A Meta-Analysis of the Prevalence of Wheat Allergy Worldwide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Definition
2.4. Study Selection and Data Extraction
2.5. Risk of Bias Assessment
2.6. Meta-Analysis of the Prevalence of Wheat Allergy
3. Results
3.1. Study Selection
3.2. Study Characteristics
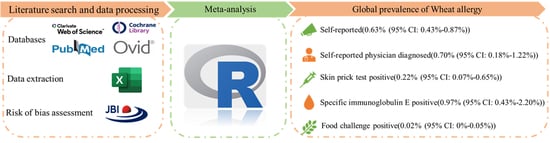
3.3. Prevalence of Wheat Allergy
3.3.1. SR Wheat Allergy
3.3.2. SRPD Wheat Allergy
3.3.3. Wheat Allergy for SPT Positive
3.3.4. Wheat Allergy for sIgE Positive
3.3.5. Wheat Allergy Confirmed by FC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and Burden of Food Allergy. Curr. Allergy Asthma Rep. 2020, 20, 6. [Google Scholar] [CrossRef]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 13, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Piboonpocanun, S.; Thongngarm, T.; Wongsa, C.; Pacharn, P.; Reamtong, O.; Sompornrattanaphan, M. Omega-5 and Gamma Gliadin are the Major Allergens in Adult-Onset IgE-Mediated Wheat Allergy: Results from Thai Cohort with Oral Food Challenge. J. Asthma Allergy 2021, 14, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ji, H.; Chen, Y.; Li, Z.; Timira, V. A systematic review on the recent advances of wheat allergen detection by mass spectrometry: Future prospects. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Matsuo, H.; Kohno, K.; Yokooji, T.; Yano, H.; Endo, T. A Narrative Mini Review on Current Status of Hypoallergenic Wheat Development for IgE-Mediated Wheat Allergy, Wheat-Dependent Exercise-Induced Anaphylaxis. Foods 2023, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Doelle-Bierke, S.; Renaudin, J.-M.; Rueff, F.; Scherer Hofmeier, K.; Treudler, R.; Pfoehler, C.; Hawranek, T.; Poziomkowska-Gesicka, I.; Jappe, U.; et al. Wheat Anaphylaxis in Adults Differs from Reactions to Other Types of Food. J. Allergy Clin. Immunol.-Pract. 2021, 9, 2844–2852. [Google Scholar] [CrossRef]
- Jiang, N.; Yin, J.; Wen, L.; Li, H. Characteristics of Anaphylaxis in 907 Chinese Patients Referred to a Tertiary Allergy Center: A Retrospective Study of 1,952 Episodes. Allergy Asthma Immunol. Res. 2016, 8, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat Allergy in Children: A Comprehensive Update. Medicina 2019, 55, 400. [Google Scholar] [CrossRef] [Green Version]
- Anvari, S.; Miller, J.; Yeh, C.-Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef] [Green Version]
- FAO/WHO. Review and Validation of CODEX Priority Allergen List through Risk Assessment; Food & Agriculture Org.: Rome, Italy, 2020. [Google Scholar]
- Cabanillas, B. Gluten-related disorders: Celiac disease, wheat allergy, and nonceliac gluten sensitivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 2606–2621. [Google Scholar] [CrossRef]
- Rona, R.J.; Keil, T.; Summers, C.; Gislason, D.; Zuidmeer, L.; Sodergren, E.; Sigurdardottir, S.T.; Lindner, T.; Goldhahn, K.; Dahlstrom, J.; et al. The prevalence of food allergy: A meta-analysis. J. Allergy Clin. Immunol. 2007, 120, 638–646. [Google Scholar] [CrossRef]
- Country Classification. Available online: https://www.un.org/en/development/desa/policy/wesp/wesp_current/2014wesp_country_classification.pdf (accessed on 6 February 2023).
- WHO Regional Offices. Available online: https://www.who.int/about/who-we-are/regional-offices (accessed on 27 January 2023).
