Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat Metabolic Disorders to Maintain Health
Abstract
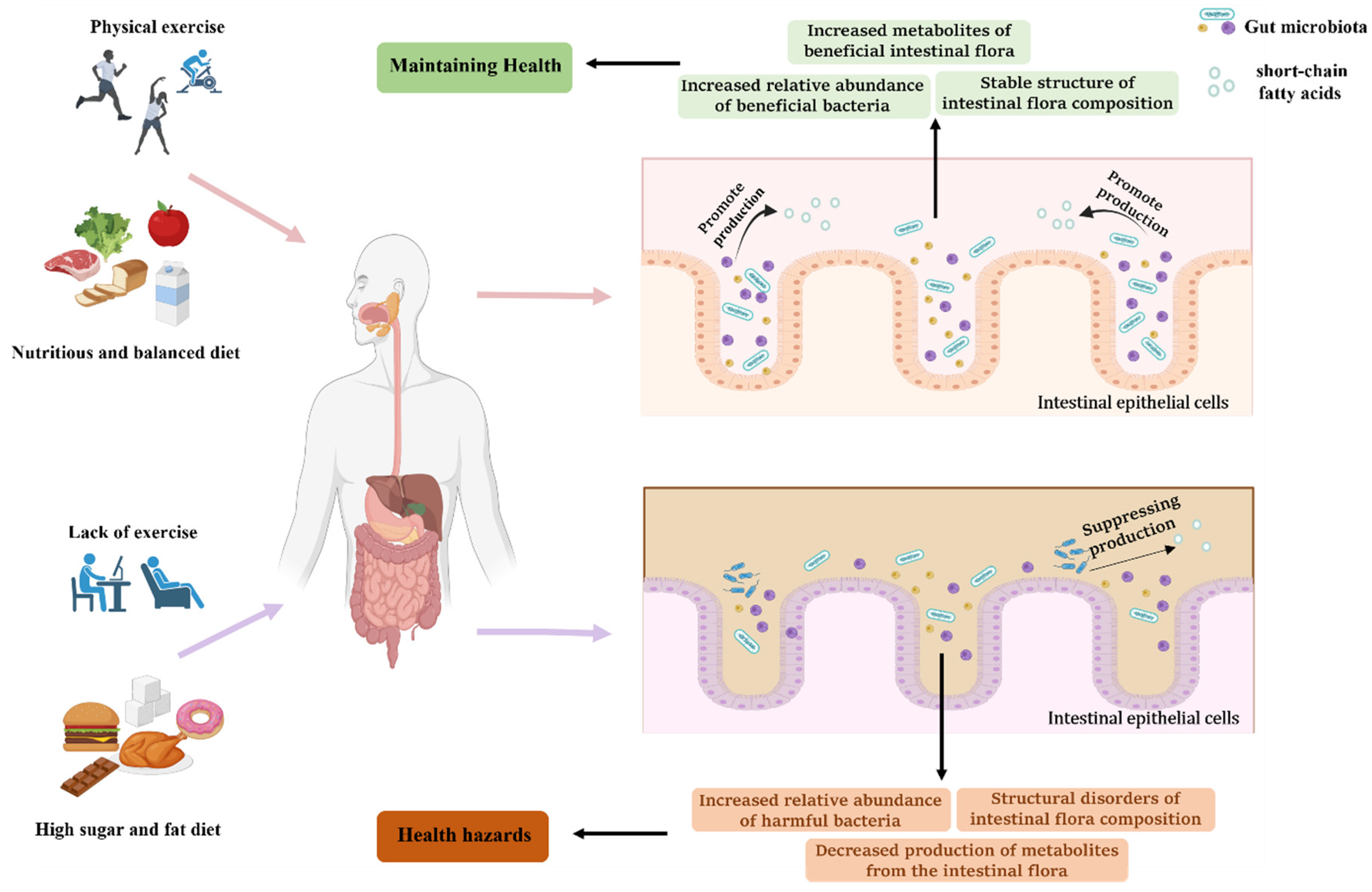
:1. Introduction
2. Physical Activity, Diet, and Gut Microbiota
2.1. Effect of Physical Exercise on Gut Microbiota
2.2. Dietary Proteins
3. The Role of Gut Microbiota in Metabolic Disorders
3.1. Metabolic Disorders and Metabolic Diseases
3.2. The Close Association between Gut Microbiota and Metabolic Disorders
4. Physical Exercise and Diet, Together, Regulate Gut Microbiota to Prevent and Treat Metabolic Diseases
4.1. Treatment and Prevention of Type 2 Diabetes
4.2. Treatment and Prevention of Hyperlipidemia
| Diet Composition | Experimental Model | Symptoms | Treatment Methods | Affected Gut Microbiota | Experimental Results | Reference |
|---|---|---|---|---|---|---|
| TP | C57BL/6 mice (6 weeks old), high-fat diet feeding | Mice with hyperlipidemia | Daily intake of TP | Restores disturbed gut microbiota and affects the distribution of gut microbiota in the intestine | Hyperlipidemia symptoms have improved | [127] |
| Auricularia auricula polysaccharide | Sprague-Dawley rats (5 weeks old), high-fat diet feeding | Daily intake of Auricularia auricula polysaccharide | Increased relative abundance of Lactobacillus, Roseburia, and Oscillibacter and increased production of SCFAs in the intestine | [128] | ||
| Oat Flavonoids | C57BL/6N mice (5-week-old), high-fat diet feeding | Daily intake of Oat Flavonoids | The relative abundance of Akkermansia increased and the relative abundance of Lachnoclostridium, Blautia, and Colidextribacter decreased | [12] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsen, O.F.A.; Claassen, E. The mechanistic link between health and gut microbiota diversity. Sci. Rep. 2018, 8, 2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agus, A.; Clement, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 2019, 22, 592–602. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef] [Green Version]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, gut microbiota, and obesity: Links with host genetics and epigenetics and potential applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from whole-grain oat alleviated high-fat diet-induced hyperlipidemia via regulating bile acid metabolism and gut microbiota in mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beverly, J.K.; Budoff, M.J. Atherosclerosis: Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J. Diabetes 2020, 12, 102–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Yang, Y.; Li, Y.; Han, R. Physical exercise as therapy for type 2 diabetes mellitus: From mechanism to orientation. Ann. Nutr. Metab. 2019, 74, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Brial, F.; Le Lay, A.; Dumas, M.E.; Gauguier, D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell. Mol. Life Sci. 2018, 75, 3977–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.J.; Smits, L.P.; Pekmez, C.T.; Prodan, A.; Meijnikman, A.S.; Troelstra, M.A.; Bouter, K.E.C.; Herrema, H.; Levin, E.; Holleboom, A.G.; et al. Donor fecal microbiota transplantation alters gut microbiota and metabolites in obese individuals with steatohepatitis. Hepatol. Commun. 2020, 4, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Shively, C.A.; Appt, S.E.; Vitolins, M.Z.; Uberseder, B.; Michalson, K.T.; Silverstein-Metzler, M.G.; Register, T.C. Mediterranean versus western diet effects on caloric intake, obesity, metabolism, and hepatosteatosis in nonhuman primates. Obesity 2019, 27, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montero, C.; Fraile-Martinez, O.; Gomez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; Garcia-Honduvilla, N.; Asunsolo, A.; et al. Nutritional components in western diet versus mediterranean diet at the gut microbiota-immune system interplay. Implications for health and disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of mediterranean diet on human gut microbiota. Nutrients 2021, 13, 7. [Google Scholar] [CrossRef]
