Crosstalk between Gut Microbiota and Epigenetic Markers in Obesity Development: Relationship between Ruminococcus, BMI, and MACROD2/SEL1L2 Methylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric Measurements
2.3. Biochemical Measurements
2.4. Gut Microbiota Analysis
2.4.1. Fecal Sample Collection and DNA Isolation
2.4.2. 16 S rRNA Sequencing and Sequence Analysis
2.5. DNA Methylation Studies
2.5.1. DNA Isolation and Bisulfite Conversion
2.5.2. Microarray Analysis
2.6. Protein Expression of MACROD2
2.7. Statistical Analysis
3. Results
3.1. Anthropometric and Clinical Data of the Sample
3.2. Microbiota and DNA Methylation Analysis
3.3. MACROD2 Protein Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, Y.; Qin, B.; Poti, J.; Sokol, R.; Gordon-Larsen, P. Epidemiology of Obesity in Adults: Latest Trends. Curr. Obes. Rep. 2018, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Aoki, Y.; Ogden, C.L. Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level among Adults in the United States, 2013–2016. J. Am. Med. Assoc. 2018, 319, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Ramírez, P.; Cadena-Ullauri, S.; Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Paz-Cruz, E.; Simancas-Racines, D.; Zambrano, A.K. Genetics, Genomics, and Diet Interactions in Obesity in the Latin American Environment. Front. Nutr. 2022, 9, 1063286. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Microbiota and Metabolites in Metabolic Diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef]
- Hu, J.; Guo, P.; Mao, R.; Ren, Z.; Wen, J.; Yang, Q.; Yan, T.; Yu, J.; Zhang, T.; Liu, Y. Gut Microbiota Signature of Obese Adults Across Different Classifications. Diabetes Metab. Syndr. Obes. 2022, 15, 3933–3947. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut Microbiota Influence in Type 2 Diabetes Mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of Diet and the Gut Microbiome on Epigenetic Modulation in Cancer and Other Diseases. Clin. Epigenetics 2015, 7, 112. [Google Scholar] [CrossRef]
- Sharma, M.; Li, Y.; Stoll, M.L.; Tollefsbol, T.O. The Epigenetic Connection between the Gut Microbiome in Obesity and Diabetes. Front. Genet. 2020, 10, 1329. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr. Physiol. 2018, 8, 1091–1115. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Microbiota or Short-Chain Fatty Acids: Which Regulates Diabetes? Cell. Mol. Immunol. 2017, 15, 88–91. [Google Scholar] [CrossRef]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of Appetite and Energy Intake by SCFA: What Are the Potential Underlying Mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-Chain Fatty Acids in Control of Energy Metabolism. Crit. Rev. Food Sci. Nutr. 2017, 58, 1243–1249. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. MBio 2015, 6, e02481-14. [Google Scholar] [CrossRef]
- Liang, F.; Quan, Y.; Wu, A.; Chen, Y.; Xu, R.; Zhu, Y.; Xiong, J. Insulin-Resistance and Depression Cohort Data Mining to Identify Nutraceutical Related DNA Methylation Biomarker for Type 2 Diabetes. Genes Dis. 2021, 8, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Carnero-Montoro, E.; van Dongen, J.; Lent, S.; Nedeljkovic, I.; Ligthart, S.; Tsai, P.C.; Martin, T.C.; Mandaviya, P.R.; Jansen, R.; et al. An Integrative Cross-Omics Analysis of DNA Methylation Sites of Glucose and Insulin Homeostasis. Nat. Commun. 2019, 10, 2581. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Sánchez-Alcoholado, L.; Cabrera-Mulero, A.; Lopez-Dominguez, R.; Carmona-Saez, P.; Garcia-Fuentes, E.; Moreno-Indias, I.; Tinahones, F.J. Gut Microbiota Composition Is Associated with the Global DNA Methylation Pattern in Obesity. Front. Genet. 2019, 10, 613. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline, Other Methyl-Donors and Epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients That Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef]
- Strozzi, G.P.; Mogna, L. Quantification of Folic Acid in Human Feces after Administration of Bifidobacterium Probiotic Strains. J. Clin. Gastroenterol. 2008, 42 Pt 2 (Suppl. 3), S179–S184. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Chaykin, S. The Biosynthesis of Trimethylamine-N-Oxide. J. Biol. Chem. 1962, 237, 1309–1313. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Minireview Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. DRUG Metab. Dispos. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; De Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-Oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-Oxide Induces Inflammation and Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells via Activating ROS-TXNIP-NLRP3 Inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Ávila, J.G.O.; Echeverri, I.; de Plata, C.A.; Castillo, A. Impact of Oxidative Stress during Pregnancy on Fetal Epigenetic Patterns and Early Origin of Vascular Diseases. Nutr. Rev. 2015, 73, 12–21. [Google Scholar] [CrossRef]
- Romano, K.A.; Martinez-del Campo, A.; Kasahara, K.; Chittim, C.L.; Vivas, E.I.; Amador-Noguez, D.; Balskus, E.P.; Rey, F.E. Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host Microbe 2017, 22, 279–290.e7. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Database on Body Mass Index; WHO: Geneva, Switzerland, 2011.
