The Interplay between Cardiovascular Risk, Cardiovascular Events, and Disease Activity in Primary Sjögren’s Syndrome: Is Uric Acid the Missing Link?
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Study and Inclusion Criteria
2.2. Clinical and Serological Features
2.2.1. Demographic and Disease-Specific Features
2.2.2. Cardiovascular Risk Factors
2.2.3. Cardiovascular Events
2.2.4. Adherence to the Mediterranean Diet
2.3. Statistical Analysis
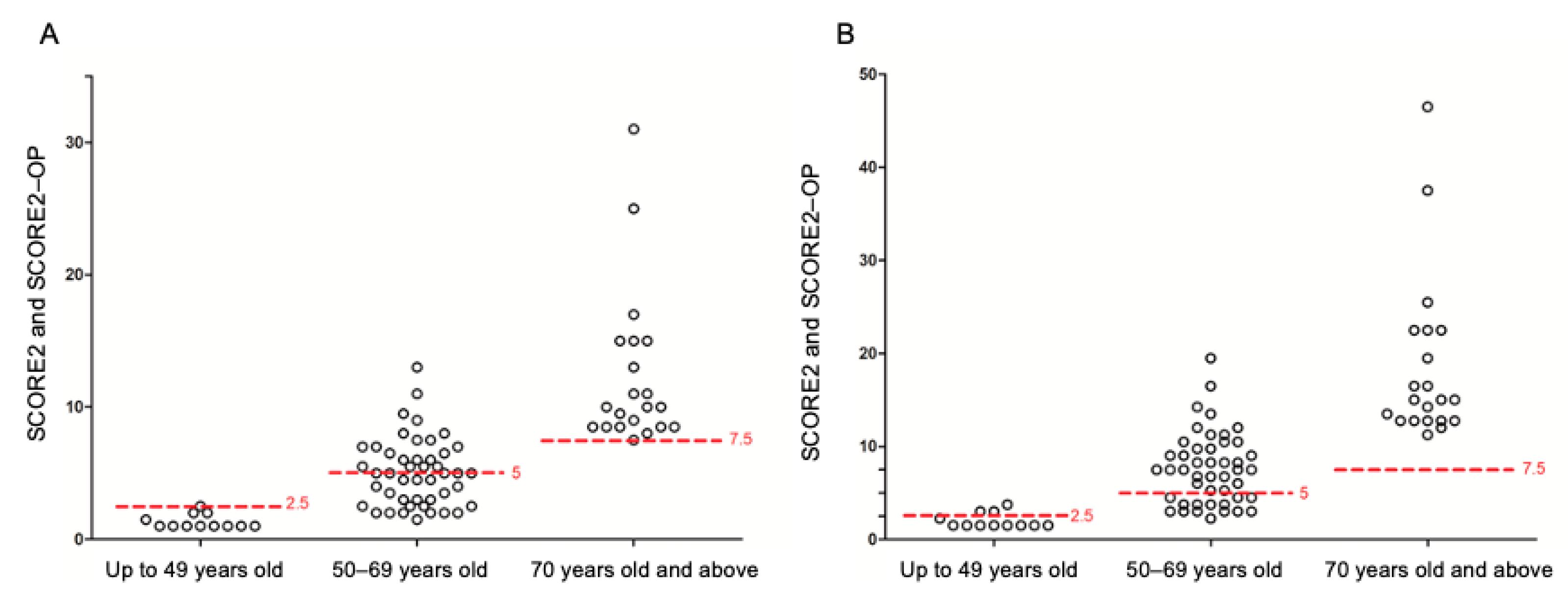
3. Results
3.1. Analysis of the Full Cohort
3.2. Analysis of pSS Patients with Arterial Hypertension
3.3. Analysis of pSS Patients without Previous CV Events
3.4. Domains of Disease Activity, SUA Levels and CV Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Givertz, M.M. Treating gour in patients with cardiovascular disease: Mutual benefit or unintended consequences? J. Am. Coll. Cardiol. 2018, 71, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. ESC Scientific document group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; Bombelli, M.; et al. Identification of the Uric Acid Thresholds Predicting an Increased Total and Cardiovascular Mortality over 20 Years. Hypertension 2020, 75, 302–308. [Google Scholar] [CrossRef]
- Muiesan, M.L.; Salvetti, M.; Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Barbagallo, C.M.; Bombelli, M.; Cicero, A.F.; Cirillo, M.; et al. Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension Serum uric acid, predicts heart failure in a large Italian cohort: Search for a cut-off value the URic acid Right for heArt Health study. J. Hypertens. 2021, 39, 62–69. [Google Scholar] [CrossRef]
- Mariette, X.; Criswell, L.A. Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef]
- Carubbi, F.; Alunno, A.; Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Berardicurti, O.; Bartoloni, E.; Bistoni, O.; Caterbi, S.; Ciccia, F.; et al. Is minor salivary gland biopsy more than a diagnostic tool in primary Sjögren’s syndrome? Association between clinical, histopathological, and molecular features: A retrospective study. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 2014; Volume 44, pp. 314–324. [Google Scholar]
- Carubbi, F.; Alunno, A.; Cipriani, P.; Bartoloni, E.; Baldini, C.; Quartuccio, L.; Priori, R.; Valesini, G.; De Vita, S.; Bombardieri, S.; et al. A retrospective, multicenter study evaluating the prognostic value of minor salivary gland histology in a large cohort of patients with primary Sjögren’s syndrome. Lupus 2015, 24, 315–320. [Google Scholar] [CrossRef]
