Combinatory Effects of Training and Nutritive Administration of Carbohydrates and Protein via Food on Strength in Postmenopausal Women, and Old Men and Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study A: Effect of Training and Protein/Carbohydrate Supplementation in Postmenopausal Women
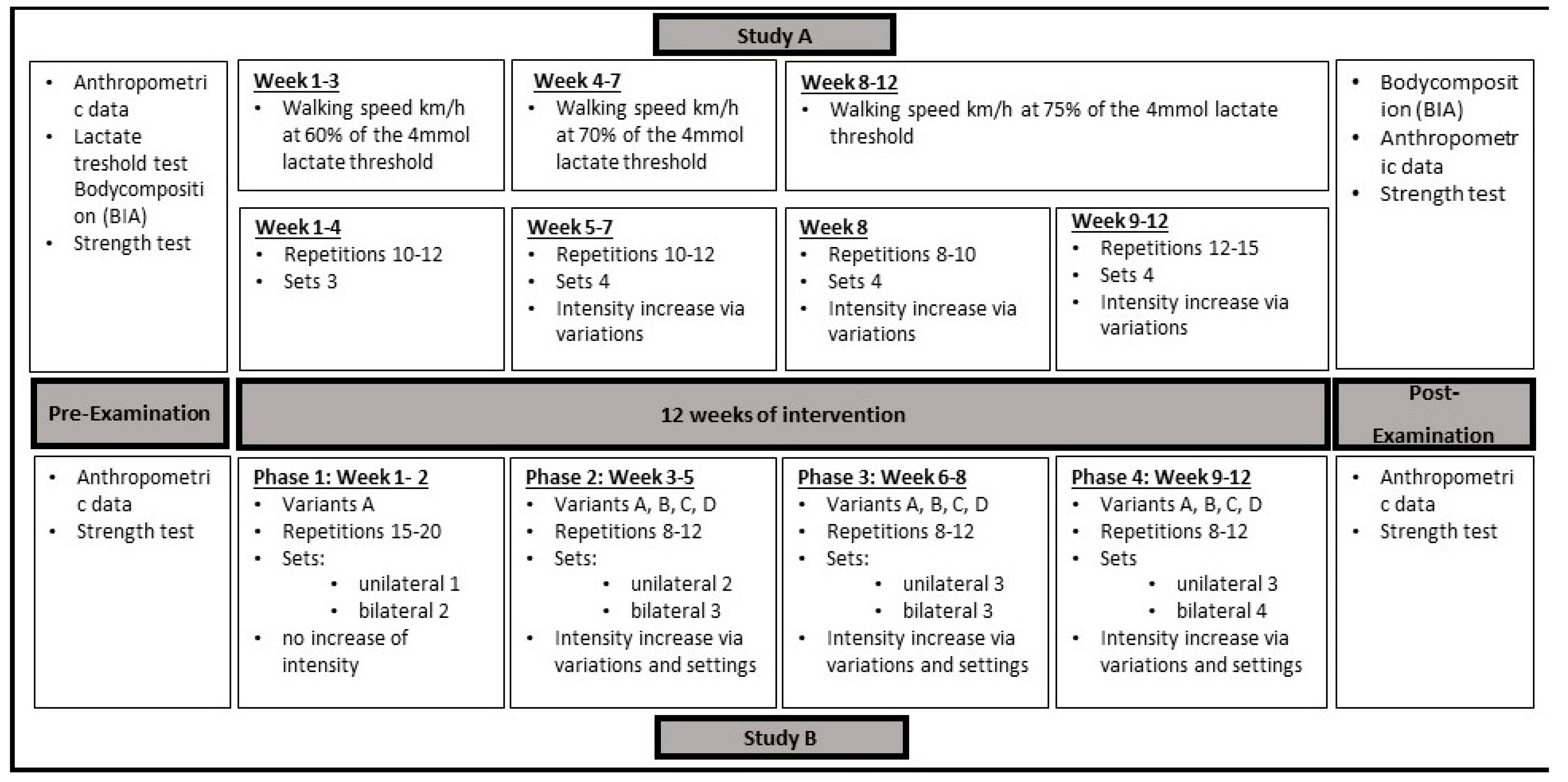
2.1.1. Study Design and Participants
2.1.2. Test Day
2.1.3. Training Intervention
2.1.4. Nutritional Intervention
2.2. Study B: Effect of Sling Training and Protein/Carbohydrate Supplementation in Elderly Men and Women
2.2.1. Study Design and Participants
2.2.2. Test Day
2.2.3. Training Intervention
- Two exercises for the upper body (rowing and chest press).
