Prevalence of Four Sarcopenia Criteria in Older Patients with Cancer, and Their Predictive Value for 6-Month Mortality: The NutriAgeCancer National Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Criteria for Sarcopenia Case-Finding, Assessment, Diagnosis and Severity Determination
2.3.1. The SARC-F Questionnaire
2.3.2. Hand-Grip Strength
2.3.3. Arm Circumference
2.3.4. The TUG Test
2.4. Outcome
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
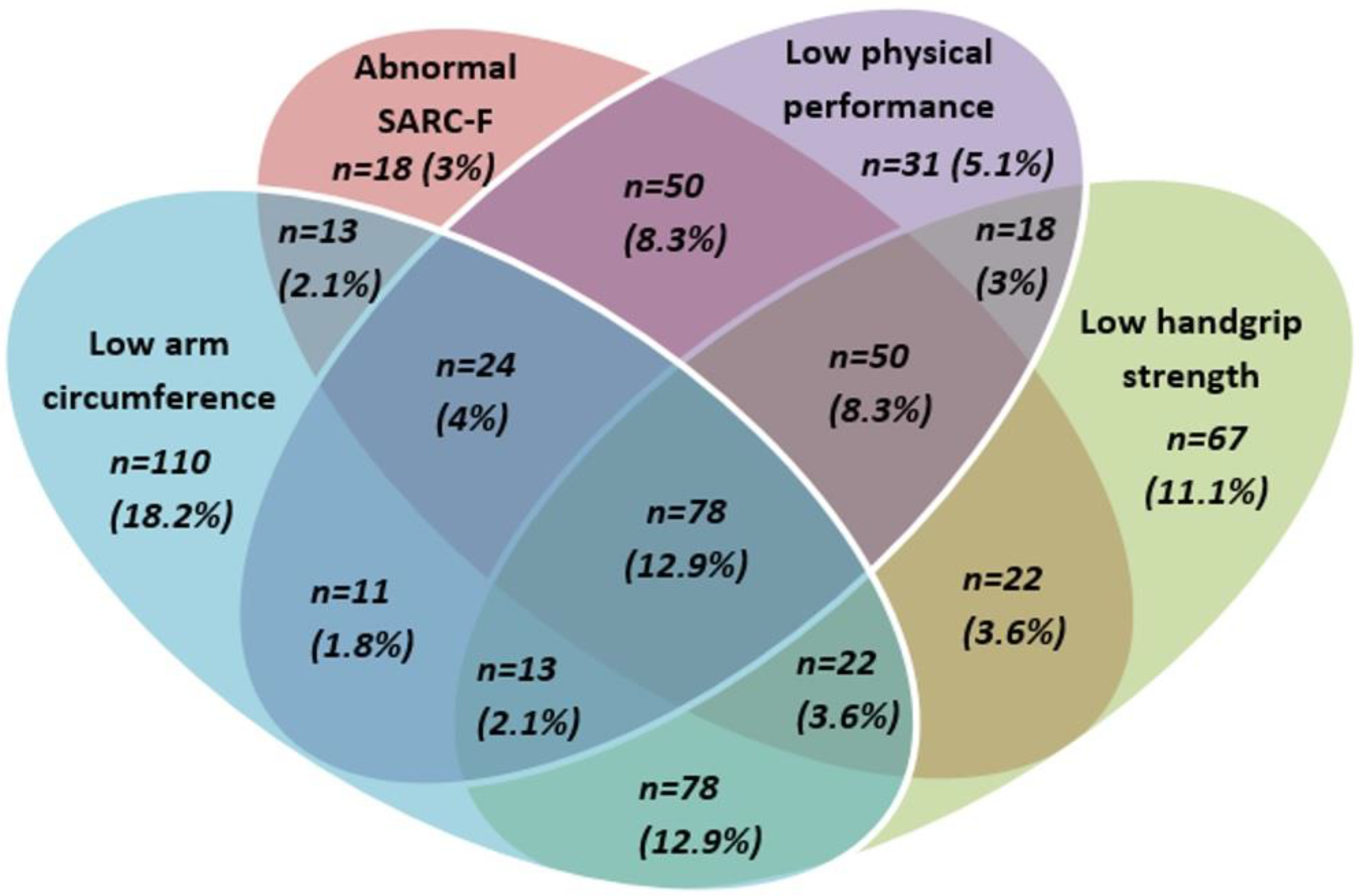
3.2. Prevalence of and Relationships between Criteria for Sarcopenia
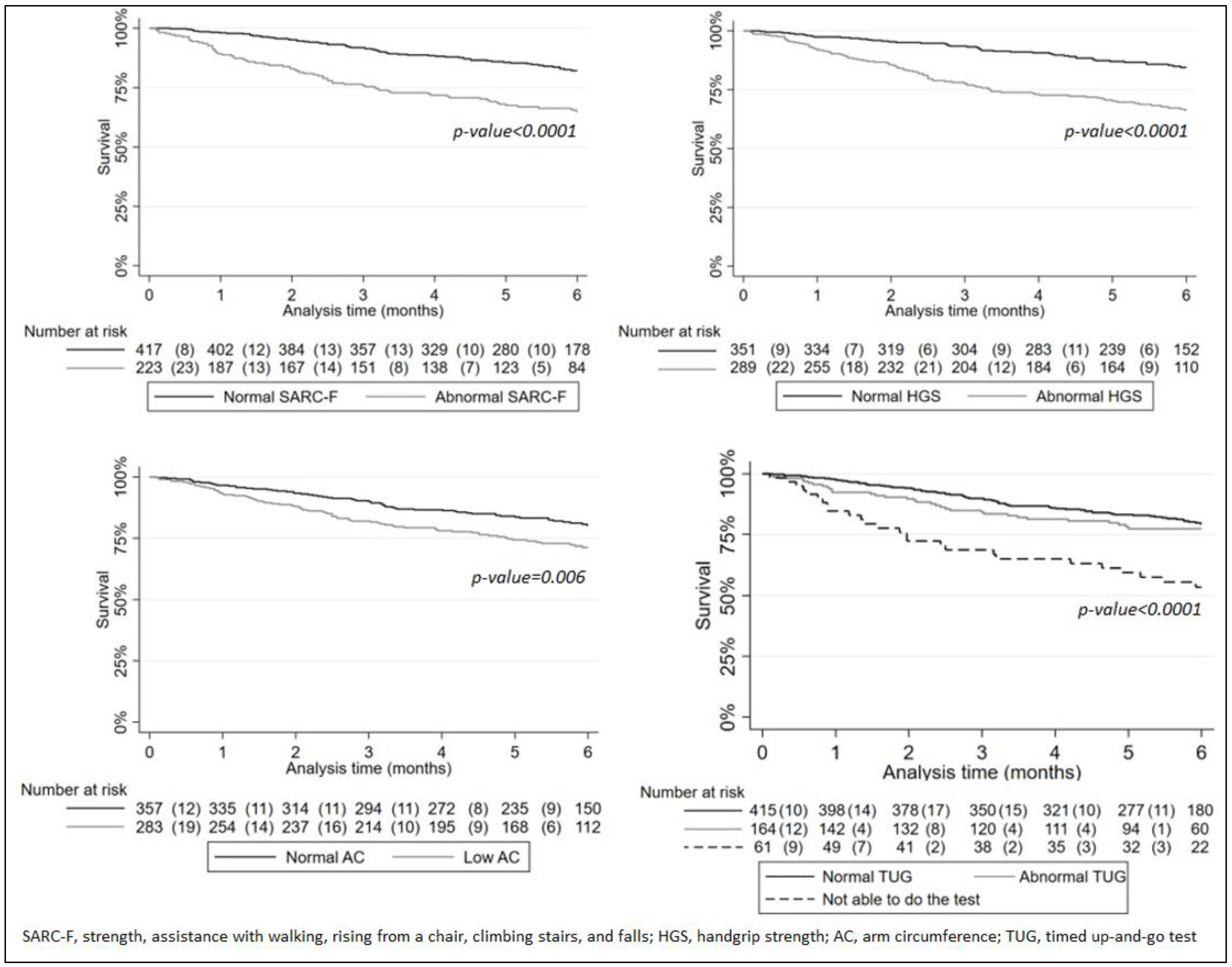
3.3. The Survival Analysis
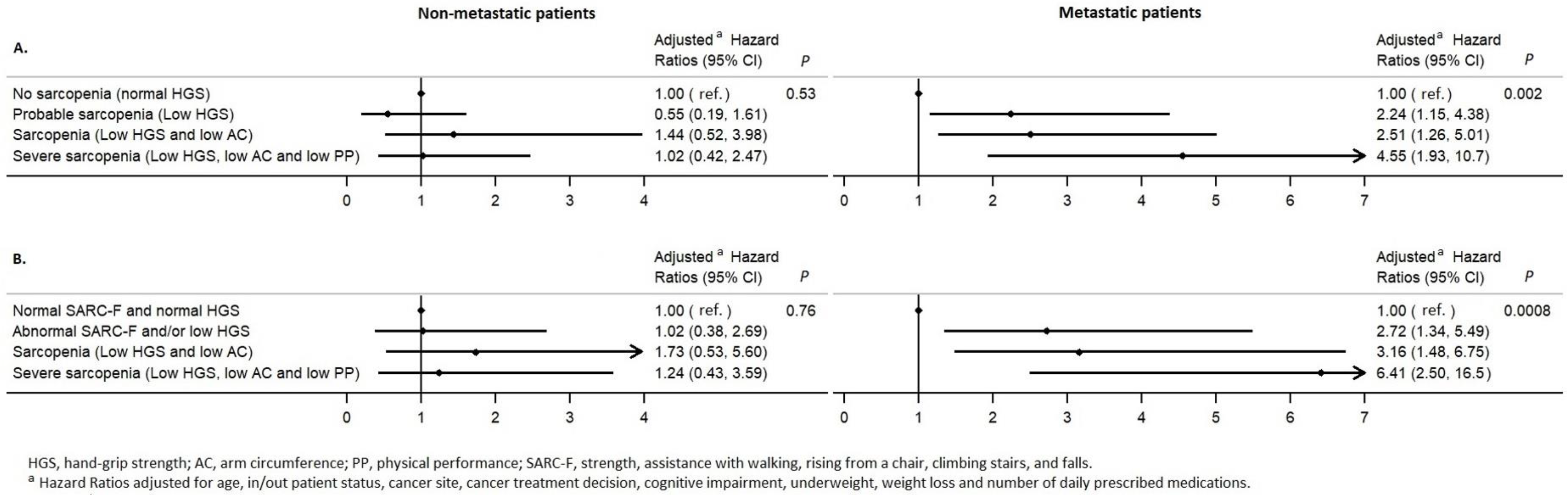
3.4. Sensitivity Analysis
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Marhold, M.; Topakian, T.; Unseld, M. Sarcopenia in cancer—A focus on elderly cancer patients. Memo Mag. Eur. Med. Oncol. 2021, 14, 20–23. [Google Scholar] [CrossRef]
- Williams, G.R.; Rier, H.N.; McDonald, A.; Shachar, S.S. Sarcopenia & aging in cancer. J. Geriatr. Oncol. 2019, 10, 374–377. [Google Scholar]
- Pamoukdjian, F.; Bouillet, T.; Levy, V.; Soussan, M.; Zelek, L.; Paillaud, E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin. Nutr. 2018, 37, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.O.; Cagney, D.; Hassan, F.; Lim, J.Y.; O’Leary, D.P.; Liew, A.; Redmond, H.P.; Corrigan, M.; O’Sullivan, M.; Kelly, L. Prevalence of sarcopenia and its impact on survival in breast cancer—A systematic review and meta-analysis. Mesentery Peritoneum 2019, 3, AB020. [Google Scholar] [CrossRef]
- Catikkas, N.M.; Bahat, Z.; Oren, M.M.; Bahat, G. Older cancer patients receiving radiotherapy: A systematic review for the role of sarcopenia in treatment outcomes. Aging Clin. Exp. Res. 2022, 34, 1747–1759. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, R.; Wan, Q.; Wu, Y.; Zhou, Y.; Wang, Y.; Cui, Y.; Shen, X.; Wu, X. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies. Cancer Med. 2020, 9, 7964–7978. [Google Scholar] [CrossRef]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef]