- Beltai, A.; Barnetche, T.; Daien, C.; Lukas, C.; Gaujoux-Viala, C.; Combe, B.; Morel, J. Cardiovascular morbidity and mortality in primary Sjögren’s Syndrome: A systematic review and meta-analysis. Arthritis Care Res. 2020, 72, 131–139. [Google Scholar] [CrossRef]
- Drosos, G.C.; Vedder, D.; Houben, E.; Boekel, L.; Atzeni, F.; Badreh, S.; Boumpas, D.T.; Brodin, N.; Bruce, I.N.; González-Gay, M.Á.; et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann. Rheum. Dis. 2022, 81, 768–779. [Google Scholar] [CrossRef]
- Luo, Q.; Qin, L.; Zhang, Y.; Yang, X.; Wang, H. Relationship between serum uric acid and hypertension in patients with primary Sjögren’s syndrome: A retrospective cohort study. J. Clin. Hypertens. 2022, 24, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Ravaud, P.; Bowman, S.J.; Baron, G.; Tzioufas, A.; Theander, E.; Gottenberg, J.-E.; Bootsma, H.; Mariette, X.; Vitali, C. EULAR Sjögren’s Task Force. EULAR Sjogren’s syndrome disease activity index: Development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann. Rheum. Dis. 2010, 69, 1103–1109. [Google Scholar] [CrossRef]
- Seror, R.; Ravaud, P.; Mariette, X.; Bootsma, H.; Theander, E.; Hansen, A.; Ramos-Casals, M.; Dörner, T.; Bombardieri, S.; Hachulla, E.; et al. EULAR Sjögren’s Task Force. EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI): Development of a consensus patient index for primary Sjögren’s syndrome. Ann. Rheum. Dis. 2011, 70, 968–972. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program (US). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- The Interactive Tool for Predicting and Managing the Fatal and Non-Fatal Cardiovascular Disease (CVD) Risk in Individuals without Previous CVD in Europe. Available online: https://www.heartscore.org/en_GB/ (accessed on 1 January 2023).
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. PREDIMED Study Investigators. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Santos-Beneit, G.; Bodega, P.; Pocock, S.; Mattei, J.; Penalvo, J.L. Validation of a questionnaire to measure overall Mediterranean lifestyle habits for research application: The Mediterranean lifestyle index (MEDLIFE). Nutr. Hosp. 2015, 32, 1153–1163. [Google Scholar] [PubMed]
- CuoreData, Il Progetto Cuore, Istituto Superiore di Sanità. Available online: https://www.cuore.iss.it/ (accessed on 30 June 2022).