- Barber, C.; Mego, M.; Sabater, C.; Vallejo, F.; Bendezu, R.A.; Masihy, M.; Guarner, F.; Espin, J.C.; Margolles, A.; Azpiroz, F. Differential effects of western and mediterranean-type diets on gut microbiota: A metagenomics and metabolomics approach. Nutrients 2021, 13, 2638. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Wang, R.; An, Y.; Gao, W.; Bai, L.; Li, Y.; Zhao, S.; Fan, J.; Liu, E. Western diet feeding influences gut microbiota profiles in apoE knockout mice. Lipids Health Dis. 2018, 17, 159. [Google Scholar] [CrossRef] [Green Version]
- Bortolin, R.C.; Vargas, A.R.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Martinello, K.B.; Silveira, A.K.; Rabelo, T.K.; Gelain, D.P.; Moreira, J.C.F. A new animal diet based on human Western diet is a robust diet-induced obesity model: Comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. 2018, 42, 525–534. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Aburub, A.; Ledger, S.J.; Sim, J.; Hunter, S.M. Cardiopulmonary function and aerobic exercise in parkinson’s: A systematic review of the literature. Mov. Disord. Clin. Pract. 2020, 7, 599–606. [Google Scholar] [CrossRef]
- Yu, W.W.; Lee, S.; Arslanian, S.; Tamim, H.; Kuk, J.L. Effects of exercise on resting metabolic rate in adolescents with overweight and obesity. Child. Obes. 2021, 17, 249–256. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Sanchez-Delgado, G.; Ruiz, J.R.; Castillo, M.J. Metabolic rate in sedentary adults, following different exercise training interventions: The fit-ageing randomized controlled trial. Clin. Nutr. 2020, 39, 3230–3240. [Google Scholar] [CrossRef]
- Queipo-Ortuno, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, 65465. [Google Scholar] [CrossRef] [Green Version]
- Denou, E.; Marcinko, K.; Surette, M.G.; Steinberg, G.R.; Schertzer, J.D. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, 982–993. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Alvarez, L.; Xu, H.; Martinez-Tellez, B. Influence of exercise on the human gut microbiota of healthy adults: A systematic review. Clin. Transl. Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef]
- Chekroud, S.R.; Gueorguieva, R.; Zheutlin, A.B.; Paulus, M.; Krumholz, H.M.; Krystal, J.H.; Chekroud, A.M. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry 2018, 5, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Fernandez, M.; Porras, D.; Garcia-Mediavilla, M.V.; Roman-Saguillo, S.; Gonzalez-Gallego, J.; Nistal, E.; Sanchez-Campos, S. Aging, gut microbiota and metabolic diseases: Management through physical exercise and nutritional interventions. Nutrients 2021, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, Y.; Amamoto, R.; Park, S.; Honda, Y.; Shimamoto, K.; Kushiro, A.; Tsuji, H.; Matsumoto, H.; Shimizu, K.; Miyazaki, K.; et al. Independent and interactive effects of habitually ingesting fermented milk products containing lactobacillus casei strain shirota and of engaging in moderate habitual daily physical activity on the intestinal health of older people. Front. Microbiol. 2019, 10, 1477. [Google Scholar] [CrossRef] [Green Version]