- Two exercises for the legs (squat and hip abduction).
- Two exercises for the trunk (crunches and side bend).
- One exercise for the entire ventral chain (body stretching).
2.2.4. Nutritional Intervention
2.3. Statistical Analysis
3. Results
3.1. Study A
3.1.1. PCA
3.1.2. LME
3.2. Study B
3.2.1. PCA
3.2.2. LME
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ko, S.-H.; Kim, H.-S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostini, D.; Zeppa Donati, S.; Lucertini, F.; Annibalini, G.; Gervasi, M.; Ferri Marini, C.; Piccoli, G.; Stocchi, V.; Barbieri, E.; Sestili, P. Muscle and Bone Health in Postmenopausal Women: Role of Protein and Vitamin D Supplementation Combined with Exercise Training. Nutrients 2018, 10, 1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, M.A.; Zunzunegui, M.V.; Vafaei, A.; Da Câmara, S.M.A.; Oliveira, T.S.; Maciel, Á.C.C. Sarcopenic obesity and physical performance in middle aged women: A cross-sectional study in Northeast Brazil. BMC Public Health 2016, 16, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemmler, W.; Engelke, K.; von Stengel, S.; Weineck, J.; Lauber, D.; Kalender, W.A. Long-term four-year exercise has a positive effect on menopausal risk factors: The Erlangen Fitness Osteoporosis Prevention Study. J. Strength Cond. Res. 2007, 21, 232–239. [Google Scholar] [PubMed]
- Skrypnik, D.; Bogdański, P.; Mądry, E.; Karolkiewicz, J.; Ratajczak, M.; Kryściak, J.; Pupek-Musialik, D.; Walkowiak, J. Effects of Endurance and Endurance Strength Training on Body Composition and Physical Capacity in Women with Abdominal Obesity. Obes. Facts 2015, 8, 175–187. [Google Scholar] [CrossRef]
- Nunes, P.R.P.; Barcelos, L.C.; Oliveira, A.A.; Furlanetto Júnior, R.; Martins, F.M.; Orsatti, C.L.; Resende, E.A.M.R.; Orsatti, F.L. Effect of resistance training on muscular strength and indicators of abdominal adiposity, metabolic risk, and inflammation in postmenopausal women: Controlled and randomized clinical trial of efficacy of training volume. Age 2016, 38, 40. [Google Scholar] [CrossRef] [Green Version]
- Manojlović, M.; Protić-Gava, B.; Maksimović, N.; Šćepanović, T.; Poček, S.; Roklicer, R.; Drid, P. Effects of Combined Resistance and Aerobic Training on Arterial Stiffness in Postmenopausal Women: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 9450. [Google Scholar] [CrossRef]
- Nascimento, C.M.; Ingles, M.; Salvador-Pascual, A.; Cominetti, M.R.; Gomez-Cabrera, M.C.; Viña, J. Sarcopenia, frailty and their prevention by exercise. Free Radic. Biol. Med. 2019, 132, 42–49. [Google Scholar] [CrossRef]
- Kirk, B.; Al Saedi, A.; Duque, G. Osteosarcopenia: A case of geroscience. Aging Med. 2019, 2, 147–156. [Google Scholar] [CrossRef]
- Maltais, M.L.; Desroches, J.; Dionne, I.J. Changes in muscle mass and strength after menopause. J. Musculoskelet. Neuronal Interact. 2009, 9, 186–197. [Google Scholar]
- Gorzelitz, J.; Trabert, B.; Katki, H.A.; Moore, S.C.; Watts, E.L.; Matthews, C.E. Independent and joint associations of weightlifting and aerobic activity with all-cause, cardiovascular disease and cancer mortality in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Br. J. Sport. Med. 2022, 56, 1277–1283. [Google Scholar] [CrossRef]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv. Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseeb, M.A.; Volpe, S.L. Protein and exercise in the prevention of sarcopenia and aging. Nutr. Res. 2017, 40, 1–20. [Google Scholar] [CrossRef]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and Menopause: The Role of Estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef]