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. JBI Evid. Implement. 2015, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Al-Hammadi, S.; Al-Maskari, F.; Bernsen, R. Prevalence of Food Allergy among Children in Al-Ain City, United Arab Emirates. Int. Arch. Allergy Immunol. 2010, 151, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Ali, F. A Survey of Self-Reported Food Allergy and Food-Related Anaphylaxis among Young Adult Students at Kuwait University, Kuwait. Med. Princ. Pract. 2017, 26, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Althumiri, N.A.; Basyouni, M.H.; AlMousa, N.; AlJuwaysim, M.F.; BinDhim, N.F.; Alqahtani, S.A. Prevalence of Self-Reported Food Allergies and Their Association with Other Health Conditions among Adults in Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 347. [Google Scholar] [CrossRef] [PubMed]
- Basera, W.; Botha, M.; Gray, C.L.; Lunjani, N.; Watkins, A.S.; Venter, C.; Allen, K.J.; Hlela, C.; Zar, H.J.; Levin, M.E. The South African Food Sensitisation and Food Allergy population-based study of IgE-mediated food allergy: Validity, safety, and acceptability. Ann. Allergy Asthma. Immunol. 2015, 115, 113–119. [Google Scholar] [CrossRef]
- Beltrán-Cárdenas, C.E.; Granda-Restrepo, D.M.; Franco-Aguilar, A.; Lopez-Teros, V.; Arvizu-Flores, A.A.; Cárdenas-Torres, F.I.; Ontiveros, N.; Cabrera-Chávez, F.; Arámburo-Gálvez, J.G. Prevalence of Food-Hypersensitivity and Food-Dependent Anaphylaxis in Colombian Schoolchildren by Parent-Report. Medicina 2021, 57, 146. [Google Scholar] [CrossRef] [PubMed]
- Botha, M.; Basera, W.; Facey-Thomas, H.E.; Gaunt, B.; Gray, C.L.; Ramjith, J.; Watkins, A.; Levin, M.E. Rural and urban food allergy prevalence from the South African Food Allergy (SAFFA) study. J. Allergy Clin. Immunol. 2019, 143, 662–668.e2. [Google Scholar] [CrossRef] [PubMed]
- Da, S.C.J.A.; Antunes, A.A.; Taborda-Barata, L.; Boechat, J.L.; Sarinho, E.S.C. Prevalence of reported food allergies in Brazilian preschoolers living in a small Brazilian city. Allergy Asthma. Clin. Immunol. 2022, 18, 74. [Google Scholar]
- Dean, T.; Venter, C.; Pereira, B.; Arshad, S.H.; Grundy, J.; Clayton, C.B.; Higgins, B. Patterns of sensitization to food and aeroallergens in the first 3 years of life. J. Allergy Clin. Immunol. 2007, 120, 1166–1171. [Google Scholar] [CrossRef]
- Dogruel, D.; Bingol, G.; Altintas, D.U.; Yilmaz, M.; Kendirli, S.G. Clinical Features of Food Allergy during the 1st Year of Life: The ADAPAR Birth Cohort Study. Int. Arch. Allergy Immunol. 2016, 169, 171–180. [Google Scholar] [CrossRef]
- Gaspar-Marques, J.; Carreiro-Martins, P.; Papoila, A.L.; Caires, I.; Pedro, C.; Araújo-Martins, J.; Virella, D.; Rosado-Pinto, J.; Leiria-Pinto, P.; Neuparth, N. Food allergy and anaphylaxis in infants and preschool-age children. Clin. Pediatr. 2014, 53, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.C.P.; Guimaraes, T.C.P.; Silva, R.M.; Cheik, M.F.A.; Napolis, A.C.D.R.; Silva, G.B.E.; Segundo, G.R.S. Prevalence of food allergy in infants and pre-schoolers in Brazil. Allergol. Et. Immunopathol. 