- Castellanos, N.; Diez, G.G.; Antunez-Almagro, C.; Bailen, M.; Bressa, C.; Gonzalez Soltero, R.; Perez, M.; Larrosa, M. A critical mutualism—Competition interplay underlies the loss of microbial diversity in sedentary lifestyle. Front. Microbiol. 2019, 10, 3142. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, N.; Diez, G.G.; Antunez-Almagro, C.; Bressa, C.; Bailen, M.; Gonzalez-Soltero, R.; Perez, M.; Larrosa, M. Key bacteria in the gut microbiota network for the transition between sedentary and active lifestyle. Microorganisms 2020, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, 92193. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef]
- Wang, Y.; You, Y.; Tian, Y.; Sun, H.; Li, X.; Wang, X.; Wang, Y.; Liu, J. Pediococcus pentosaceus pp04 ameliorates high-fat diet-induced hyperlipidemia by regulating lipid metabolism in C57BL/6N mice. J. Agric. Food Chem. 2020, 68, 15154–15163. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Fang, D.; Yang, B.; Su, C.; Li, R.; Xiao, X.; et al. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol. 2021, 6, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; An, Y.; Tang, H.; Wang, Y. Alterations of bile acids and gut microbiota in obesity induced by high fat diet in rat model. J. Agric. Food Chem. 2019, 67, 3624–3632. [Google Scholar] [CrossRef]
- Cao, W.; Chin, Y.; Chen, X.; Mi, Y.; Xue, C.; Wang, Y.; Tang, Q. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food Sci. Nutr. 2020, 71, 453–463. [Google Scholar] [CrossRef]
- Velazquez, K.T.; Enos, R.T.; Bader, J.E.; Sougiannis, A.T.; Carson, M.S.; Chatzistamou, I.; Carson, J.A.; Nagarkatti, P.S.; Nagarkatti, M.; Murphy, E.A. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 2019, 11, 619–637. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Gao, Y.; Xue, Y.; Yan, Y.; Guo, X. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef]
- Cao, Y.; Zou, L.; Li, W.; Song, Y.; Zhao, G.; Hu, Y. Dietary quinoa (Chenopodium quinoa Willd.) polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 163, 55–65. [Google Scholar] [CrossRef]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2691–2709. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Reciprocal Interactions between epigallocatechin-3-gallate (EGCG) and human gut microbiota in vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green tea polyphenols modulate colonic microbiota diversity and lipid metabolism in high-fat diet treated hfa mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- Aakko, J.; Pietila, S.; Toivonen, R.; Rokka, A.; Mokkala, K.; Laitinen, K.; Elo, L.; Hanninen, A. A carbohydrate-active enzyme (CAZy) profile links successful metabolic specialization of prevotella to its abundance in gut microbiota. Sci. Rep. 2020, 10, 12411. [Google Scholar] [CrossRef]
- Yin, H.-M.; Wang, S.-N.; Nie, S.-P.; Xie, M.-Y. Coix polysaccharides: Gut microbiota regulation and immunomodulatory. Bioact. Carbohydr. Diet. Fibre 2018, 16, 53–61. [Google Scholar] [CrossRef]
- Tang, C.; Sun, J.; Zhou, B.; Jin, C.; Liu, J.; Kan, J.; Qian, C.; Zhang, N. Effects of polysaccharides from purple sweet potatoes on immune response and gut microbiota composition in normal and cyclophosphamide treated mice. Food Funct. 2018, 9, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Fan, H.; Zeng, S.; Nie, S.; Zhang, Y.; Tan, L.; Li, C.; Xu, F.; Liu, Q.; Wu, G. Polysaccharide from artocarpus heterophyllus lam. (jackfruit) pulp modulates gut microbiota composition and improves short-chain fatty acids production. Food Chem. 2021, 364, 130434. [Google Scholar] [CrossRef]