- Del Brutto, O.H.; Mera, R.M.; Gillman, J.; Castillo, P.R.; Zambrano, M.; Ha, J.E. Dietary oily fish intake and blood pressure levels: A population-based study. J. Clin. Hypertens. 2016, 18, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.L.; Catena, C.; Dialti, V.; Pezzutto, F.; Mos, L.; Sechi, L.A. Fish meal supplementation and ambulatory blood pressure in patients with hypertension: Relevance of baseline membrane fatty acid composition. Am. J. Hypertens. 2014, 27, 471–481. [Google Scholar] [CrossRef]
- Carubbi, F.; Alunno, A.; Mai, F.; Mercuri, A.; Centorame, D.; Cipollone, J.; Mariani, F.M.; Rossi, M.; Bartoloni, E.; Grassi, D.; et al. Adherence to the Mediterranean diet and the impact on clinical features in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 133), 190–196. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart. J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Landolfo, M.; Borghi, C. Hyperuricaemia and vascular risk: The debate continues. Curr. Opin. Cardiol. 2019, 34, 399–405. [Google Scholar] [CrossRef]
- Quartuccio, L.; Baldini, C.; Bartoloni, E.; Priori, R.; Carubbi, F.; Alunno, A.; Gandolfo, S.; Gattamelata, A.; Giacomelli, R.; Gerli, R.; et al. Correlation between ESSDAI and ClinESSDAI in a real-life cohort of patients with Sjögren’s syndrome. Clin. Exp. Rheumatol. 2017, 35, 546–547. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Xiang, T.; Gong, B.; Xie, J. Serum uric acid as a diagnostic biomarker for rheumatoid arthritis-associated interstitial lung disease. Inflammation 2022, 45, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Gasse, P.; Riteau, N.; Charron, S.; Girre, S.; Fick, L.; Pétrilli, V.; Tschopp, J.; Lagente, V.; Quesniaux, V.F.J.; Ryffel, B.; et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2009, 179, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.M.; Lee, S.Y.; Lee, S.H.; Kim, S.S.; Park, H.W. Lung function decline is associated with serum uric acid in Korean health screening individuals. Sci. Rep. 2021, 11, 10183. [Google Scholar] [CrossRef]
- Bartoloni, E.; Baldini, C.; Schillaci, G.; Quartuccio, L.; Priori, R.; Carubbi, F.; Bini, V.; Alunno, A.; Bombardieri, S.; De Vita, S.; et al. Cardiovascular disease risk burden in primary Sjögren’s syndrome: Results of a population-based multicentre cohort study. J. Intern. Med. 2015, 278, 185–192. [Google Scholar] [CrossRef]
