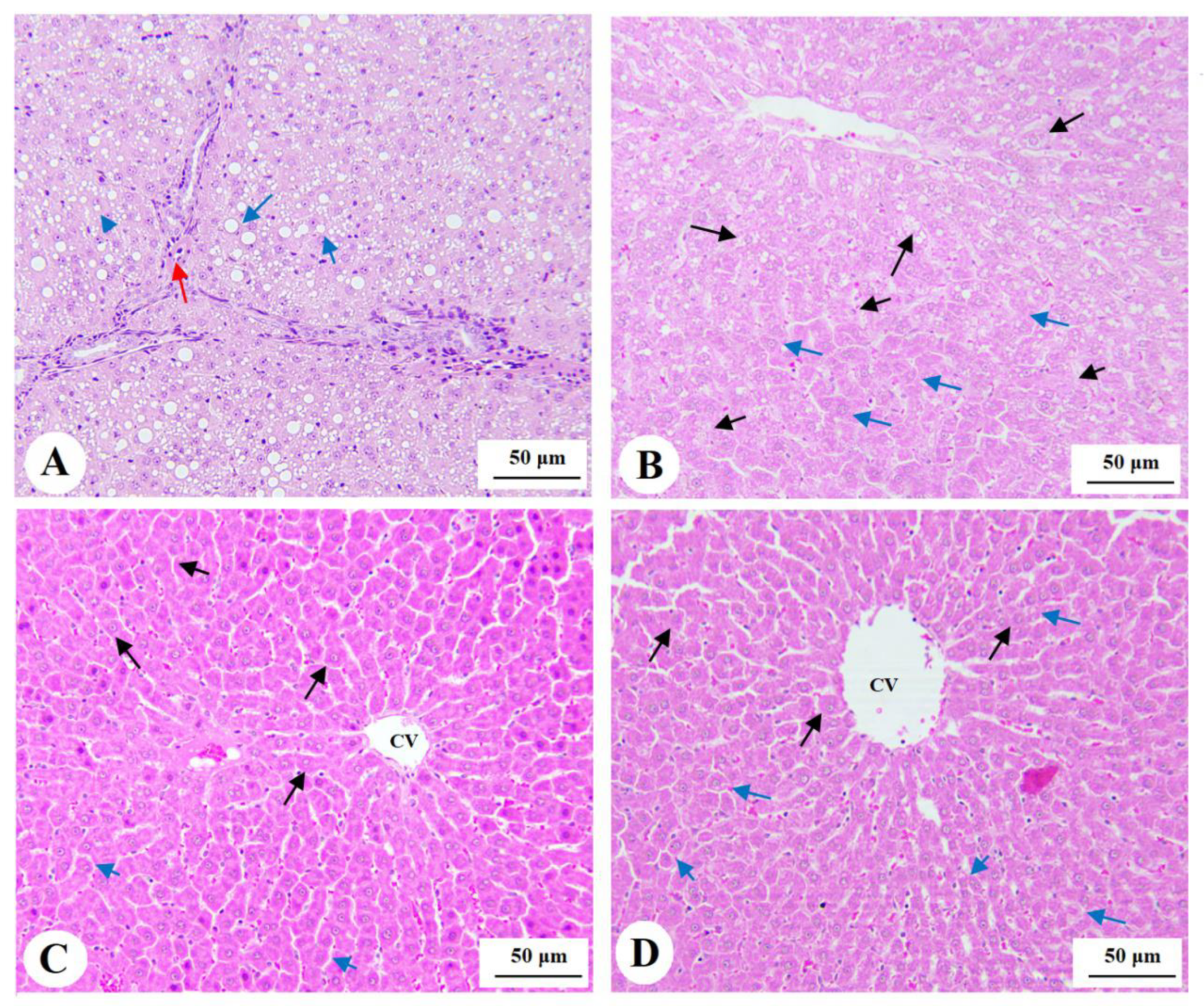
3.7. Histological Findings for the Livers
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2014, 42, 698–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, C.C.; Karuranga, S.; Salpea, P.; Saeedi, P.; Dahlquist, G.; Soltesz, G.; Ogle, G.D. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107842. [Google Scholar] [CrossRef] [Green Version]
- Al-Hussaini, A.A.; Sulaiman, N.M.; Alzahrani, M.D.; Alenizi, A.S.; Khan, M. Prevalence of hepatopathy in type 1 diabetic children. BMC Pediatr. 2012, 12, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef] [PubMed]
- Barros, B.S.V.; Santos, D.C.; Pizarro, M.H.; de Melo, L.G.N.; Gomes, M.B. Type 1 Diabetes and Non-Alcoholic Fatty Liver Disease: When Should We Be Concerned? A Nationwide Study in Brazil. Nutrients 2017, 9, 878. [Google Scholar] [CrossRef] [Green Version]
- Mertens, J.; De Block, C.; Spinhoven, M.; Driessen, A.; Francque, S.M.; Kwanten, W.J. Hepatopathy Associated with Type 1 Diabetes: Distinguishing Non-alcoholic Fatty Liver Disease from Glycogenic Hepatopathy. Front. Pharmacol. 2021, 12, 768576. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141, Erratum in 2021, 162, 174. [Google Scholar] [CrossRef]
- Qu, L.-L.; Yu, B.; Li, Z.; Jiang, W.-X.; Jiang, J.-D.; Kong, W.-J. Gastrodin Ameliorates Oxidative Stress and Proinflammatory Response in Nonalcoholic Fatty Liver Disease through the AMPK/Nrf2 Pathway. Phytother. Res. 2015, 30, 402–411. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.; Yang, C.; Li, B.; Wei, J.; Lin, Y.; Ye, P.; Hu, G.; Li, J. Liraglutide reduces hepatic glucolipotoxicity-induced liver cell apoptosis through NRF2 signaling in Zucker diabetic fatty rats. Mol. Med. Rep. 2018, 17, 8316–8324. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Luo, J.; Han, H.; Xie, W.; Wang, L.; Han, R.; Chen, H.; Cai, Y.; Huang, H.; Xia, Z. Allopurinol ameliorates liver injury in type 1 diabetic rats through activating Nrf2. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211031417. [Google Scholar] [CrossRef]
- Chowdhry, S.; Nazmy, M.H.; Meakin, P.; Dinkova-Kostova, A.; Walsh, S.V.; Tsujita, T.; Dillon, J.; Ashford, M.; Hayes, J.D. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2010, 48, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-K.J.; Yeager, R.L.; Tanaka, Y.; Klaassen, C.D. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol. Appl. Pharmacol. 2010, 245, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Aleksunes, L.M.; Yeager, R.L.; Gyamfi, M.A.; Esterly, N.; Guo, G.L.; Klaassen, C.D. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharm. Exp. Ther. 2008, 325, 655–664. [Google Scholar] [CrossRef]