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Lopez-Legarrea, P.; De La Iglesia, R.; Abete, I.; Bondia-Pons, I.; Navas-Carretero, S.; Forga, L.; Martinez, J.A.; Zulet, M.A. Short-Term Role of the Dietary Total Antioxidant Capacity in Two Hypocaloric Regimes on Obese with Metabolic Syndrome Symptoms: The RESMENA Randomized Controlled Trial. Nutr. Metab. 2013, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Assmann, T.S.; Cuevas-Sierra, A.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez, J.A. Comprehensive Analysis Reveals Novel Interactions between Circulating MicroRNAs and Gut Microbiota Composition in Human Obesity. Int. J. Mol. Sci. 2020, 21, 9509. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Milagro, F.I.; Aranaz, P.; Martínez, J.A.; Riezu-Boj, J.I. Gut Microbiota Differences According to Ultra-Processed Food Consumption in a Spanish Population. Nutrients 2021, 13, 2710. [Google Scholar] [CrossRef]
- Hildebrand, F.; Tadeo, R.; Voigt, A.Y.; Bork, P.; Raes, J. LotuS: An Efficient and User-Friendly OTU Processing Pipeline. Microbiome 2014, 2, 30. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C. UCHIME2: Improved Chimera Prediction for Amplicon Sequencing. bioRxiv 2016, 074252. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Ritari, J.; Salojärvi, J.; Lahti, L.; de Vos, W.M. Improved Taxonomic Assignment of Human Intestinal 16S RRNA Sequences by a Dedicated Reference Database. BMC Genom. 2015, 16, 1056. [Google Scholar] [CrossRef]
- Pasolli, E.; Schiffer, L.; Manghi, P.; Renson, A.; Obenchain, V.; Truong, D.T.; Beghini, F.; Malik, F.; Ramos, M.; Dowd, J.B.; et al. Accessible, Curated Metagenomic Data through ExperimentHub. Nat. Methods 2017, 14, 1023–1024. [Google Scholar] [CrossRef]
- Lawrence, M.; Huber, W.; Pagès, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for Computing and Annotating Genomic Ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef] [PubMed]