- Shen, Y.; Hao, Q.; Zhou, J.; Dong, B. The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: A systematic review and meta-analysis. BMC Geriatr. 2017, 17, 188. [Google Scholar] [CrossRef]
- Chan, M.Y.; Chok, K.S.H. Sarcopenia in pancreatic cancer—Effects on surgical outcomes and chemotherapy. World J. Gastrointest. Oncol. 2019, 11, 527–537. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Hanaoka, J.; Ohshio, Y.; Okamoto, K.; Kaku, R.; Hayashi, K.; Shiratori, T.; Akazawa, A. Does sarcopenia affect postoperative short- and long-term outcomes in patients with lung cancer?—A systematic review and meta-analysis. J. Thorac. Dis. 2021, 13, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Otten, L.; Stobäus, N.; Franz, K.; Genton, L.; Müller-Werdan, U.; Wirth, R.; Norman, K. Impact of sarcopenia on 1-year mortality in older patients with cancer. Age Ageing 2019, 48, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.J.; Liu, H.; Liu, X.L.; Jia, S.L.; Hou, L.S.; Xia, X.; Dong, B.R. Mid-Upper Arm Circumference as an Alternative Screening Instrument to Appendicular Skeletal Muscle Mass Index for Diagnosing Sarcopenia. Clin. Interv. Aging 2021, 16, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Esteves, C.L.; Ohara, D.G.; Matos, A.P.; Ferreira, V.T.K.; Iosimuta, N.C.R.; Pegorari, M.S. Anthropometric indicators as a discriminator of sarcopenia in community-dwelling older adults of the Amazon region: A cross-sectional study. BMC Geriatr. 2020, 20, 518. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Li, Y.; Xia, X.; Deng, C.; Wu, X.; Hou, L.; Yue, J.; Dong, B. Associations of geriatric nutrition risk index and other nutritional risk-related indexes with sarcopenia presence and their value in sarcopenia diagnosis. BMC Geriatr. 2022, 22, 327. [Google Scholar] [CrossRef]
- Williams, G.R.; Al-Obaidi, M.; Dai, C.; Bhatia, S.; Giri, S. SARC-F for screening of sarcopenia among older adults with cancer. Cancer 2021, 127, 1469–1475. [Google Scholar] [CrossRef]