- Xie, J.; Song, Q.; Yu, Q.; Chen, Y.; Hong, Y.; Shen, M. Dietary polysaccharide from mung bean [Vigna radiate (Linn.) Wilczek] skin modulates gut microbiota and short-chain fatty acids in mice. Int. J. Food Sci. Technol. 2022, 57, 2581–2589. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Li, J.; Li, T.; Zhang, Y.; Liang, Y.; Mei, Y. Phellinus linteus polysaccharide extract improves insulin resistance by regulating gut microbiota composition. FASEB J. 2020, 34, 1065–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Alotaibi, A.; Perry, L.; Gholizadeh, L.; Al-Ganmi, A. Incidence and prevalence rates of diabetes mellitus in Saudi Arabia: An overview. J. Epidemiol. Glob. Health 2017, 7, 211–218. [Google Scholar] [CrossRef]
- El-Tantawy, W.H.; Temraz, A. Natural products for controlling hyperlipidemia: Review. Arch. Physiol. Biochem. 2019, 125, 128–135. [Google Scholar] [CrossRef]
- Yao, Y.S.; Li, T.D.; Zeng, Z.H. Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakumar, P.; Babbar, L. Preconditioning the hyperlipidemic myocardium: Fact or fantasy? Cell. Signal. 2012, 24, 589–595. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Xing, R.; Cui, X.; Xiao, Y.; Xie, L.; You, P.; Wang, T.; Zeng, L.; Peng, W.; et al. Hyperlipidemia induces typical atherosclerosis development in ldlr and apoe deficient rats. Atherosclerosis 2018, 271, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Fenk, S.; Fischer, M.; Strack, C.; Schmitz, G.; Loew, T.; Lahmann, C.; Baessler, A. Successful weight reduction improves left ventricular diastolic function and physical performance in severe obesity. Int. Heart J. 2015, 56, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Suzuki, H.; Miwa, K.; Ushijima, T.; Nagasu, S.; Fukahori, M.; Ishii, K.; Nakamura, T.; Iwamoto, H.; Masuda, A.; et al. Hyperlipidemia as a risk factor for Trousseau syndrome-related cerebral infarction in patients with advanced gastrointestinal cancer. Oncol. Lett. 2022, 24, 318. [Google Scholar] [CrossRef] [PubMed]
- Warrington, N.M.; Beaumont, R.N.; Horikoshi, M.; Day, F.R.; Helgeland, Ø.; Laurin, C.; Bacelis, J.; Peng, S.; Hao, K.; Feenstra, B.; et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat. Genet. 2019, 51, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; An, J.; Lee, H.; Kim, K.; Lee, S.J.; Choi, H.R.; Kwon, J.W.; Lee, T.B.; Song, Y.; Kong, H. Multifaceted effect of rubus occidentalis on hyperglycemia and hypercholesterolemia in mice with diet-induced metabolic diseases. Nutrients 2018, 10, 1846. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Wu, S.; Zheng, H.M.; Li, P.; Sheng, H.F.; Chen, M.X.; Chen, Z.H.; Ji, G.Y.; Zheng, Z.D.; et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome 2018, 6, 172. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [Green Version]
- Remely, M.; Tesar, I.; Hippe, B.; Gnauer, S.; Rust, P.; Haslberger, A.G. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef. Microbes 2015, 6, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Collado, M.C.; Garcia-Valdes, L.; Segura, M.T.; Martin-Lagos, J.A.; Anjos, T.; Marti-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidu, C.; Escoula, Q.; Bellenger, S.; Spor, A.; Galan, M.; Geissler, A.; Bouchot, A.; Dardevet, D.; Morio, B.; Cani, P.D.; et al. The transplantation of omega3 pufa-altered gut microbiota of fat-1 mice to wild-type littermates prevents obesity and associated metabolic disorders. Diabetes 2018, 67, 1512–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.J.; Zhang, Q.M.; Ni, W.W.; Zhang, X.; Li, Y.; Li, A.L.; Du, P.; Li, C.; Yu, S.S. Modulatory effect of lactobacillus acidophilus KLDS 1.0738 on intestinal short-chain fatty acids metabolism and GPR41/43 expression in beta-lactoglobulin-sensitized mice. Microbiol. Immunol. 2019, 63, 303–315. [Google Scholar]