- Salas-Pérez, F.; Ramos-Lopez, O.; Mansego, M.L.; Milagro, F.I.; Santos, J.L.; Riezu-Boj, J.I.; Alfredo Martínez, J. DNA Methylation in Genes of Longevity-Regulating Pathways: Association with Obesity and Metabolic Complications. Aging 2019, 11, 1874–1899. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, J.; Gordon, L.; Oshlack, A. SWAN: Subset-Quantile within Array Normalization for Illumina Infinium HumanMethylation450 BeadChips. Genome Biol. 2012, 13, R44. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Schillert, A.; Röthemeier, C.; Trégouët, D.-A.; Proust, C.; Binder, H.; Pfeiffer, N.; Beutel, M.; Lackner, K.J.; Schnabel, R.B.; et al. Removing Batch Effects from Longitudinal Gene Expression—Quantile Normalization Plus ComBat as Best Approach for Microarray Transcriptome Data. PLoS ONE 2016, 11, e0156594. [Google Scholar] [CrossRef]
- Houseman, E.; Accomando, W.P.; Koestler, D.C.; Christensen, B.C.; Marsit, C.J.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. DNA Methylation Arrays as Surrogate Measures of Cell Mixture Distribution. BMC Bioinform. 2012, 13, 86. [Google Scholar] [CrossRef]
- Salas-Pérez, F.; Cuevas-Sierra, A.; Cuervo, M.; Goni, L.; Milagro, F.I.; Martínez, J.A.; Riezu-Boj, J.I. Differentially Methylated Regions (DMRs) in PON3 Gene between Responders and Non-Responders to a Weight Loss Dietary Intervention: A New Tool for Precision Management of Obesity. Epigenetics 2022, 17, 81. [Google Scholar] [CrossRef]
- Zhao, X.; Lynch, J.G., Jr.; Chen, Q. Reconsidering Baron and Kenny: Myths and Truths about Mediation Analysis. J. Consum. Res. 2010, 37, 197–206. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut Microbiota in Patients with Obesity and Metabolic Disorders—A Systematic Review. Genes Nutr. 2022, 17, 2. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Yang, S.; Lu, J.; Jin, X.; Wu, M. Diet-Gut Microbiota-Epigenetics in Metabolic Diseases: From Mechanisms to Therapeutics. Biomed. Pharmacother. 2022, 153, 113290. [Google Scholar] [CrossRef]