- Chiang, C.H. Primary Sjogren’s syndrome and risk of ischemic stroke: A nationwide study. Eur. Heart J. 2013, 34 (Suppl. 1), P400. [Google Scholar] [CrossRef]
- Qiao, T.; Wu, H.; Peng, W. The relationship between elevated serum uric acid and risk of stroke in adult: An updated and dose-response meta-analysis. Front. Neurol. 2021, 12, 674398. [Google Scholar] [CrossRef]
- Vedder, D.; Walrabenstein, W.; Heslinga, M.; de Vries, R.; Nurmohamed, M.; van Schaardenburg, D.; Gerritsen, M. Dietary interventions for gout and effect on the cardiovascular risk factors: A systematic review. Nutrients 2019, 11, 2955. [Google Scholar] [CrossRef]
- Alunno, A.; Carubbi, F. Unmet needs in primary Sjögren’s syndrome and the never-ending quest for the perfect biomarker. Acta Reumatol. Port. 2020, 45, 167–169. [Google Scholar] [PubMed]
| Variable | Mean | SD |
| Age, years | 61.9 | 11.5 |
| Age at diagnosis, years | 56.3 | 12.5 |
| Disease duration, years | 5.7 | 5.0 |
| ESSDAI | 6.3 | 4.8 |
| ClinESSDAI | 6.0 | 4.6 |
| ESSPRI | 6.3 | 2.3 |
| VAS dryness | 6.0 | 2.4 |
| VAS xerostomia | 6.3 | 2.7 |
| VAS xerophthalmia | 5.8 | 2.9 |
| VAS pain | 5.8 | 3.2 |
| VAS fatigue | 7.1 | 2.7 |
| Total cholesterol (mg/dL) | 202.5 | 35.7 |
| Triglycerides (mg/dL) | 109.3 | 49.5 |
| HDL-c (mg/dL) | 58.0 | 14.4 |
| LDL-c (mg/dL) | 126.0 | 34.6 |
| SUA (mg/dL) | 4.4 | 1.1 |
| Variable | N | % |
| Female gender | 99 | 94 |
| Autoantibodies | ||
| Neither anti-Ro nor anti-La | 62 | 59 |
| Anti-Ro only | 26 | 25 |
| Both anti-Ro and anti-La | 17 | 16 |
| Rheumatoid factor | 25 | 24 |
| Never smoked | 75 | 71 |
| Former smoker | 19 | 18 |
| Current smoker | 11 | 11 |
| Obesity | 17 | 16 |
| Arterial hypertension | 46 | 44 |
| Hypercholesterolemia | 41 | 39 |
| LDL-c > 115 mg/dL | 41 | 39 |
| HDL-c < 40 mg/dL | 18 | 17 |
| Hypertriglyceridemia | 12 | 11 |
| Type 2 diabetes | 3 | 3 |
| BMI (kg/m2) | 25.2 | 4.4 |
| Obesity | 17 | 16 |
| Myocardial infarction | 4 | 4 |
| Angina | 6 | 6 |
| Heart failure | 3 | 3 |
| Stroke | 3 | 3 |
| TIA | 14 | 13 |
| PAD | 5 | 5 |
| CV Risk Factors | ||
| 0 | 31 | 30 |
| 1–2 | 48 | 46 |
| >2 | 26 | 25 |
| CV events | ||
| 0 | 80 | 76 |
| 1 | 17 | 16 |
| >1 | 8 | 8 |
| SUA < 4.79 mg/dL (N = 81) | SUA ≥ 4.79 mg/dL (N = 24) | ||||
| Variable | Mean | SD | Mean | SD | p Value |
| Age, years | 62.6 | 12.1 | 59.7 | 9.0 | 0.167 |
| Age at diagnosis, years | 56.9 | 13.2 | 54.1 | 10.1 | 0.254 |