- Ezzatvar, Y.; Ramírez-Vélez, R.; Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Izquierdo, M.; García-Hermoso, A. Physical Function and All-Cause Mortality in Older Adults Diagnosed with Cancer: A Systematic Review and Meta-Analysis. J. Gerontol. Biol. Sci. Med. Sci. 2021, 76, 1447–1453. [Google Scholar] [CrossRef]
- Wijnhoven, H.A.; van Bokhorst-de van der Schueren, M.A.; Heymans, M.W.; de Vet, H.C.; Kruizenga, H.M.; Twisk, J.W.; Visser, M. Low mid-upper arm circumference, calf circumference, and body mass index and mortality in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1107–1114. [Google Scholar] [CrossRef]
- Poisson, J.; Martinez-Tapia, C.; Heitz, D.; Geiss, R.; Albrand, G.; Falandry, C.; Gisselbrecht, M.; Couderc, A.L.; Boulahssass, R.; Liuu, E.; et al. Prevalence and prognostic impact of cachexia among older patients with cancer: A nationwide cross-sectional survey (NutriAgeCancer). J. Cachexia Sarcopenia Muscle 2021, 12, 1477–1488. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- D’Ath, P.; Katona, P.; Mullan, E.; Evans, S.; Katona, C. Screening, detection and management of depression in elderly primary care attenders. I: The acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam. Pract. 1994, 11, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the yield of medical tests. JAMA 1982, 247, 2543–2546. [Google Scholar] [CrossRef]
- Gönen, M.; Heller, G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika 2005, 92, 965–970. [Google Scholar] [CrossRef]
- Yourman, L.C.; Lee, S.J.; Schonberg, M.A.; Widera, E.W.; Smith, A.K. Prognostic indices for older adults: A systematic review. JAMA 2012, 307, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Behne, T.E.G.; Dock-Nasimento, D.B.; Sierra, J.C.; Rodrigues, H.H.N.P.; Palauro, M.L.; Andreo, F.O.; Silva-The, M.B.; DE-Aguilar-Nascimento, J.E. Association between preoperative potential sarcopenia and survival of cancer patients undergoing major surgical procedures. Rev. Col. Bras. Cir. 2020, 47, e20202528. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.V.; Paiva, A.E.G.; Silva, A.C.B.; de Castro, I.C.; Santiago, A.F.; de Oliveira, E.P.; Porto, L.C.J. Prevalence of sarcopenia according to EWGSOP1 and EWGSOP2 in older adults and their associations with unfavorable health outcomes: A systematic review. Aging Clin. Exp. Res. 2022, 34, 505–514. [Google Scholar] [CrossRef]