- Liberman, K.; Forti, L.N.; Beyer, I.; Bautmans, I. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: A systematic review. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 30–53. [Google Scholar] [CrossRef]
- Juopperi, S.; Sund, R.; Rikkonen, T.; Kröger, H.; Sirola, J. Cardiovascular and musculoskeletal health disorders associate with greater decreases in physical capability in older women. BMC Musculoskelet. Disord. 2021, 22, 192. [Google Scholar]
- Awick, E.A.; Ehlers, D.K.; Aguiñaga, S.; Daugherty, A.M.; Kramer, A.F.; McAuley, E. Effects of a randomized exercise trial on physical activity, psychological distress and quality of life in older adults. Gen. Hosp. Psychiatry 2017, 49, 44–50. [Google Scholar] [CrossRef]
- McAuley, E.; Konopack, J.F.; Motl, R.W.; Morris, K.S.; Doerksen, S.E.; Rosengren, K.R. Physical activity and quality of life in older adults: Influence of health status and self-efficacy. Ann. Behav. Med. Publ. Soc. Behav. Med. 2006, 31, 99–103. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.; Walrand, S.; Boirie, Y. Protein redistribution from skeletal muscle to splanchnic tissue on fasting and refeeding in young and older healthy individuals. J. Am. Med. Dir. Assoc. 2013, 14, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Cholewa, J.M.; Dardevet, D.; Huang, T.; Zhao, Y.; Shang, H.; Yang, Y.; Ding, X.; Zhang, C.; Wang, H.; et al. Effects of oat protein supplementation on skeletal muscle damage, inflammation and performance recovery following downhill running in untrained collegiate men. Food Funct. 2018, 9, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, L.; Brindisi, J.; Kleppinger, A.; Sullivan, R.; Mangano, K.M.; Bihuniak, J.D.; Kenny, A.M.; Kerstetter, J.E.; Insogna, K.L. Adequate dietary protein is associated with better physical performance among post-menopausal women 60–90 years. J. Nutr. Health Aging 2014, 18, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reidy, P.T.; Rasmussen, B.B. Role of Ingested Amino Acids and Protein in the Promotion of Resistance Exercise-Induced Muscle Protein Anabolism. J. Nutr. 2016, 146, 155–183. [Google Scholar] [CrossRef] [Green Version]
- van Loon, L.J.C.; Saris, W.H.M.; Verhagen, H.; Wagenmakers, A.J.M. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate1–3. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isenmann, E.; Blume, F.; Bizjak, D.A.; Hundsdörfer, V.; Pagano, S.; Schibrowski, S.; Simon, W.; Schmandra, L.; Diel, P. Comparison of Pro-Regenerative Effects of Carbohydrates and Protein Administrated by Shake and Non-Macro-Nutrient Matched Food Items on the Skeletal Muscle after Acute Endurance Exercise. Nutrients 2019, 11, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenberg, T.; von Stengel, S.; Sieber, C.; Kemmler, W. The Favorable Effects of a High-Intensity Resistance Training on Sarcopenia in Older Community-Dwelling Men with Osteosarcopenia: The Randomized Controlled FrOST Study. Clin. Interv. Aging 2019, 14, 2173–2186. [Google Scholar] [CrossRef] [Green Version]
- Dedeyne, L.; Deschodt, M.; Verschueren, S.; Tournoy, J.; Gielen, E. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: A systematic review. Clin. Interv. Aging 2017, 12, 873–896. [Google Scholar] [CrossRef] [Green Version]