2016, 44, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Grabenhenrich, L.; Trendelenburg, V.; Bellach, J.; Yürek, S.; Reich, A.; Fiandor, A.; Rivero, D.; Sigurdardottir, S.; Clausen, M.; Papadopoulos, N.G.; et al. Frequency of food allergy in school-aged children in eight European countries-The EuroPrevall-iFAAM birth cohort. Allergy 2020, 75, 2294–2308. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, K.E.; Bryant, T.; Oliver, E.M.; Martin, J.; Maskell, J.; Kemp, T.; Clare Mills, E.N.; Foote, K.D.; Margetts, B.M.; Beyer, K.; et al. Incidence and risk factors for food hypersensitivity in UK infants: Results from a birth cohort study. Clin. Transl. Allergy 2015, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States. Pediatrics 2011, 128, E9–E17. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef] [Green Version]
- Hoyos-Bachiloglu, R.; Ivanovic-Zuvic, D.; Álvarez, J.; Linn, K.; Thöne, N.; de los Ángeles Paul, M.; Borzutzky, A. Prevalence of parent-reported immediate hypersensitivity food allergy in Chilean school-aged children. Allergol Immunopathol. 2014, 42, 527–532. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Li, H. Comparison of food allergy prevalence among Chinese infants in Chongqing, 2009 versus 1999. Pediatr. Int. 2010, 52, 820–824. [Google Scholar] [CrossRef]
- Irani, C.; Maalouly, G. Prevalence of Self-Reported Food Allergy in Lebanon: A Middle-Eastern Taste. Int. Sch. Res. Not. 2015, 2015, 639796. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Jeon, H.Y.; Yang, H.K.; Lee, K.J.; Han, Y.; Kim, Y.H.; Kim, J.; Ahn, K. Prevalence of Immediate-Type Food Allergy in Korean Schoolchildren in 2015: A Nationwide, Population-based Study. Allergy Asthma. Immunol. Res. 2017, 9, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lao-araya, M.; Trakultivakorn, M. Prevalence of food allergy among preschool children in northern Thailand. Pediatr. Int. 2012, 54, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.K.; Nguyen, D.H.; Vu, A.T.L.; Ruethers, T.; Taki, A.C.; Lopata, A.L. A cross-sectional, population-based study on the prevalence of food allergies among children in two different socio-economic regions of Vietnam. Pediatr. Allergy Immunol. 2019, 30, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ogorodova, L.M.; Mahesh, P.A.; Wang, M.H.; Fedorova, O.S.; Leung, T.F.; Fernandez-Rivas, M.; Mills, E.N.C.; Potts, J.; Kummeling, I.; et al. Comparative Study of Food Allergies in Children from China, India, and Russia: The EuroPrevall-INCO Surveys. J. Allergy Clin. Immunol. Pract. 2020, 8, 1349–1358. [Google Scholar] [CrossRef]
- Lyons, S.A.; Burney, P.G.J.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Clausen, M.; Dubakiene, R.; Fernandez-Perez, C.; Fritsche, P.; Jedrzejczak-Czechowicz, M.; et al. Food Allergy in Adults: Substantial Variation in Prevalence and Causative Foods Across Europe. J. Allergy Clin. Immunol. Pract. 2019, 7, 1920–1928.e11. [Google Scholar] [CrossRef]
- Mahesh, P.A.; Wong, G.W.; Ogorodova, L.; Potts, J.; Leung, T.F.; Fedorova, O.; Holla, A.D.; Fernandez-Rivas, M.; Clare Mills, E.N.; Kummeling, I.; et al. Prevalence of food sensitization and probable food allergy among adults in India: The EuroPrevall INCO study. Allergy 2016, 71, 1010–1019. [Google Scholar] [CrossRef]