- Huang, D.D.; Cai, H.Y.; Wang, W.B.; Song, H.N.; Luo, X.; Dong, W.X.; Dong, Q.T.; Chen, X.L.; Yan, J.Y. Measurement of muscle quantity/quality has additional predictive value for postoperative complications and long-term survival after gastrectomy for gastric cancer in patients with probable sarcopenia as defined by the new EWGSOP2 consensus: Analysis from a large-scale prospective study. Nutrition 2021, 86, 111156. [Google Scholar]
- Trussardi Fayh, A.P.; de Sousa, I.M. Comparison of revised EWGSOP2 criteria of sarcopenia in patients with cancer using different parameters of muscle mass. PLoS ONE 2021, 16, e0257446. [Google Scholar] [CrossRef]
- Mori, N.; Maeda, K.; Fukami, Y.; Matsuyama, R.; Nonogaki, T.; Kato, R.; Ishida, Y.; Shimizu, A.; Ueshima, J.; Nagano, A. High SARC-F score predicts poor survival of patients with cancer receiving palliative care. Support Care Cancer 2022, 30, 4065–4072. [Google Scholar] [CrossRef]
- Matsui, M.; Nishikawa, H.; Goto, M.; Asai, A.; Ushiro, K.; Ogura, T.; Takeuchi, T.; Nakamura, S.; Kakimoto, K.; Miyazaki, T.; et al. Prognostic Impact of the SARC-F Score in Gastrointestinal Advanced Cancers. Cancers 2021, 14, 10. [Google Scholar] [CrossRef]
- Weng, C.H.; Tien, C.P.; Li, C.I.; L’Heureux, A.; Liu, C.S.; Lin, C.H.; Lin, C.C.; Lai, S.W.; Lai, M.M.; Lin, W.Y. Mid-upper arm circumference, calf circumference and mortality in Chinese long-term care facility residents: A prospective cohort study. BMJ Open 2018, 8, e020485. [Google Scholar] [CrossRef]
- Tsai, A.C.; Chang, T.L. The effectiveness of BMI, calf circumference and mid-arm circumference in predicting subsequent mortality risk in elderly Taiwanese. Br. J. Nutr. 2011, 105, 275–281. [Google Scholar] [CrossRef]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N | % | |