- Lakshmanan, A.P.; Al Zaidan, S.; Bangarusamy, D.K.; Al-Shamari, S.; Elhag, W.; Terranegra, A. Increased Relative Abundance of Ruminoccocus Is Associated with Reduced Cardiovascular Risk in an Obese Population. Front. Nutr. 2022, 9, 849005. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Shao, C.; Zhong, B. Compositional Alterations of Gut Microbiota in Nonalcoholic Fatty Liver Disease Patients: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2021, 20, 22. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut Microbial Metabolites, Short-Chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Wu, J.; Ye, T.; Wang, S.; Wang, P.; Xing, D. Butyrate-Producing Bacteria and the Gut-Heart Axis in Atherosclerosis. Clin. Chim. Acta 2020, 507, 236–241. [Google Scholar] [CrossRef]
- Gao, F.; Lv, Y.W.; Long, J.; Chen, J.M.; He, J.M.; Ruan, X.Z.; Zhu, H.B. Butyrate Improves the Metabolic Disorder and Gut Microbiome Dysbiosis in Mice Induced by a High-Fat Diet. Front. Pharmacol. 2019, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-Induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Weng, H.; Endo, K.; Li, J.; Kito, N.; Iwai, N. Induction of Peroxisomes by Butyrate-Producing Probiotics. PLoS ONE 2015, 10, e0117851. [Google Scholar] [CrossRef]
- Kumar, H.; Lund, R.; Laiho, A.; Lundelin, K.; Ley, R.E.; Isolauri, E.; Salminen, S.; Ravel, J. Gut Microbiota as an Epigenetic Regulator: Pilot Study Based on Whole-Genome Methylation Analysis. MBio 2014, 5, e02113-14. [Google Scholar] [CrossRef]
- Manterola, M.; Palominos, M.F.; Calixto, A. The Heritability of Behaviors Associated with the Host Gut Microbiota. Front. Immunol. 2021, 12, 1497. [Google Scholar] [CrossRef]