- Matsyura, O.; Besh, L.; Borysiuk, O.; Gutor, T.; Malska, A.; Kovalska, O.; Besh, O.; Sorokopud, O.; Vari, S.G. Food Hypersensitivity in Children Aged 0-3 Years of the Lviv Region in Ukraine: A Cross-Sectional Study. Front Pediatr. 2021, 9, 800331. [Google Scholar] [CrossRef]
- McGowan, E.C.; Keet, C.A. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007–2010. J. Allergy Clin. Immunol. 2013, 132, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Morillo-Argudo, D.A.; Andrade Tenesaca, D.S.; Rodas-Espinoza, C.R.; Perkin, M.R.; Gebreegziabher, T.L.; Zuniga, G.A.; Andrade Munoz, D.D.; Ramirez, P.L.; Garcia Garcia, A.A.; Ochoa-Aviles, A.M. Food allergy, airborne allergies, and allergic sensitisation among adolescents living in two disparate socioeconomic regions in Ecuador: A cross-sectional study. World Allergy Organ. J. 2020, 13, 100478. [Google Scholar] [CrossRef]
- Nachshon, L.; Schwartz, N.; Elizur, A.; Schon, Y.; Cheryomukhin, M.; Katz, Y.; Goldberg, M.R. The Prevalence of Food Allergy in Young Israeli Adults. J. Allergy Clin. Immunol. Pract. 2019, 7, 2782–2789. [Google Scholar] [CrossRef]
- Okada, Y.; Yamashita, T.; Kumagai, H.; Morikawa, Y.; Akasawa, A. Accurate Determination of Childhood Food Allergy Prevalence and Correction of Unnecessary Avoidance. Allergy Asthma Immunol. Res. 2017, 9, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ontiveros, N.; Emilio Real-Delor, R.; Antonio Mora-Melgem, J.; Eduardo Beltran-Cardenas, C.; Gerardo Figueroa-Salcido, O.; de Jesus Vergara-Jimenez, M.; Isidro Cardenas-Torres, F.; Karem Flores-Mendoza, L.; Gilberto Aramburo-Galvez, J.; Cabrera-Chavez, F. Prevalence of Wheat/Gluten-Related Disorders and Gluten-Free Diet in Paraguay: An Online Survey-Based Study. Nutrients 2021, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; Valdez-Meza, E.E.; Vergara-Jimenez, M.J.; Canizalez-Roman, A.; Borzutzky, A.; Cabrera-Chavez, F. Parent-reported prevalence of food allergy in Mexican schoolchildren: A population-based study. Allergol. Et. Immunopathol. 2016, 44, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ostblom, E.; Lilja, G.; Ahlstedt, S.; van Hage, M.; Wickman, M. Patterns of quantitative food-specific IgE-antibodies and reported food hypersensitivity in 4-year-old children. Allergy 2008, 63, 418–424. [Google Scholar] [CrossRef]
- Ostblom, E.; Wickman, M.; van Hage, M.; Lilja, G. Reported symptoms of food hypersensitivity and sensitization to common foods in 4-year-old children. Acta. Paediatr. 2008, 97, 85–90. [Google Scholar] [CrossRef]
- Osterballe, M.; Mortz, C.G.; Hansen, T.K.; Andersen, K.E.; Bindslev-Jensen, C. The Prevalence of food hypersensitivity in young adults. Pediatr. Allergy Immunol. 2009, 20, 686–692. [Google Scholar] [CrossRef]
- Pyrhonen, K.; Nayha, S.; Kaila, M.; Hiltunen, L.; Laara, E. Occurrence of parent-reported food hypersensitivities and food allergies among children aged 1–4 years. Pediatr. Allergy Immunol. 2009, 20, 328–338. [Google Scholar] [CrossRef]
- Sasaki, M.; Koplin, J.J.; Dharmage, S.C.; Field, M.J.; Sawyer, S.M.; McWilliam, V.; Peters, R.L.; Gurrin, L.C.; Vuillermin, P.J.; Douglass, J.; et al. Prevalence of clinic-defined food allergy in early adolescence: The SchoolNuts study. J. Allergy Clin. Immunol. 2018, 141, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, R.; Ellert, U.; Kalcklösch, M.; Dahm, S.; Thamm, M. Patterns of sensitization to inhalant and food allergens—findings from the German Health Interview and Examination Survey for Children and Adolescents. Int. Arch. Allergy Immunol. 2013, 162, 263–270. [Google Scholar] [CrossRef]