| Disease duration, years | 5.9 | 5.2 | 4.8 | 4.0 | 0.971 |
| ESSDAI | 5.5 | 4.2 | 9.0 | 5.6 | 0.005 |
| ClinESSDAI | 5.2 | 4.1 | 8.8 | 5.4 | 0.003 |
| ESSPRI | 6.3 | 2.3 | 6.5 | 2.3 | 0.662 |
| VAS dryness | 6.1 | 2.4 | 6.0 | 2.4 | 0.903 |
| VAS xerostomia | 6.3 | 2.6 | 6.2 | 3.1 | 0.984 |
| VAS xerophthalmia | 5.8 | 3.0 | 5.7 | 2.6 | 0.677 |
| VAS vaginal dryness | 5.1 | 3.3 | 6.8 | 2.9 | 0.044 |
| VAS pain | 5.7 | 3.3 | 6.2 | 2.9 | 0.663 |
| VAS fatigue | 7.1 | 2.7 | 7.3 | 2.9 | 0.628 |
| Total cholesterol (mg/dL) | 204.5 | 35.7 | 197.6 | 36.3 | 0.561 |
| Triglycerides (mg/dL) | 98.9 | 38.2 | 134.7 | 64.0 | 0.009 |
| HDL-c (mg/dL) | 59.2 | 15.1 | 54.2 | 11.7 | 0.275 |
| LDL-c (mg/dL) | 127.6 | 34.6 | 122.4 | 35.4 | 0.6 |
| BMI (kg/m2) | 24.6 | 4.3 | 27.2 | 3.9 | 0.009 |
| Variable | N | % | N | % | p Value |
| Female gender | 80 | 99 | 19 | 79 | <0.001 |
| Autoantibodies | 0.408 | ||||
| Neither anti-Ro nor anti-La | 50 | 62 | 12 | 50 | |
| Anti-Ro only | 17 | 21 | 9 | 38 | |
| Both anti-Ro and anti-La | 14 | 17 | 3 | 13 | |
| Rheumatoid factor | 19 | 23 | 6 | 25 | 0.876 |
| Smoking habit | 0.014 | ||||
| Never | 63 | 78 | 12 | 50 | |
| Former | 10 | 12 | 9 | 38 | |
| Current | 8 | 10 | 3 | 13 | |
| Obesity | 9 | 11 | 8 | 33 | 0.023 |
| Arterial hypertension | 32 | 40 | 14 | 58 | 0.159 |
| Hypercholesterolemia | 31 | 38 | 10 | 42 | 0.814 |
| LDL-c > 115 mg/dL | 31 | 38 | 10 | 42 | 0.814 |
| HDL-c < 40 mg/dL | 13 | 16 | 5 | 21 | |
| Hypertriglyceridemia | 6 | 7 | 6 | 25 | 0.028 |
| Type 2 diabetes | 2 | 2 | 1 | 4 | 0.545 |
| Myocardial infarction | 3 | 4 | 1 | 4 | 0.917 |
| Stroke | 0 | 0 | 3 | 13 | 0.011 |
| TIA | 11 | 14 | 3 | 13 | 0.891 |
| Heart failure | 2 | 2 | 1 | 4 | 0.661 |
| Angina | 4 | 5 | 2 | 8 | 0.618 |
| PAD | 3 | 4 | 2 | 8 | 0.321 |
| Hydroxychloroquine | 39 | 48 | 14 | 58 | 0.49 |
| Glucocorticoids | 13 | 16 | 6 | 25 | 0.37 |
| Other immunosuppressant | 20 | 25 | 4 | 17 | |
| CV Risk Factors | 0.024 | ||||
| 0 | 27 | 33 | 4 | 17 | |
| 1–2 | 38 | 47 | 10 | 42 | |
| >2 | 16 | 20 | 10 | 42 | |
| CV events | 0.07 | ||||
| No | 65 | 80 | 15 | 63 | |
| Yes | 16 | 20 | 9 | 38 | |
| Without Arterial Hypertension | With Arterial Hypertension | ||||
| Number of Patients = 59 | Number of Patients = 46 | ||||
| Variable | Mean | SD | Mean | SD | p Value |
| Age, years | 59.0 | 13.0 | 65.7 | 8.0 | 0.009 |
| Age at diagnosis, years | 53.9 | 13.6 | 59.3 | 10.3 | 0.036 |
| Disease duration, years | 5.2 | 5.4 | 6.4 | 4.3 | 0.014 |