- Liu, Z.; Dou, W.; Ni, Z.; Wen, Q.; Zhang, R.; Qin, M.; Wang, X.; Tang, H.; Cao, Y.; Wang, J.; et al. Deletion of Nrf2 leads to hepatic insulin resistance via the activation of NF-κB in mice fed a high-fat diet. Mol. Med. Rep. 2016, 14, 1323–1331. [Google Scholar] [CrossRef] [Green Version]
- Ou, Q.; Weng, Y.; Wang, S.; Zhao, Y.; Zhang, F.; Zhou, J.; Wu, X. Silybin Alleviates Hepatic Steatosis and Fibrosis in NASH Mice by Inhibiting Oxidative Stress and Involvement with the Nf-κB Pathway. Dig. Dis. Sci. 2018, 63, 3398–3408. [Google Scholar] [CrossRef]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludtmann, M.H.R.; Angelova, P.R.; Zhang, Y.; Abramov, A.Y.; Dinkova-Kostova, A.T. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem. J. 2014, 457, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Smolková, K.; Mikó, E.; Kovács, T.; Leguina-Ruzzi, A.; Sipos, A.; Bai, P. Nuclear Factor Erythroid 2-Related Factor 2 in Regulating Cancer Metabolism. Antioxid. Redox Signal. 2020, 33, 966–997. [Google Scholar] [CrossRef] [Green Version]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Upadhyay, K.K.; Devkar, R.V.; Khurana, S. Naturally Occurring Nrf2 Activators: Potential in Treatment of Liver Injury. Oxid. Med. Cell. Longev. 2016, 2016, 3453926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nithiya, T.; Udayakumar, R. In Vitro Antioxidant Properties of Phloretin—An Important Phytocompound. J. Biosci. Med. 2016, 4, 85–94. [Google Scholar]
- Nithiya, T.; Udayakumar, R. Protective effect of phloretin on hyperglycemia mediated oxidative stress in experimental diabetic rats. IFNM 2017, 5, 1–6. [Google Scholar]
- Yang, Q.; Han, L.; Li, J.; Xu, H.; Liu, X.; Wang, X.; Pan, C.; Lei, C.; Chen, H.; Lan, X. Activation of Nrf2 by Phloretin Attenuates Palmitic Acid-Induced Endothelial Cell Oxidative Stress via AMPK-Dependent Signaling. J. Agric. Food Chem. 2018, 67, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Paluszczak, J.; Baer-Dubowska, W. Xanthohumol induces phase II enzymes via Nrf2 in human hepatocytes in vitro. Toxicol. Vitr. 2013, 27, 149–156. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.; Celewicz, L.; Barciszewski, J.; Baer-Dubowska, W. Phloretamide, an apple phenolic compound, activates the Nrf2/ARE pathway in human hepatocytes. Food Chem. Toxicol. Int. J. 2013, 51, 202–209. [Google Scholar]
- Akinlade, O.M.; Owoyele, B.V.; Soladoye, A.O. Streptozotocin-induced type 1 and 2 diabetes in rodents: A model for studying diabetic cardiac autonomic neuropathy. Afr. Heal. Sci. 2021, 21, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, J.Z.; AlFaris, N.A.; Alshammari, G.M.; Alagal, R.I.; Aljabryn, D.H.; Aldera, H.; Alrfaei, B.M.; Alkhateeb, M.A.; Yahya, M.A. Ellagic acid protects against diabetic nephropathy in rats by regulating the transcription and activity of Nrf2. J. Funct. Foods 2021, 79, 104397. [Google Scholar] [CrossRef]