- Feijs, K.L.H.; Cooper, C.D.O.; Žaja, R. The Controversial Roles of ADP-Ribosyl Hydrolases MACROD1, MACROD2 and TARG1 in Carcinogenesis. Cancers 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, F.; Feijs, K.L.H.; Frugier, E.; Bonalli, M.; Forst, A.H.; Imhof, R.; Winkler, H.C.; Fischer, D.; Caflisch, A.; Hassa, P.O.; et al. Macrodomain-Containing Proteins Are New Mono-ADP-Ribosylhydrolases. Nat. Struct. Mol. Biol. 2013, 20, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Iny Stein, T.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinforma. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Hee, S.-W.; Lee, W.-J.; Li, H.-Y.; Chang, T.-J.; Lin, M.-W.; Hung, Y.-J.; Lee, I.-T.; Hung, K.-Y.; Assimes, T.; et al. Genome-Wide Scan for Circulating Vascular Adhesion Protein-1 Levels: MACROD2 as a Potential Transcriptional Regulator of Adipogenesis. J. Diabetes Investig. 2018, 9, 1067–1074. [Google Scholar] [CrossRef]
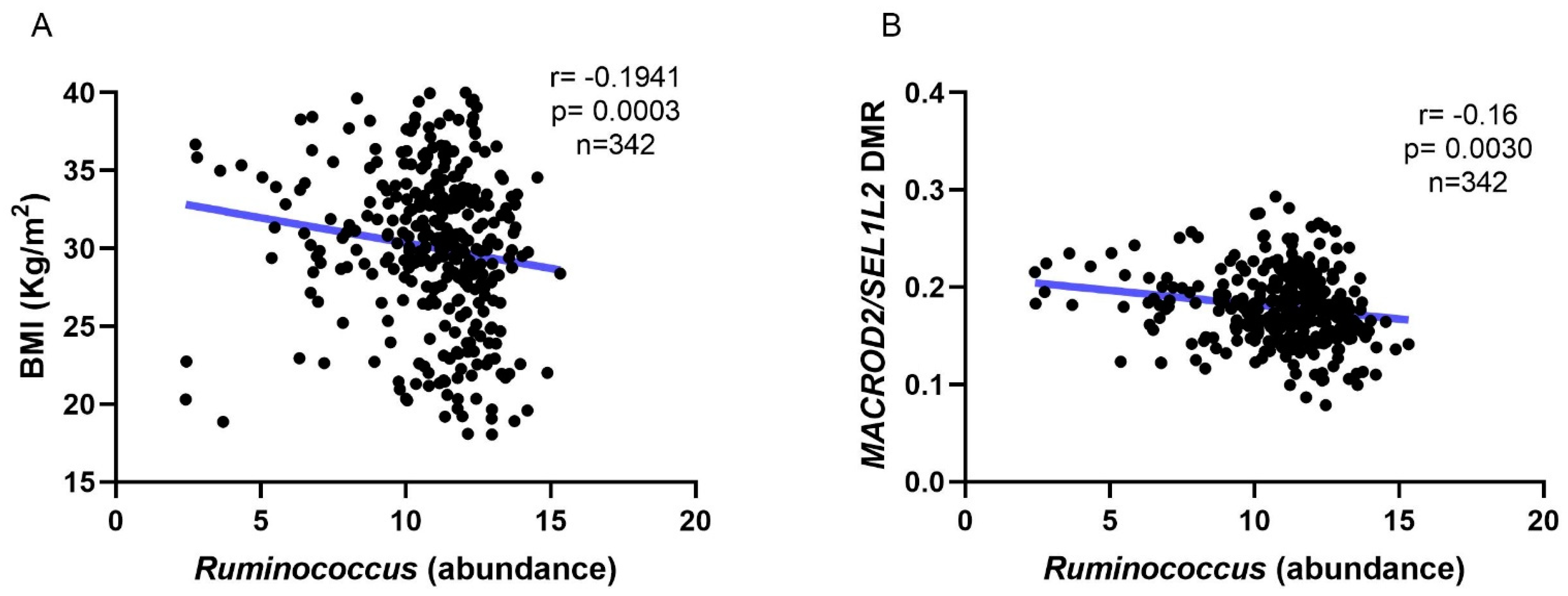

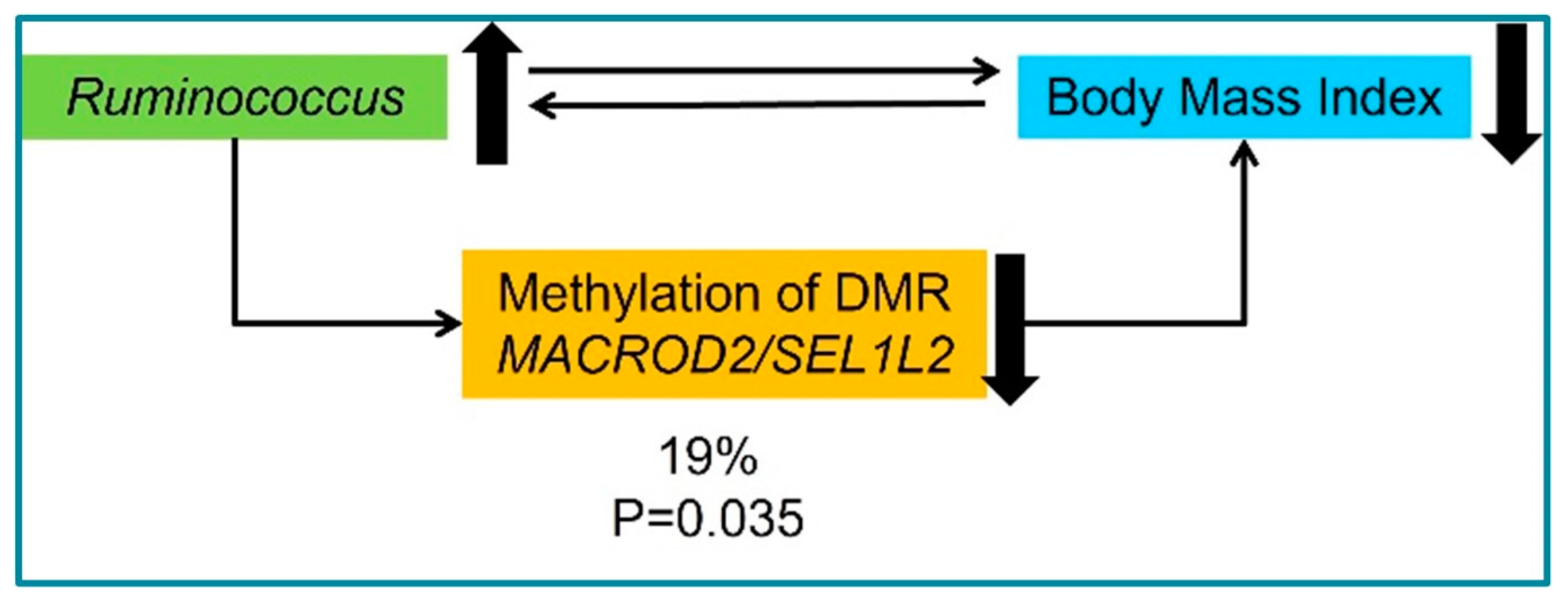

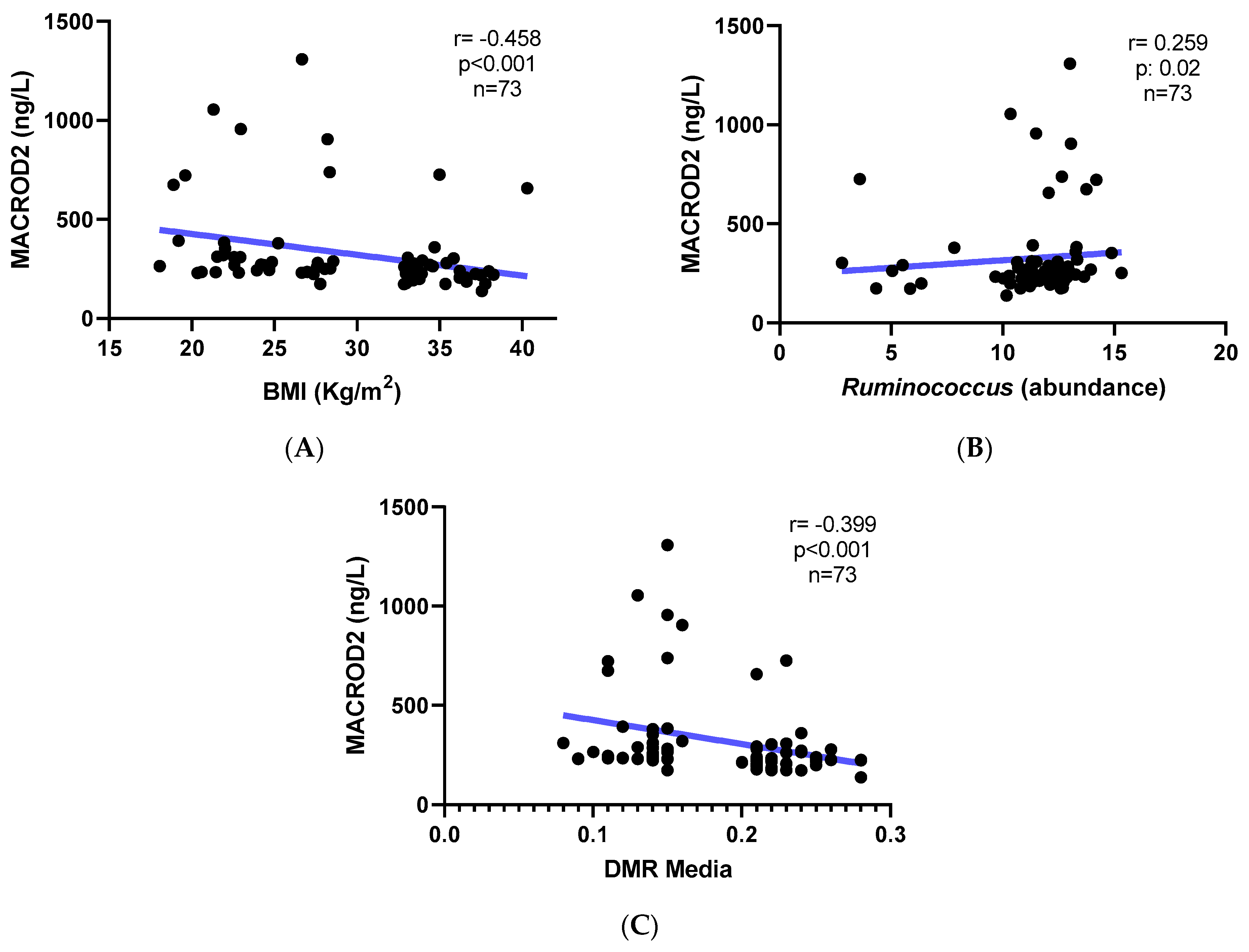
| Parameter | Eutrophic Individuals (Controls, n = 64) | Obese Individuals (Cases, n = 278) | p-Value |
|---|---|---|---|
| Age (years) | 39.6 ± 9.2 | 45.9 ± 10.2 | <0.001 |
| Gender (M%) | 28.1 | 31.3 | - |
| BMI (kg/m2) | 22.1 ± 1.8 | 37.9 ± 3.4 | <0.001 |
| WC (cm) | 75.6 ± 7.2 | 102.8 ± 10.4 | <0.001 |
| HC (cm) | 94.7 ± 6.0 | 112.1 ± 8.0 | <0.001 |
| SBP (mmHg) | 110 ± 13 | 129 ± 18 | <0.001 |
| DBP (mmHg) | 69 ± 9 | 80 ± 11 | <0.001 |
| Fasting Glucose (mg/dL) | 85 ± 7 | 97 ± 14 | <0.001 |
| Total Cholesterol (mg/dL) | 193 ± 34 | 217 ± 38 | <0.001 |
| HDL Cholesterol (mg/dL) | 63 ± 11 | 55 ± 13 | <0.001 |
| Triglycerides (mg/dL) | 68 ± 33 | 106 ± 58 | <0.001 |
| HOMA-IR index | 0.9 ± 0.5 | 2.1 ± 1.4 | <0.001 |