| ESSDAI | 5.8 | 4.4 | 6.9 | 5.3 | 0.317 |
| ClinESSDAI | 5.5 | 4.2 | 6.6 | 5.1 | 0.3 |
| ESSPRI | 6.0 | 2.2 | 6.6 | 2.3 | 0.147 |
| VAS dryness | 5.8 | 2.4 | 6.3 | 2.4 | 0.335 |
| VAS xerostomia | 6.0 | 2.8 | 6.6 | 2.5 | 0.301 |
| VAS xerophthalmia | 5.6 | 2.9 | 6.0 | 3.0 | 0.512 |
| VAS vaginal dryness | 5.0 | 3.2 | 5.8 | 3.4 | 0.221 |
| VAS pain | 5.6 | 3.2 | 6.1 | 3.2 | 0.479 |
| VAS fatigue | 6.9 | 2.7 | 7.5 | 2.8 | 0.137 |
| Total cholesterol (mg/dL) | 194.7 | 29.4 | 209.6 | 39.6 | 0.111 |
| Triglycerides (mg/dL) | 98.2 | 44.0 | 119.0 | 52.6 | 0.035 |
| HDL-c (mg/dL) | 62.1 | 15.3 | 53.9 | 12.5 | 0.043 |
| LDL-c (mg/dL) | 117.9 | 31.2 | 132.2 | 36.2 | 0.085 |
| BMI (kg/m2) | 24.4 | 4.4 | 26.1 | 4.3 | 0.020 |
| Variable | N | % | N | % | p Value |
| Female gender | 56 | 95 | 43 | 93 | 0.753 |
| Autoantibodies | 0.676 | ||||
| Neither anti-Ro nor anti-La | 37 | 63 | 25 | 54 | |
| Anti-Ro only | 13 | 22 | 13 | 28 | |
| Both anti-Ro and anti-La | 9 | 15 | 8 | 17 | |
| Rheumatoid factor | 13 | 22 | 12 | 26 | 0.651 |
| Smoking habit | 0.04 | ||||
| Never | 45 | 76 | 30 | 65 | |
| Former | 6 | 10 | 13 | 28 | |
| Current | 8 | 14 | 3 | 7 | |
| Obesity | 8 | 14 | 9 | 20 | 0.435 |
| Hypercholesterolemia | 14 | 24 | 27 | 56 | <0.001 |
| LDL-c > 115 mg/dL | 12 | 22 | 29 | 63 | <0.001 |
| HDL-c < 40 mg/dL | 8 | 14 | 10 | 22 | 0.341 |
| Hypertriglyceridemia | 4 | 7 | 8 | 17 | 0.124 |
| Type 2 diabetes | 0 | 0 | 3 | 7 | 0.08 |
| Myocardial infarction | 1 | 2 | 3 | 6 | 0.317 |
| Angina | 1 | 2 | 5 | 11 | 0.08 |
| Heart failure | 1 | 2 | 2 | 4 | 0.580 |
| Any cardiac event | 2 | 3 | 8 | 17 | 0.02 |
| Stroke | 1 | 2 | 2 | 4 | 0.418 |
| TIA | 7 | 12 | 7 | 15 | 0.774 |
| PAD | 2 | 3 | 3 | 6 | |
| CV Risk Factors other than hypertension | <0.0001 | ||||
| 0 | 31 | 53 | 11 | 24 | |
| 1–2 | 23 | 39 | 26 | 56 | |
| >2 | 5 | 8 | 9 | 20 | |
| Previous CV event(s) | 9 | 17 | 16 | 33 | 0.02 |
| SUA < 4.79 mg/dL (N = 32) | SUA ≥ 4.79 mg/dL (N = 14) | ||||
| Variable | Mean | SD | Mean | SD | p Value |
| Age, years | 66.9 | 8.8 | 63.1 | 5.0 | 0.12 |
| Age at diagnosis, years | 60.5 | 10.8 | 56.6 | 8.9 | 0.19 |
| Disease duration, years | 6.5 | 4.5 | 6.1 | 3.9 | 0.97 |
| ESSDAI | 5.5 | 4.1 | 10.1 | 6.4 | 0.02 |
| ClinESSDAI | 5.2 | 4.0 | 9.8 | 6.1 | 0.02 |
| ESSPRI | 6.6 | 2.2 | 6.6 | 2.6 | 1.00 |
| VAS dryness | 6.6 | 2.2 | 5.7 | 2.8 | 0.32 |
| VAS xerostomia | 6.9 | 2.2 | 5.9 | 3.1 | 0.25 |