- Rybicka, H. 3-[p-hydroxyphenyl]-propionic acid amide (phloretamide) in root exudate of Malus. Biochem. Physiol. Pflanz. 1984, 179, 303–309. [Google Scholar] [CrossRef]
- Rybicka, H. Phloretamid in fruitlets of apple tree (Malus domestica). Acta Physiol. Plant. 1996, 18, 359–363. [Google Scholar]
- Struck, M.B.; Andrutis, K.A.; Ramirez, H.E.; Battles, A.H. Effect of a short-term fast on ketamine-xylazine anesthesia in rats. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 344–348. [Google Scholar] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Evelson, P.A.; Travacio, M.; Repetto, M.; Escobar, J.; Llesuy, S.F.; Lissi, E.A. Evaluation of Total Reactive Antioxidant Potential (TRAP) of Tissue Homogenates and Their Cytosols. Arch. Biochem. Biophys. 2001, 388, 261–266. [Google Scholar] [CrossRef]
- Azahar, M.A.; Al-Naqeb, G.; Hasan, M.; Adam, A. Hypoglycemic effect of Octomeles sumatrana aqueous extract in streptozoto-cin-induced diabetic rats and its molecular mechanisms. Asian Pac. J. Trop. Med. 2012, 5, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Wang-Fischer, Y.; Garyantes, T. Improving the Reliability and Utility of Streptozotocin-Induced Rat Diabetic Model. J. Diabetes Res. 2018, 2018, 8054073. [Google Scholar] [CrossRef]
- Nabiyouni, F.; Vaezi, G.; Malekirad, A.A. Hypoglycaemic and hypolipidemic activities of Alhagi camelorum in streptozotocin-induced diabetes in Wistar rats. Arch. Physiol. Biochem. 2021, 127, 296–303. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Kawashima, I.; Koshiisi, M.; Kumagai, T.; Suzuki, M.; Suzuki, J.; Mitsumori, T.; Kirito, K. Glycolytic enzyme hexokinase II is a putative therapeutic target in B-cell malignant lymphoma. Exp. Hematol. 2019, 78, 46–55.e3. [Google Scholar] [CrossRef]
- Timson, D.J. Fructose 1,6-bisphosphatase: Getting the message across. Biosci. Rep. 2019, 39, BSR20190124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischof, M.G.; Bernroider, E.; Krssak, M.; Krebs, M.; Stingl, H.; Nowotny, P.; Yu, C.; Shulman, G.I.; Waldhäusl, W.; Roden, M. Hepatic Glycogen Metabolism in Type 1 Diabetes After Long-Term Near Normoglycemia. Diabetes 2002, 51, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadler, M.; Krššák, M.; Jankovic, D.; Göbl, C.; Winhofer, Y.; Pacini, G.; Bischof, M.; Haidinger, M.; Säemann, M.; Mühlbacher, F.; et al. Fasting and postprandial liver glycogen content in patients with type 1 diabetes mellitus after successful pancreas-kidney transplantation with systemic venous insulin delivery. Clin. Endocrinol. 2013, 80, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.A.; Forbes, J.M. Glucose and glycogen in the diabetic kidney: Heroes or villains? EBioMedicine 2019, 47, 590–597. [Google Scholar] [CrossRef] [Green Version]