| Adiponectin (ng/mL) | 13.8 ± 5.2 | 11.3 ± 5 | <0.001 |
| Insulin (mU/L) | 4.4 ± 2 | 8.3 ± 4.9 | <0.001 |
| Leptin (ng/dL) | 10.9 ± 8.8 | 38.2 ± 28.7 | <0.001 |
| C-reactive Protein (µg/mL) | 1.3 ± 4.7 | 3.0 ± 3.2 | <0.001 |
| TNF (pg/mL) | 0.8 ± 0.3 | 0.9 ± 0.4 | 0.303 |
| ID | Genera | Correlation Coefficient | p-Value |
|---|---|---|---|
| 1 | Allisonella | −0.220 | 0.0001 |
| 2 | Bifidobacterium | −0.130 | 0.014 |
| 3 | Christensenella | −0.152 | 0.004 |
| 4 | Coprococcus | −0.224 | 0.0001 |
| 5 | Faecalibacterium | −0.189 | 0.0001 |
| 6 | Fusicatenibacter | −0.123 | 0.02 |
| 7 | Lactobacilus | −0.135 | 0.01 |
| 8 | Oscillospira | −0.223 | 0.001 |
| 9 | Prevotella | 0.136 | 0.01 |
| 10 | Ruminococcus | −0.188 | 0.0001 |
| ID | CHR 1 | MAPINFO | Strand 2 | Gene | Region 3 | Cgi 4 |
|---|---|---|---|---|---|---|
| cg04624110 | 20 | 13976093 | R | MACROD2 | TSS200 | Island |
| cg01552272 | 20 | 13976096 | R | MACROD2 | TSS200 | Island |
| cg23169957 | 20 | 13976106 | R | MACROD2 | TSS200 | Island |
| cg25557432 | 20 | 13976117 | R | MACROD2 | TSS200 | Island |
| cg06571075 | 20 | 13976143 | R | MACROD2 | TSS200 | Island |
| cg26059153 | 20 | 13976190 | R | MACROD2 | TSS200 | Island |
| cg05677624 | 20 | 13976218 | R | MACROD2 | TSS200 | Island |
| Parameter | Lowest MACROD2 DMR of the Lower BMI Tertile (n = 36) | Highest MACROD2 DMR of the Upper BMI Tertile (n = 37) | p-Value |
|---|---|---|---|
| BMI (kg/m2) | 24.0 ± 3.1 | 35.0 ± 1.9 | <0.001 |
| MACROD2/SEL1L2 DMR | 0.1344 ± 0.0196 | 0.2288 ± 0.0196 | <0.001 |
| WC (cm) | 81.5 ± 10.3 | 110.8 ± 7.0 | <0.001 |
| HC (cm) | 99.7 ± 6.9 | 116.9 ± 7.1 | <0.001 |
| SBP (mmHg) | 112.3 ± 10.9 | 134.3 ± 15.4 | <0.001 |
| DBP (mmHg) | 70.5 ± 8.1 | 84.0 ± 9.5 | <0.001 |
| Fasting Glucose (mg/dL) | 87.5 ± 6.9 | 102.3 ± 13.1 | <0.001 |