| VAS xerophthalmia | 6.2 | 2.9 | 5.6 | 3.1 | 0.48 |
| VAS vaginal dryness | 5.3 | 3.6 | 7.1 | 2.6 | 0.20 |
| VAS pain | 5.9 | 3.2 | 6.6 | 3.0 | 0.50 |
| VAS fatigue | 7.5 | 2.8 | 7.4 | 2.9 | 0.98 |
| Total cholesterol (mg/dL) | 213.1 | 41.1 | 202.5 | 37.1 | 0.57 |
| Triglycerides (mg/dL) | 113.5 | 40.7 | 129.1 | 70.4 | 0.65 |
| HDL-c (mg/dL) | 53.3 | 14.2 | 55.4 | 6.3 | 0.61 |
| LDL-c (mg/dL) | 135.3 | 37.6 | 126.0 | 34.2 | 0.63 |
| BMI (kg/m2) | 25.0 | 3.9 | 28.7 | 4.2 | 0.03 |
| Variable | N | % | N | % | p Value |
| Female gender | 31 | 97 | 12 | 86 | 0.216 |
| Autoantibodies | 0.246 | ||||
| Neither anti-Ro nor anti-La | 18 | 56 | 7 | 50 | |
| Anti-Ro only | 7 | 22 | 6 | 43 | |
| Both anti-Ro and anti-La | 7 | 22 | 1 | 7 | |
| Rheumatoid factor | 10 | 31 | 2 | 14 | 0.294 |
| Smoking habit | 0.02 | ||||
| Never | 25 | 78 | 5 | 36 | |
| Former | 6 | 19 | 7 | 50 | |
| Current | 1 | 3 | 2 | 14 | |
| Obesity | 3 | 9 | 6 | 43 | 0.015 |
| Hypercholesterolemia | 20 | 62 | 7 | 50 | 0.522 |
| LDL-c > 115 mg/dL | 21 | 66 | 8 | 57 | 0.742 |
| HDL-c < 40 mg/dL | 8 | 25 | 2 | 14 | |
| Hypertriglyceridemia | 6 | 19 | 2 | 14 | 0.54 |
| Type 2 diabetes | 2 | 6 | 1 | 7 | |
| Myocardial infarction | 2 | 6 | 1 | 7 | 0.673 |
| Stroke | 0 | 0 | 2 | 14 | 0.088 |
| TIA | 4 | 12 | 3 | 21 | 0.658 |
| Heart failure | 1 | 3 | 1 | 7 | 0.521 |
| Angina | 3 | 9 | 2 | 14 | 0.633 |
| PAD | 2 | 6 | 1 | 7 | 0.910 |
| CV Risk Factors other than hypertension | 0.18 | ||||
| 0 | 10 | 31 | 1 | 7 | |
| 1–2 | 18 | 56 | 8 | 57 | |
| 2–3 | 13 | 41 | 7 | 50 | |
| >3 | 0 | 0 | 1 | 7 | |
| CV events | 8 | 25 | 7 | 50 | 0.18 |
| Eligible for SCORE (N = 79) | Not Eligible for SCORE (N = 26) | ||||
| Variable | Mean | SD | Mean | SD | p Value |
| Age, years | 61.3 | 11.7 | 63.8 | 11.0 | 0.389 |
| Age at diagnosis, years | 55.8 | 12.7 | 57.7 | 12.1 | 0.506 |
| Disease duration, years | 5.8 | 5.2 | 5.5 | 4.3 | 0.946 |
| ESSDAI | 6.2 | 4.7 | 6.5 | 5.2 | 0.884 |
| ClinESSDAI | 5.9 | 4.6 | 6.2 | 4.9 | 0.875 |
| ESSPRI | 6.0 | 2.2 | 7.3 | 2.1 | 0.018 |
| VAS dryness | 5.8 | 2.3 | 6.8 | 2.7 | 0.086 |
| VAS xerostomia | 6.1 | 2.6 | 6.7 | 2.8 | 0.362 |
| VAS xerophthalmia | 5.5 | 2.9 | 6.8 | 2.8 | 0.037 |
| VAS vaginal dryness | 5.2 | 3.5 | 6.2 | 2.6 | 0.283 |
| VAS pain | 5.4 | 3.2 | 7.0 | 2.7 | 0.03 |
| VAS fatigue | 6.9 | 2.9 | 8.0 | 2.0 | 0.106 |
| Total cholesterol (mg/dL) | 194.9 | 31.4 | 228.3 | 38.3 | 0.002 |