- López-Soldado, I.; Guinovart, J.J.; Duran, J. Increasing hepatic glycogen moderates the diabetic phenotype in insulin-deficient Akita mice. J. Biol. Chem. 2021, 296, 100498. [Google Scholar] [CrossRef]
- Li, X.; Jayachandran, M.; Xu, B. Antidiabetic effect of konjac glucomannan via insulin signaling pathway regulation in high-fat diet and streptozotocin-induced diabetic rats. Food Res. Int. 2021, 149, 110664. [Google Scholar] [CrossRef]
- Wickramasinghe, A.S.D.; Kalansuriya, P.; Attanayake, A.P. Herbal Medicines Targeting the Improved β-Cell Functions and β-Cell Regeneration for the Management of Diabetes Mellitus. Evid. Based Complement. Alternat. Med. 2021, 2021, 2920530. [Google Scholar] [CrossRef]
- Mata-Torres, G.; Andrade-Cetto, A.; Espinoza-Hernández, F.A.; Cárdenas-Vázquez, R. Hepatic Glucose Output Inhibition by Mexican Plants Used in the Treatment of Type 2 Diabetes. Front Pharmacol. 2020, 11, 215. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Kong, B.; Yang, N.; Cao, B.; Feng, D.; Yu, X.; Ge, C.; Feng, S.; Fei, F.; Huang, J.; et al. The Hypoglycemic Effect of Berberine and Berberrubine Involves Modulation of Intestinal Farnesoid X Receptor Signaling Pathway and Inhibition of Hepatic Gluconeogenesis. Drug Metab. Dispos. 2021, 49, 276–286. [Google Scholar] [CrossRef]
- Sok Yen, F.; Shu Qin, C.; Tan Shi Xuan, S.; Jia Ying, P.; Yi Le, H.; Darmarajan, T.; Gunasekaran, B.; Salvamani, S. Hypoglycemic Effects of Plant Flavonoids: A Review. Evid. Based Complement. Alternat. Med. 2021, 2021, 2057333. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Bugianesi, E. NASH in Lean Individuals. Semin. Liver Dis. 2019, 39, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Sillars, A.; Sattar, N. Management of Lipid Abnormalities in Patients with Diabetes. Curr. Cardiol. Rep. 2019, 21, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regnell, S.E.; Lernmark, A. Hepatic Steatosis in Type 1 Diabetes. Rev. Diabet. Stud. 2011, 8, 454–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018, 27, 22–41. [Google Scholar] [CrossRef] [Green Version]
- Liou, C.J.; Wu, S.J.; Shen, S.C.; Chen, L.C.; Chen, Y.L.; Huang, W.C. Phloretin ameliorates hepatic steatosis through regulation of lipo-genesis and Sirt1/AMPK signaling in obese mice. Cell Biosci. 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Schmatz, R.; Perreira, L.B.; Stefanello, N.; Mazzanti, C.; Spanevello, R.; Gutierres, J.; Bagatini, M.; Martins, C.C.; Abdalla, F.H.; Serres, J.D.D.S.; et al. Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie 2012, 94, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Wu, B.; Shen, G.; Li, X.; Wu, Q. Curcumin alleviates liver oxidative stress in type 1 diabetic rats. Mol. Med. Rep. 2017, 17, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Marques, V.; Marinho, H.S.; Cyrne, L.; Antunes, F. Role of hydrogen peroxide in NF-kappaB activation: From inducer to modulator. Antioxid. Redox Signal. 2009, 11, 2223–2243. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, W.; Wang, J.; Yang, H.; Zhao, X.; Zhou, Q.; Wang, H.; Li, L.; Du, G. Activation of Nrf2 signaling by salvianolic acid C attenuates NF κB mediated inflammatory response both in vivo and in vitro. Int. Immunopharmacol. 2018, 63, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Jiang, C.; Zhang, M.; Jin, J.; Ge, S.; Wang, X. Phloretin protects against cardiac damage and remodeling via restoring SIRT1 and anti-inflammatory effects in the streptozotocin-induced diabetic mouse model. Aging 2019, 11, 2822–2835. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primers (5′→3′) | Accession | BP |
|---|---|---|---|
| Nrf2 | F:-AAAATCATTAACCTCCCTGTTGAT R: R:′-CGGCGACTTTATTCTTACCTCTC | NM_031789 | 118 |
| NF-κB | F: GTGCAGAAAGAAGACATTGAGGTG R: AGGCTAGGGTCAGCGTATGG | XM_342346.4 | 176 |
| Β-actin | F: GACCTCTATGCCAACACAGT R: CACCAATCCACACAGAGTAC | NM_031144 | 154 |
| Control | Phloretamide (100 mg/kg) | Phloretamide (200 mg/kg) | STZ | STZ + Phloretamide (100 mg/kg) | STZ + Phloretamide (200 mg/kg) | |
|---|---|---|---|---|---|---|
| Final body weight (g) | 482 ± 32 | 471 ± 41 | 489 ± 54 | 382 ± 29 | 431 ± 32 abcd | 473 ± 36 de |
| Fasting glucose (mg/dL) | 122 ± 8.1 | 107.7 ± 8.5 a | 91.5 ± 7.9 ab | 354 ± 29 abc | 235 ± 15.7 abcd | 153 ± 11.6 abcde |
| Fasting insulin (ng/mL) | 4.5 ± 0.68 | 5.1 ± 0.43 | 4.8 ± 0.65 | 1.2 ± 0.27 abc | 1.89 ± 0.32 abcd | 2.68 ± 0.34 abcde |
| Hepatic Hexokinase (pg/mL) | 18.6 ± 1.3 | 22.4 ± 1.8 a | 39.5 ± 3.3 ab | 6.7 ± 0.82 abc | 9.2 ± 1.5 acbd | 14.5 ± 2.1 abcde |
| Hepatic G-6-Pase (U/mg) | 9.8 ± 0.79 | 6.8 ± 0.73 a | 4.6 ± 0.53 ab | 22.7 ± 2.9 abc | 16.7 ± 2.1 acbd | 12.4 ± 1.1 abcde |
| Hepatic FBP-1 (pg/mg) | 114 ± 7.6 | 91.6 ± 7.1 a | 81.8 ± 6.8 ab | 422 ± 32 abc | 312 ± 27 acbd | 178 ± 14.7 abcde |
| Liver glycogen (mg/mg) | 35.7 ± 2.4 | 48.8 ± 5.6 a | 61.5 ± 6.8 ab | 17.6 ± 2.1 abc | 24.7± 12.5 acbd | 29.1 ± 1.9 abcde |
| Control | Phloretamide (100 mg/kg) | Phloretamide (200 mg/kg) | STZ | STZ + Phloretamide (100 mg/kg) | STZ + Phloretamide (200 mg/kg) | ||
|---|---|---|---|---|---|---|---|
| Serum | TGs (mg/dL) | 67.5 ± 5.4 | 61.7 ± 7.1 | 68.9 ± 6.5 | 182 ± 15.6 | 124.5 ± 12.3 abcd | 101 ± 8.7 abcde |