|---|---|---|---|
| Age | Mean ± standard deviation | 83.1 ± 5.99 | |
| ≥85 years | 314 | 40.2 | |
| Sex | Males | 365 | 46.7 |
| Poor performance status | ECOG-PS ≥ 2 | 334 | 43.8 |
| Cancer type | Head and neck | 34 | 4.4 |
| Esophageal/stomach | 45 | 5.8 | |
| Pancreas/liver | 62 | 7.9 | |
| Colorectal | 118 | 15.0 | |
| Prostate | 58 | 7.4 | |
| Urinary tract | 67 | 8.6 | |
| Lung | 79 | 10.1 | |
| Breast | 134 | 17.2 | |
| Gynecological | 71 | 9.1 | |
| Hematological | 42 | 5.4 | |
| Others a | 71 | 9.1 | |
| Metastasis | 328 | 42.3 | |
| Treatment, missing: n = 4 | Curative | 388 | 49.9 |
| Palliative | 314 | 40.4 | |
| Supportive care | 76 | 9.8 | |
| Score G-8 | Abnormal: ≤14/17 | 547 | 86.7 |
| Dependency | ADL ≤5/6 | 249 | 32.0 |
| Cognitive impairment | MMSE ≤23/30 or physician-diagnosed disorder | 277 | 38.0 |
| Risk of depression | mini-GDS ≥1/4 | 284 | 40.6 |
| Comorbidities | Updated Charlson Comorbidity Index, median (IQR) | 5 (3–7) | |
| Prescription medications | Number taken daily | 6 (3–8) | |
| Malnutrition | MNA score <17/30 | 105 | 14.8 |
| Underweight, missing: n = 3 | BMI <22 kg/m2 | 213 | 27.3 |
| Weight loss, missing: n = 75 | >5% in the previous 6 months | 335 | 47.2 |
| Low serum albumin | <35 | 200 | 37.9 |
| High serum CRP | >10 | 242 | 50.1 |
| SARC-F | Normal: <4/10 pts | 504 | 64.5 |
| Abnormal: ≥4/10 pts | 277 | 35.5 | |
| Hand-grip strength | Median (IQR), men | 26 (20–33) | |
| Median (IQR), women | 18 (12.5–20) | ||
| Normal: ≥27 kg for men; ≥16 kg for women | 433 | 55.4 | |
| Low: <27 kg for men; <16 kg for women | 348 | 44.6 | |
| Arm circumference | Normal: ≥26 cm for men; ≥25 cm for women | 432 | 55.3 |
| Low: <26 cm for men; <25 cm for women | 349 | 44.7 | |
| Timed up-and-go test | Normal: ≤20 sec. | 506 | 64.8 |
| Abnormal: >20 sec. | 199 | 25.5 | |
| Unable to perform the test | 76 | 9.7 | |
| SARC-F | Hand-Grip Strength | Arm Circumference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | p-Values | Normal | Low a | p-Values | Normal | Low b | p-Values | ||