| Triglycerides (mg/dL) | 97.7 | 32.6 | 147.6 | 73.5 | 0.005 |
| HDL-c (mg/dL) | 58.7 | 13.8 | 55.5 | 16.7 | 0.709 |
| LDL-c (mg/dL) | 119.6 | 30.7 | 150.8 | 38.9 | 0.014 |
| SUA (mg/dL) | 4.3 | 1.0 | 4.9 | 1.2 | 0.05 |
| BMI (kg/m2) | 24.5 | 4.0 | 27.5 | 4.9 | 0.005 |
| Variable | N | % | N | % | p Value |
| Female gender | 76 | 96 | 23.0 | 88 | 0.160 |
| Autoantibodies | 0.234 | ||||
| Neither anti-Ro nor anti-La | 47 | 60 | 15 | 58 | |
| Anti-Ro only | 17 | 21 | 9 | 34 | |
| Both anti-Ro and anti-La | 15 | 19 | 2 | 8 | |
| Rheumatoid factor | 19 | 24 | 6 | 23 | 0.574 |
| Smoking habit | 0.42 | ||||
| Never | 59 | 75 | 16 | 62 | |
| Former | 13 | 16 | 6 | 23 | |
| Current | 7 | 9 | 4 | 15 | |
| Obesity | 9 | 11 | 8 | 31 | 0.031 |
| Hypertension | 30 | 38 | 16 | 62 | 0.04 |
| Hypercholesterolemia | 23 | 29 | 18 | 69 | <0.001 |
| LDL-c > 115 mg/dL | 28 | 35 | 0.241 | ||
| HDL-c < 40 mg/dL | 9 | 11 | 9 | 35 | |
| Hypertriglyceridemia | 5 | 6 | 7 | 27 | 0.009 |
| CV Risk Factors | |||||
| 0 | 28 | 35 | 3 | 11 | 0.0001 |
| 1–2 | 38 | 48 | 10 | 38 | |
| 2–3 | 26 | 33 | 12 | 46 | |
| >3 | 2 | 3 | 8 | 31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alunno, A.; Carubbi, F.; Mariani, F.M.; Martini, C.; Campanozzi, E.; Ferri, C. The Interplay between Cardiovascular Risk, Cardiovascular Events, and Disease Activity in Primary Sjögren’s Syndrome: Is Uric Acid the Missing Link? Nutrients 2023, 15, 1563. https://doi.org/10.3390/nu15071563
Alunno A, Carubbi F, Mariani FM, Martini C, Campanozzi E, Ferri C. The Interplay between Cardiovascular Risk, Cardiovascular Events, and Disease Activity in Primary Sjögren’s Syndrome: Is Uric Acid the Missing Link? Nutrients. 2023; 15(7):1563. https://doi.org/10.3390/nu15071563
Chicago/Turabian StyleAlunno, Alessia, Francesco Carubbi, Francesco Maria Mariani, Cecilia Martini, Elena Campanozzi, and Claudio Ferri. 2023. "The Interplay between Cardiovascular Risk, Cardiovascular Events, and Disease Activity in Primary Sjögren’s Syndrome: Is Uric Acid the Missing Link?" Nutrients 15, no. 7: 1563. https://doi.org/10.3390/nu15071563
APA StyleAlunno, A., Carubbi, F., Mariani, F. M., Martini, C., Campanozzi, E., & Ferri, C. (2023). The Interplay between Cardiovascular Risk, Cardiovascular Events, and Disease Activity in Primary Sjögren’s Syndrome: Is Uric Acid the Missing Link? Nutrients, 15(7), 1563. https://doi.org/10.3390/nu15071563