- Arora, T.; Rudenko, O.; Egerod, K.L.; Husted, A.S.; Kovatcheva-Datchary, P.; Akrami, R.; Kristensen, M.; Schwartz, T.W.; Backhed, F. Microbial fermentation of flaxseed fibers modulates the transcriptome of GPR41-expressing enteroendocrine cells and protects mice against diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, 453–463. [Google Scholar] [CrossRef]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver. Physiol. 2018, 315, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafferty, R.A.; Flatt, P.R.; Irwin, N. Established and emerging roles peptide YY (PYY) and exploitation in obesity–diabetes. Curr. Opin. Endocrinol. 2021, 28, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Behary, P.; Tharakan, G.; Alexiadou, K.; Johnson, N.; Wewer Albrechtsen, N.J.; Kenkre, J.; Cuenco, J.; Hope, D.; Anyiam, O.; Choudhury, S.; et al. Combined GLP-1, oxyntomodulin, and peptide YY improves body weight and glycemia in obesity and prediabetes/type 2 diabetes: A randomized, single-blinded, placebo-controlled study. Diabetes Care 2019, 42, 1446–1453. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.S.; Preston, T.; Brignardello, J.; Garcia-Perez, I.; Holmes, E.; Frost, G.S.; Morrison, D.J. The effect of L-rhamnose on intestinal transit time, short chain fatty acids and appetite regulation: A pilot human study using combined (13)CO2/H2 breath tests. J. Breath. Res. 2018, 12, 046006. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-h.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2021, 372, 4573. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsboll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroen. 2014, 30, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki-Anzai, S.; Masuda, M.; Levi, M.; Keenan, A.L.; Miyazaki, M. Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS ONE 2014, 9, 108270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Han, X.; Tan, H.; Huang, W.; You, Y.; Zhan, J. Blueberry extract improves obesity through regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. iScience 2019, 19, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Huang, S.; Xu, W.; Chen, L.; Su, J.; Ni, H.; Feng, X.; Chen, J.; Wang, X.; Huang, Q. Compound K attenuates hyperglycemia by enhancing glucagon-like peptide-1 secretion through activating TGR5 via the remodeling of gut microbiota and bile acid metabolism. J. Ginseng Res. 2022, 46, 780–789. [Google Scholar] [CrossRef]
- Tveter, K.M.; Villa-Rodriguez, J.A.; Cabales, A.J.; Zhang, L.; Bawagan, F.G.; Duran, R.M.; Roopchand, D.E. Polyphenol-induced improvements in glucose metabolism are associated with bile acid signaling to intestinal farnesoid X receptor. BMJ Open Diabetes Res. Care 2020, 8, e001386. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between lipopolysaccharide and gut microbiota in inflammatory bowel diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Kim, K.A.; Jeong, J.J.; Yoo, S.Y.; Kim, D.H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Hrncir, T.; Stepankova, R.; Kozakova, H.; Hudcovic, T.; Tlaskalova-Hogenova, H. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: Studies in germ-free mice. BMC Immunol. 2008, 9, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salguero, M.V.; Al-Obaide, M.A.I.; Singh, R.; Siepmann, T.; Vasylyeva, T.L. Dysbiosis of gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 2019, 18, 3461–3469. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, F.; Considine, R.V.; Abdelhadi, O.A.; Acton, A.J. Saturated fat ingestion promotes lipopolysaccharide-mediated inflammation and insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 934–946. [Google Scholar] [CrossRef] [Green Version]