- Denison, H.J.; Cooper, C.; Sayer, A.A.; Robinson, S.M. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging 2015, 10, 859–869. [Google Scholar]
- Trommelen, J.; Betz, M.W.; van Loon, L.J.C. The Muscle Protein Synthetic Response to Meal Ingestion Following Resistance-Type Exercise. Sport. Med. 2019, 49, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diel, P. Effects of a Nutritive Administration of Carbohydrates and Protein by Foodstuffs on Skeletal Muscle Inflammation and Damage After Acute Endurance Exercise. JNHFS 2017, 5, 1–7. [Google Scholar] [CrossRef]
- Wacker, A. Kardiovaskuläre und metabolische Risikofaktoren nach der Menopause. Einfluss Unterschiedlicher Trainingsinterventionen auf die Körperliche Leistungsfähigkeit, das Kardiovaskuläre Risikoprofil und die Mechanismen des Energiestoffwechsels der Postmenopausalen Frau. Ph.D. Dissertation, Deutsche Sporthochschule Köln, Köln, Germany, 2018. [Google Scholar]
- Rühl, J.; Schuba, V. Funktionelles Fitnesskrafttraining, 1. Auflage; Meyer & Meyer Sport: Aachen, Germany, 2003. [Google Scholar]
- Gaedtke, A. Erstellung und Effektivitätsprüfung eines Sling-Trainings zur Verbesserung der funktionellen Mobilität, der Kraft- und der Gleichgewichtsfähigkeit von Älteren; Deutsche Sporthochschule Köln: Köln, Germany, 2014. [Google Scholar]
- Gaedtke, A.; Morat, T. Effects of Two 12-week Strengthening Programmes on Functional Mobility, Strength and Balance of Older Adults: Comparison between TRX Suspension Training versus an Elastic Band Resistance Training. Cent. Eur. J. Sport Sci. Med. 2016, 13, 49–64. [Google Scholar] [CrossRef]
- Gaedtke, A.; Morat, T. TRX Suspension Training: A New Functional Training Approach for Older Adults—Development, Training Control and Feasibility. Int. J. Exerc. Sci. 2015, 8, 224–233. [Google Scholar]
- Kolster, B. Medizinische Trainingstherapie. In Leitfaden Physiotherapie—Befund, Techniken, Behandlungen, Rehabilitation; Kolster, B., Ebelt-Paprotny, M., Hirsch, M., Eds.; Urban und Fischer: Stuttgart, Germany, 1994; pp. 618–636. [Google Scholar]
- Tschopp, M.; Bourban, P.; Hübner, K.; Marti, B. Messgenauigkeit eines 4-teiligen, standardisierten dynamischen Rumpfkrafttests: Erfahrungen mit gesunden männlichen Spitzensportlern. Schweiz. Z. Für Sportmed. Und Sport. 2001, 49, 67–72. [Google Scholar]
- Bourban, P.; Hübner, K.; Tschopp, M.; Marti, B. Grundkraftanforderungen im Spitzensport: Ergebnisse eines 3-teiligen Rumpfkrafttests. Schweiz. Z. Für Sportmed. Und Sport. 2001, 49, 73–78. [Google Scholar]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sport. Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 24 February 2023).
- Pinheiro, J.; Bates, D.; R Core Team. NLME: Linear and Nonlinear Mixed Effects Models; R Core Team: Vienna, Austria, 2007. [Google Scholar]
- Jiménez-García, J.D.; Hita-Contreras, F.; de La Torre-Cruz, M.J.; Aibar-Almazán, A.; Achalandabaso-Ochoa, A.; Fábrega-Cuadros, R.; Martínez-Amat, A. Effects of HIIT and MIIT Suspension Training Programs on Sleep Quality and Fatigue in Older Adults: Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 1211. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Schoenfeld, B.J.; Marini, E.; Stagi, S.; Mauro, M.; Toselli, S. Effects of a 12-Week Suspension versus Traditional Resistance Training Program on Body Composition, Bioimpedance Vector Patterns, and Handgrip Strength in Older Men: A Randomized Controlled Trial. Nutrients 2021, 13, 2267. [Google Scholar] [CrossRef]