| (<4/10 pts) | (≥4/10 pts) | |||||||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||
| Hand-grip strength | Normal | 328 (65.1) | 105 (37.9) | <0.0001 | ||||||
| Low a | 176 (34.9) | 172 (62.1) | ||||||||
| Arm circumference | Normal | 292 (57.9) | 140 (50.5) | 0.047 | 275 (63.5) | 157 (45.1) | <0.0001 | |||
| Low b | 212 (42.1) | 137 (49.5) | 158 (36.5) | 191 (54.9) | ||||||
| Timed up-and-go test | Normal (≤20 s) | 431 (85.5) | 75 (27.1) | <0.0001 | 317 (73.2) | 189 (54.3) | <0.0001 | 283 (65.5) | 223 (63.9) | 0.33 |
| Abnormal (>20 s) | 69 (13.7) | 130 (46.9) | 99 (22.9) | 100 (28.7) | 113 (26.2) | 86 (24.6) | ||||
| Unable to perform the test | 4 (0.8) | 72 (26.0) | 17 (3.9) | 59 (17.0) | 36 (8.3) | 40 (11.5) | ||||
| Overall Population N = 536 | Non-Metastatic Cancer n = 309 | Metastatic Cancer n = 227 | |||||
|---|---|---|---|---|---|---|---|
| Criteria | aHR a | p-value | aHR a | p-value | aHR a | p-value | |
| SARC-F | Normal (score < 4) | 1(ref) | 0.005 | 1(ref) | 1(ref) | ||
| Abnormal (score ≥ 4) | 1.82 (1.20–2.77) | 1.61 (0.78–3.35) | 0.20 | 1.94 (1.10–3.43) | 0.022 | ||
| Hand-grip strength | Normal | 1(ref) | 0.008 | 1(ref) | 1(ref) | ||
| Low (<27, men; <16, women) | 1.79 (1.16–2.75) | 0.90 (0.44–1.85) | 0.78 | 2.61 (1.52–4.47) | <0.0001 | ||
| Arm circumference | Normal | 1(ref) | 0.074 | 1(ref) | 1(ref) | ||
| Low (<26, men; <25, women) | 1.49 (0.96–2.32) | 1.04 (0.49–2.17) | 0.92 | 1.73 (0.98–3.04) | 0.057 | ||
| Timed up-and-go test | Normal (≤20 s) | 1(ref) | 0.004 | 1(ref) | 0.06 | 1(ref) | 0.13 |
| Abnormal (>20 s) | 0.90 (0.55–1.48) | 0.67 | 0.49 (0.19–1.23) | 0.13 | 1.04 (0.56–1.94) | 0.90 | |
| Unable to perform the test | 2.29 (1.32–3.97) | 0.003 | 1.51 (0.63–3.61) | 0.36 | 2.32 (1.01–5.32) | 0.048 | |
| Composite Variables for Sarcopenia | aHR a [95%CI] | p-Value |
|---|---|---|
| According to the EWGSOP2 definition | ||
| No sarcopenia (normal HGS) | 1.00 (ref) | 0.009 |
| Probable sarcopenia (Low HGS) | 1.31 [0.75–2.30] | |
| Sarcopenia (Low HGS and low AC) | 2.07 [1.19–3.61] | |