| Total Cholesterol (mg/dL) | 202.1 ± 32. | 219.2 ± 39.5 | <0.05 |
| HDL Cholesterol (mg/dL) | 62.3 ± 13.1 | 51.8 ± 12.4 | <0.001 |
| Triglycerides (mg/dL) | 76.7 ± 44.3 | 126.2 ± 68.7 | <0.001 |
| HOMA-IR index | 1.1 ± 0.7 | 2.7 ± 1.5 | <0.001 |
| Adiponectin (ng/mL) | 13.4 ± 4.7 | 10.7 ± 4.6 | <0.05 |
| Insulin (mU/L) | 5.2 ± 2.9 | 10.6 ± 5.1 | <0.001 |
| Leptin (ng/dL) | 20.1 ± 18.0 | 43.1 ± 28.1 | <0.001 |
| C-reactive Protein (µg/mL) | 0.8 ± 1.3 | 4.4 ± 3.7 | <0.001 |
| TNF (pg/mL) | 0.7 ± 0.3 | 0.8 ± 0.2 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Perez, F.; Assmann, T.S.; Ramos-Lopez, O.; Martínez, J.A.; Riezu-Boj, J.I.; Milagro, F.I. Crosstalk between Gut Microbiota and Epigenetic Markers in Obesity Development: Relationship between Ruminococcus, BMI, and MACROD2/SEL1L2 Methylation. Nutrients 2023, 15, 1550. https://doi.org/10.3390/nu15071550
Salas-Perez F, Assmann TS, Ramos-Lopez O, Martínez JA, Riezu-Boj JI, Milagro FI. Crosstalk between Gut Microbiota and Epigenetic Markers in Obesity Development: Relationship between Ruminococcus, BMI, and MACROD2/SEL1L2 Methylation. Nutrients. 2023; 15(7):1550. https://doi.org/10.3390/nu15071550
Chicago/Turabian StyleSalas-Perez, Francisca, Taís Silveira Assmann, Omar Ramos-Lopez, J. Alfredo Martínez, Jose Ignacio Riezu-Boj, and Fermín I. Milagro. 2023. "Crosstalk between Gut Microbiota and Epigenetic Markers in Obesity Development: Relationship between Ruminococcus, BMI, and MACROD2/SEL1L2 Methylation" Nutrients 15, no. 7: 1550. https://doi.org/10.3390/nu15071550
APA StyleSalas-Perez, F., Assmann, T. S., Ramos-Lopez, O., Martínez, J. A., Riezu-Boj, J. I., & Milagro, F. I. (2023). Crosstalk between Gut Microbiota and Epigenetic Markers in Obesity Development: Relationship between Ruminococcus, BMI, and MACROD2/SEL1L2 Methylation. Nutrients, 15(7), 1550. https://doi.org/10.3390/nu15071550