- Jorge, M.L.M.P.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.D.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S.; et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Fotiadis, G.; Kapelouzou, A.; Kostakis, A.; Liapis, C.D.; Vrabas, I.S. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet. Med. 2013, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nagasaki, M.; Nakai, N.; Fushimi, T. Physical exercise improves glucose metabolism in lifestyle-related diseases. Exp. Biol. Med. 2003, 228, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Mul, J.D.; Stanford, K.I.; Hirshman, M.F.; Goodyear, L.J. Exercise and regulation of carbohydrate metabolism. Prog. Mol. Biol. Transl. 2015, 135, 17–37. [Google Scholar]
- Benedini, S.; Dozio, E.; Invernizzi, P.L.; Vianello, E.; Banfi, G.; Terruzzi, I.; Luzi, L.; Corsi Romanelli, M.M. Irisin: A potential link between physical exercise and metabolism-an observational study in differently trained subjects, from elite athletes to sedentary people. J. Diabetes Res. 2017, 2017, 1039161. [Google Scholar] [CrossRef] [Green Version]
- Pasini, E.; Corsetti, G.; Assanelli, D.; Testa, C.; Romano, C.; Dioguardi, F.S.; Aquilani, R. Effects of chronic exercise on gut microbiota and intestinal barrier in human with type 2 diabetes. Minerva Med. 2019, 110, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Torquati, L.; Gajanand, T.; Cox, E.R.; Willis, C.R.G.; Zaugg, J.; Keating, S.E.; Coombes, J.S. Effects of exercise intensity on gut microbiome composition and function in people with type 2 diabetes. Eur. J. Sport Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Nemes, A.; Homoki, J.R.; Kiss, R.; Hegedus, C.; Kovacs, D.; Peitl, B.; Gal, F.; Stundl, L.; Szilvassy, Z.; Remenyik, J. Effect of anthocyanin-rich tart cherry extract on inflammatory mediators and adipokines involved in type 2 diabetes in a high fat diet induced obesity mouse model. Nutrients 2019, 11, 1966. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, D.A.; Kume, W.T.; Correia, F.S.; Queiroz, T.S.; Allebrandt Neto, E.W.; Santos, M.P.D.; Kawashita, N.H.; Franca, S.A. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: A new proposal. An. Acad. Bras. Cienc. 2019, 91, 20180314. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferre, M.; Santos, J.L.; Martinez-Gonzalez, M.A.; Clish, C.B.; Razquin, C.; Wang, D.; Liang, L.; Li, J.; Dennis, C.; Corella, D.; et al. Glycolysis/gluconeogenesis- and tricarboxylic acid cycle-related metabolites, Mediterranean diet, and type 2 diabetes. Am. J. Clin. Nutr. 2020, 111, 835–844. [Google Scholar] [CrossRef]
- Vitale, M.; Masulli, M.; Calabrese, I.; Rivellese, A.A.; Bonora, E.; Signorini, S.; Perriello, G.; Squatrito, S.; Buzzetti, R.; Sartore, G.; et al. Impact of a mediterranean dietary pattern and its components on cardiovascular risk factors, glucose control, and body weight in people with type 2 diabetes: A real-life study. Nutrients 2018, 10, 1067. [Google Scholar] [CrossRef] [Green Version]
- Collins, Q.F.; Liu, H.Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5’-AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef] [Green Version]
- Zengin, G.; Locatelli, M.; Carradori, S.; Mocan, A.M.; Aktumsek, A. Total phenolics, flavonoids, condensed tannins content of eight centaurea species and their broad inhibitory activities against cholinesterase, tyrosinase, α-amylase and α-glucosidase. Not. Bot. Horti Agrobot. 2016, 44, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Liu, A.B.; Sun, S.; Ajami, N.J.; Ross, M.C.; Wang, H.; Zhang, L.; Reuhl, K.; Kobayashi, K.; Onishi, J.C.; et al. Green tea polyphenols modify the gut microbiome in db/db mice as co-abundance groups correlating with the blood glucose lowering effect. Mol. Nutr. Food Res. 2019, 63, 1801064. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and hypolipidemic mechanism of tea polysaccharides on type 2 diabetic rats via gut microbiota and metabolism alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- Li, Z.R.; Jia, R.B.; Luo, D.; Lin, L.; Zheng, Q.; Zhao, M. The positive effects and underlying mechanisms of Undaria pinnatifida polysaccharides on type 2 diabetes mellitus in rats. Food Funct. 2021, 12, 11898–11912. [Google Scholar] [CrossRef]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Q.; Cao, J.; Xu, Y.; Pei, Z.; Fan, H.; Yuan, Y.; Shen, X.; Li, C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in goto-kakizaki rats. Food Chem. Toxicol. 2020, 135, 110886. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamamoto, F.; Taneda, Y.; Naito, Y.; Clark, D.; Lund, S.S.; Okamura, T.; Kaku, K. Effects of anti-diabetes medications on cardiovascular and kidney outcomes in asian patients with type 2 diabetes: A rapid evidence assessment and narrative synthesis. Expert Opin. Drug Saf. 2021, 20, 707–720. [Google Scholar] [CrossRef]
- Trejo-Gutierrez, J.F.; Fletcher, G. Impact of exercise on blood lipids and lipoproteins. J. Clin. Lipidol. 2007, 1, 175–181. [Google Scholar] [CrossRef]
- Zhao, S.; Zhong, J.; Sun, C.; Zhang, J. Effects of aerobic exercise on TC, HDL-C, LDL-C and TG in patients with hyperlipidemia: A protocol of systematic review and meta-analysis. Medicine 2021, 100, 25103. [Google Scholar] [CrossRef]
- Wen, J.; Su, M. A randomized trial of tai chi on preventing hypertension and hyperlipidemia in middle-aged and elderly patients. Int. J. Environ. Res. Public Health 2021, 18, 5480. [Google Scholar] [CrossRef]
- McClements, D.J. The biophysics of digestion: Lipids. Curr. Opin. Food Sci. 2018, 21, 1–6. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Kamada, K.; Ishikawa, T.; Inoue, R.; Okuda, K.; Tsujimoto, Y.; et al. Changes in the gut microbiota are associated with hypertension, hyperlipidemia, and type 2 diabetes mellitus in japanese subjects. Nutrients 2020, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fan, X.; Ye, R.; Hu, Y.; Zheng, T.; Shi, R.; Cheng, W.; Lv, X.; Chen, L.; Liang, P. The effect of simvastatin on gut microbiota and lipid metabolism in hyperlipidemic rats induced by a high-fat diet. Front. Pharmacol. 2020, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, B.; Hu, Y.; Wang, J.; Liu, J.; Qin, R.; Lv, S.; Wang, S. Correlation analysis of intestinal redox state with the gut microbiota reveals the positive intervention of tea polyphenols on hyperlipidemia in high fat diet fed mice. J. Agric. Food Chem. 2019, 67, 7325–7335. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Xie, B.; Liu, H. Effects of Auricularia auricula and its polysaccharide on diet-induced hyperlipidemia rats by modulating gut microbiota. J. Funct. Foods 2020, 72, 104038. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, Y.; Wang, X.; Zhang, X. Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat Metabolic Disorders to Maintain Health. Nutrients 2023, 15, 1539. https://doi.org/10.3390/nu15061539
Zhang L, Liu Y, Wang X, Zhang X. Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat Metabolic Disorders to Maintain Health. Nutrients. 2023; 15(6):1539. https://doi.org/10.3390/nu15061539
Chicago/Turabian StyleZhang, Li, Yuan Liu, Xinzhou Wang, and Xin Zhang. 2023. "Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat Metabolic Disorders to Maintain Health" Nutrients 15, no. 6: 1539. https://doi.org/10.3390/nu15061539
APA StyleZhang, L., Liu, Y., Wang, X., & Zhang, X. (2023). Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat Metabolic Disorders to Maintain Health. Nutrients, 15(6), 1539. https://doi.org/10.3390/nu15061539