| Severe sarcopenia (Low HGS, low AC, and low PP) | 2.52 [1.38–4.62] | |
| Including the SARC-F in the definition | ||
| Normal SARC-F and normal HGS | 1.00 (ref) | 0.003 |
| Abnormal SARC-F and/or low HGS | 1.81 [1.03–3.19] | |
| Sarcopenia (Low HGS and low AC) | 2.63 [1.41–4.91] | |
| Severe sarcopenia (Low HGS, low AC, and low PP) | 3.37 [1.70–6.70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Tapia, C.; Rougette, K.; Fossey-Diaz, V.; Cudennec, T.; Taleb, C.; Balardy, L.; Mertens, C.; Mitha, N.; Bringuier, M.; Maley, K.; et al. Prevalence of Four Sarcopenia Criteria in Older Patients with Cancer, and Their Predictive Value for 6-Month Mortality: The NutriAgeCancer National Prospective Cohort Study. Nutrients 2023, 15, 1508. https://doi.org/10.3390/nu15061508
Martinez-Tapia C, Rougette K, Fossey-Diaz V, Cudennec T, Taleb C, Balardy L, Mertens C, Mitha N, Bringuier M, Maley K, et al. Prevalence of Four Sarcopenia Criteria in Older Patients with Cancer, and Their Predictive Value for 6-Month Mortality: The NutriAgeCancer National Prospective Cohort Study. Nutrients. 2023; 15(6):1508. https://doi.org/10.3390/nu15061508
Chicago/Turabian StyleMartinez-Tapia, Claudia, Kevin Rougette, Virginie Fossey-Diaz, Tristan Cudennec, Cherifa Taleb, Laurent Balardy, Cécile Mertens, Nathalie Mitha, Michael Bringuier, Karin Maley, and et al. 2023. "Prevalence of Four Sarcopenia Criteria in Older Patients with Cancer, and Their Predictive Value for 6-Month Mortality: The NutriAgeCancer National Prospective Cohort Study" Nutrients 15, no. 6: 1508. https://doi.org/10.3390/nu15061508
APA StyleMartinez-Tapia, C., Rougette, K., Fossey-Diaz, V., Cudennec, T., Taleb, C., Balardy, L., Mertens, C., Mitha, N., Bringuier, M., Maley, K., Estivin, S., Quipourt, V., Canoui-Poitrine, F., Baldini, C., Poisson, J., & Paillaud, E. (2023). Prevalence of Four Sarcopenia Criteria in Older Patients with Cancer, and Their Predictive Value for 6-Month Mortality: The NutriAgeCancer National Prospective Cohort Study. Nutrients, 15(6), 1508. https://doi.org/10.3390/nu15061508