- Soligon, S.D.; Da Silva, D.G.; Bergamasco, J.G.A.; Angleri, V.; Júnior, R.A.M.; Dias, N.F.; Nóbrega, S.R.; de Castro Cesar, M.; Libardi, C.A. Suspension training vs. traditional resistance training: Effects on muscle mass, strength and functional performance in older adults. Eur. J. Appl. Physiol. 2020, 120, 2223–2232. [Google Scholar]
- Morat, T.; Holzer, D.; Trumpf, R. Trunk Muscle Activation During Dynamic Sling Training Exercises. Int. J. Exerc. Sci. 2019, 12, 590–601. [Google Scholar]
- Unsgaard-Tøndel, M.; Fladmark, A.M.; Salvesen, Ø.; Vasseljen, O. Motor control exercises, sling exercises, and general exercises for patients with chronic low back pain: A randomized controlled trial with 1-year follow-up. Phys. Ther. 2010, 90, 1426–1440. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.; Yang, S.-H.; Koog, Y.-H.; Jun, H.-J.; Kim, S.-H.; Kim, K.-J. Effectiveness of Sling Exercise for Chronic Low Back Pain: A Systematic Review. J. Phys. Ther. Sci. 2014, 26, 1301–1306. [Google Scholar] [CrossRef] [Green Version]
- Zawadski, K.M.; Yaspelkis, B.B., III; Ivy, J.L. Carbohydrate-protein complex increases the rate of muscle glycogen storage after exercise. Am. Physiol. Soc. 1992, 72, 1854–1859. [Google Scholar]
- Batacan, R.B.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sport. Med. 2017, 51, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Kim, H. Hand grip strength and health-related quality of life in postmenopausal women: A national population-based study. Menopause 2021, 28, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Z.; Zhuang, H.-F.; Cai, S.-Q.; Lin, C.-K.; Wang, P.-W.; Yan, L.-S.; Lin, J.-K.; Yu, H.-M. Low Grip Strength is a Strong Risk Factor of Osteoporosis in Postmenopausal Women. Orthop. Surg. 2018, 10, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Training in der Therapie. Grundlagen und Praxis, 3rd ed.; Froböse, I.; Nellessen, G.; Wilke, C. (Eds.) Elsevie: München, Germany; Jena, Germany, 2010. [Google Scholar]
- Keller, K.; Engelhardt, M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2019, 3, 346. [Google Scholar] [CrossRef]





| Study A | 100 g Sour Milk Cheese and 76 g White Bread |
| Protein (g) | 36.1 |
| carbohydrate (g) | 35.3 |
| fat (g) | 3.5 |
| kcal | 321 |
| Study B | 100 g Sour Milk Cheese and 76 g White Bread and 250 mL Buttermilk |
| Protein (g) | 44.6 |
| carbohydrate (g) | 45.8 |
| fat (g) | 5 |
| kcal | 416 |
| Total Sample | Intervention Group | Control Group | |
|---|---|---|---|
| Study A | N = 51 | N = 24 | N = 28 |
| Age (years) | 57.3 ± 3.0 | 57.9 ± 3.3 | 56.8 ± 2.8 |
| Height (cm) | 167 ± 7.3 | 169.8 ± 6.9 | 164.6 ± 6.7 |
| Weight (kg) | 69.7 ± 12.7 | 70.8 ± 15.2 | 68.8 ± 10.3 |
| BMI | 25.1 ± 4.4 | 24.5 ± 4.7 | 25.5 ± 4.2 |
| N = 51 | Intervention Group | Control Group | ||
|---|---|---|---|---|
| Pre Intervention | Post Intervention | Pre Intervention | Post Intervention | |
| Strength | ||||
| Leg (kg) | 89.9 ± 20.9 | 95.9 ± 24.2 | 91.4 ± 26.4 | 105.0 ± 25.9 |
| Chest (kg) | 28.0 ± 8.2 | 31.9 ± 8.4 | 25.5 ± 5.6 | 27.7 ± 5.7 |
| Hand-grip right (kg) | 28.9 ± 4.7 | 31.3 ± 4.0 | 27.8 ± 4.2 | 29.0 ± 3.9 |
| Hand-grip left (kg) | 27.9 ± 4.5 | 29.1 ± 3.9 | 26.8 ± 4.5 | 27.5 ± 4.3 |
| Hand-grip sum (kg) | 51.1 ± 18.9 | 54.2 ± 18.9 | 53.5 ± 9.4 | 56.5 ± 7.6 |
| Body composition | ||||
| Body weight | 70.8 ± 15.2 | 70.5 ± 15.5 | 68.8 ± 10.5 | 68.1 ± 10.1 |
| Muscle mass | 19.1 ± 2.4 | 19.3 ± 2.5 | 18.8 ± 1.8 | 19.2 ± 2.0 |