| CHOL (mg/dL) | 74.5 ± 6.5 | 75.1 ± 5.8 | 69.9 ± 6.3 | 207 ± 17.8 abc | 141 ± 11.3 abcd | 97.6 ± 8.7 abcde | |
| LDL-c (mg/dL) | 42.5 ± 4.1 | 38.9 ± 5.1 | 44.7 ± 5.9 | 147 ± 9.7 abc | 87.6 ± 7.5 abcd | 64.5 ± 5.9 abcde | |
| HDL-c (mg/dL) | 19.8 ± 1.9 | 22.4 ± 2.1 | 21.7 ± 2.4 | 8.7 ± 1.2 abc | 12.3 ± 1.3 abcd | 17.8 ± 1.1 abcde | |
| FFAs (µmol/L) | 445 ± 37 | 389 ± 25.6 a | 301± 25.8 ab | 988 ± 76.8 abc | 763 ± 65.8 abcde | 578 ± 61.3 abcde | |
| Liver | Triglycerides (mg/g) | 4.23 ± 0.35 | 3.83 ± 0.59 | 4.45 ± 0.65 | 7.64 ± 0.69 abc | 6.18 ± 0.72 abcd | 5.35 ± 0.54 abcde |
| CHOL (µg/g) | 2.56 ± 0.39 | 2.6 ± 0.7 | 2.77 ± 0.64 | 6.89 ± 0.45 abc | 5.13 ± 0.68 abcd | 3.38 ± 0.41 abcde |
| Control | Phloretamide (100 mg/kg) | Phloretamide (100 mg/kg) | STZ | STZ + Phloretamide (100 mg/kg) | STZ + Phloretamide (200 mg/kg) | |
|---|---|---|---|---|---|---|
| MDA (pmol/mg) | 567 ± 84 | 432 ± 37 a | 432 ± 37 a | 1654 ± 134 abc | 924 ± 79 abcd | 689 ± 73 abcde |
| SOD (U/mg) | 28.7 ± 2.5 | 36.7 ± 6.7 a | 36.7 ± 6.7 a | 13.4 ± 1.7 abc | 19.8 ± 2.4 abcd | 26.9 ± 2.9 bcde |
| GSH (µg/mg tissue) | 64.6 ± 6.1 | 75.4 ± 6.8 a | 75.4 ± 6.8 a | 25.6 ± 1.6 abc | 39.5 ± 4.1 abcd | 54.3 ± 4.3 abcde |
| CAT (U/mg tissue) | 8.2 ± 1.2 | 12.4 ± 1.5 a | 12.4 ± 1.5 a | 3.2 ± 0.56 abc | 7.8 ± 0.82 cbd | 12.4 ± 1.4 abcde |
| HO-1 (ng/mg tissue) | 5.6 ± 0.45 | 7.8 ± 0.37 a | 7.8 ± 0.37 a | 1.34 ± 0.28 abc | 3.54 ± 0.49 acbd | 5.8 ± 0.54 abcde |
| IL-6 (pg/mg tissue) | 22.4 ± 2.4 | 25.6 ± 3.4 | 25.6 ± 3.4 | 87.6 ± 5.9 abc | 51.2± 5.2 acbd | 33.7 ± 1.6 abcde |
| TNF-α (pg/mg tissue) | 4.5 ± 0.23 | 5.1 ± 0.73 | 5.1 ± 0.73 | 34.5 ± 2.8 abc | 17.6 ± 1.6 acbd | 9.4 ± 1.1 abcde |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hussan, R.; Albadr, N.A.; Alshammari, G.M.; Almasri, S.A.; Yahya, M.A. Phloretamide Prevent Hepatic and Pancreatic Damage in Diabetic Male Rats by Modulating Nrf2 and NF-κB. Nutrients 2023, 15, 1456. https://doi.org/10.3390/nu15061456
Al-Hussan R, Albadr NA, Alshammari GM, Almasri SA, Yahya MA. Phloretamide Prevent Hepatic and Pancreatic Damage in Diabetic Male Rats by Modulating Nrf2 and NF-κB. Nutrients. 2023; 15(6):1456. https://doi.org/10.3390/nu15061456
Chicago/Turabian StyleAl-Hussan, Rasha, Nawal A. Albadr, Ghedeir M. Alshammari, Soheir A. Almasri, and Mohammed Abdo Yahya. 2023. "Phloretamide Prevent Hepatic and Pancreatic Damage in Diabetic Male Rats by Modulating Nrf2 and NF-κB" Nutrients 15, no. 6: 1456. https://doi.org/10.3390/nu15061456
APA StyleAl-Hussan, R., Albadr, N. A., Alshammari, G. M., Almasri, S. A., & Yahya, M. A. (2023). Phloretamide Prevent Hepatic and Pancreatic Damage in Diabetic Male Rats by Modulating Nrf2 and NF-κB. Nutrients, 15(6), 1456. https://doi.org/10.3390/nu15061456