| Fat mass | 25.6 ± 11.0 | 25.2 ± 11.1 | 24.4 ± 7.7 | 23.4 ± 7.1 |
| Value | Std. Error | DF | t-Value | p-Value | |
|---|---|---|---|---|---|
| (Intercept) | −0.0888 | 0.321 | 46 | −0.276 | 0.784 |
| TimePost | 0.649 | 0.15 | 38 | 4.32 | 0.000108 |
| GrpTreat | 0.398 | 0.467 | 46 | 0.851 | 0.399 |
| TreatPost × GrpTreat | −0.0427 | 0.224 | 38 | −0.191 | 0.85 |
| Total Sample | Intervention Group | Control Group | |
|---|---|---|---|
| Study B | N = 31 | N = 15 | N = 16 |
| Age (years) | 65.9 ± 4.9 | 67.7 ± 5.9 | 64.2 ± 3.2 |
| Height (cm) | 166.1 ± 8.5 | 165.3 ± 9.2 | 166.9 ± 7.9 |
| Weight (kg) | 85.4 ± 15.6 | 87.3 ± 14.9 | 83.6 ± 15.5 |
| BMI | 30.9 ± 5.1 | 31.8 ± 4.1 | 30.0 ± 5.7 |
| N = 31 | Intervention Group | Control Group | ||
|---|---|---|---|---|
| Preintervention | Postintervention | Preintervention | Postintervention | |
| Leg strength (kg) | 117.0 ± 41.7 | 142.9 ± 50.2 | 105.3 ± 32.2 | 130.8 ± 32.3 |
| Chest strength (kg) | 39.7 ± 14.8 | 46.5 ± 15.9 | 30.4 ± 26.2 | 44.9 ± 30.8 |
| Ventral-trunk strength (sec) | 30.3 ± 18.1 | 49.4 ± 21.1 | 27.8 ± 4.2 | 29.0 ± 3.9 |
| Lateral-trunk strength—right | 12.9 ± 11.7 | 30.6 ± 16.2 | 12.4 ± 11.4 | 19.8 ± 13.9 |
| Lateral-trunk strength—left | 19.4 ± 13.5 | 30.7 ± 13.0 | 13.1 ± 9.3 | 21.8 ± 12.4 |
| Dorsal-trunk strength | 65.2 ± 41.3 | 79.6 ± 49.8 | 62.5 ± 26.4 | 85.6 ± 31.7 |
| Body weight | 87.2 ± 14.9 | 85.7 ± 14.3 | 83.6 ± 15.4 | 82.6 ± 15.2 |
| Value | Std. Error | DF | t-Value | p-Value | |
|---|---|---|---|---|---|
| (Intercept) | 0.157 | 0.551 | 25 | 0.285 | 0.778 |
| TimePost | 2.305 | 0.291 | 19 | 7.921 | 0.000 |
| AdipAdip+ | −1.683 | 0.763 | 25 | −2.207 | 0.037 |
| GrpTreat | 0.856 | 0.758 | 25 | 1.130 | 0.269 |
| TimePost × AdipAdip+ | −1.513 | 0.407 | 19 | −3.718 | 0.001 |
| TimePost × GrpTreat | 0.950 | 0.407 | 19 | 2.331 | 0.031 |
| Value | Std. Error | DF | t-Value | p-Value | |
|---|---|---|---|---|---|
| (Intercept) | −0.247 | 0.393 | 25 | −0.628 | 0.536 |
| TimePost | 0.753 | 0.274 | 19 | 2.751 | 0.013 |
| AdipAdip+ | 0.942 | 0.545 | 25 | 1.728 | 0.096 |
| GrpTreat | −0.562 | 0.543 | 25 | −1.034 | 0.311 |
| TimePost × AdipAdip+ | −0.364 | 0.383 | 19 | −0.952 | 0.353 |
| TimePost × GrpTreat | −0.352 | 0.383 | 19 | −0.917 | 0.371 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, K.; Flenker, U.; Kiewardt, G.; Diel, P.R. Combinatory Effects of Training and Nutritive Administration of Carbohydrates and Protein via Food on Strength in Postmenopausal Women, and Old Men and Women. Nutrients 2023, 15, 1531. https://doi.org/10.3390/nu15061531
Hofmann K, Flenker U, Kiewardt G, Diel PR. Combinatory Effects of Training and Nutritive Administration of Carbohydrates and Protein via Food on Strength in Postmenopausal Women, and Old Men and Women. Nutrients. 2023; 15(6):1531. https://doi.org/10.3390/nu15061531
Chicago/Turabian StyleHofmann, Katharina, Ulrich Flenker, Gina Kiewardt, and Patrick Rene Diel. 2023. "Combinatory Effects of Training and Nutritive Administration of Carbohydrates and Protein via Food on Strength in Postmenopausal Women, and Old Men and Women" Nutrients 15, no. 6: 1531. https://doi.org/10.3390/nu15061531
APA StyleHofmann, K., Flenker, U., Kiewardt, G., & Diel, P. R. (2023). Combinatory Effects of Training and Nutritive Administration of Carbohydrates and Protein via Food on Strength in Postmenopausal Women, and Old Men and Women. Nutrients, 15(6), 1531. https://doi.org/10.3390/nu15061531