- Sha, L.; Shao, M.; Liu, C.; Wu, Y.; Chen, Y. A cross-sectional study of the prevalence of food allergies among children younger than ages 14 years in a Beijing urban region. Allergy Asthma Proc. 2019, 40, E1–E7. [Google Scholar] [CrossRef]
- Soller, L.; Ben-Shoshan, M.; Harrington, D.W.; Fragapane, J.; Joseph, L.; St Pierre, Y.; Godefroy, S.B.; La Vieille, S.; Elliott, S.J.; Clarke, A.E. Overall prevalence of self-reported food allergy in Canada. J. Allergy Clin. Immunol. 2012, 130, 986–988. [Google Scholar] [CrossRef]
- Soller, L.; Ben-Shoshan, M.; Harrington, D.W.; Knoll, M.; Fragapane, J.; Joseph, L.; St Pierre, Y.; La Vieille, S.; Wilson, K.; Elliott, S.J.; et al. Adjusting for nonresponse bias corrects overestimates of food allergy prevalence. J. Allergy Clin. Immunol. Pract. 2015, 3, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Strinnholm, A.; Winberg, A.; West, C.; Hedman, L.; Ronmark, E. Food hypersensitivity is common in Swedish schoolchildren, especially oral reactions to fruit and gastrointestinal reactions to milk. Acta. Paediatr. 2014, 103, 1290–1296. [Google Scholar] [CrossRef]
- Tsai, H.J.; Kumar, R.; Pongracic, J.; Liu, X.; Story, R.; Yu, Y.; Caruso, D.; Costello, J.; Schroeder, A.; Fang, Y.; et al. Familial aggregation of food allergy and sensitization to food allergens: A family-based study. Clin. Exp. Allergy 2009, 39, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, D.; Erlewyn-Lajeunesse, M.; Kurukulaaratchy, R.J.; Potter, S.; Roberts, G.; Matthews, S.; Arshad, S.H. Prevalence and longitudinal trends of food allergy during childhood and adolescence: Results of the Isle of Wight Birth Cohort study. Clin. Exp. Allergy 2018, 48, 394–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, C.; Maslin, K.; Arshad, S.H.; Patil, V.; Grundy, J.; Glasbey, G.; Twiselton, R.; Dean, T. Very low prevalence of IgE mediated wheat allergy and high levels of cross-sensitisation between grass and wheat in a UK birth cohort. Clin. Transl. Allergy 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, C.; Maslin, K.; Zhang, H.; Kaushal, A.; Terry, W.; Patil, V.K.; Dean, T.; Arshad, S.H. Use of cluster analysis to characterize patterns of sensitization in childhood allergy. Pediatr. Allergy Immunol. 2018, 29, 644–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verrill, L.; Bruns, R.; Luccioli, S. Prevalence of self reported food allergy in US adults: 2001, 2006, and 2010. Allergy Asthma Proc. 2015, 36, 458–467. [Google Scholar] [CrossRef] [Green Version]
- Vierk, K.A.; Koehler, K.M.; Fein, S.B.; Street, D.A. Prevalence of self-reported food allergy in American adults and use of food labels. J. Allergy Clin. Immunol. 2007, 119, 1504–1510. [Google Scholar] [CrossRef]
- Wickman, M.; Asarnoj, A.; Tillander, H.; Andersson, N.; Bergstrom, A.; Kull, I.; Melen, E.; Pershagen, G.; Ahlstedt, S.; Lilja, G.; et al. Childhood-to-adolescence evolution of IgE antibodies to pollens and plant foods in the BAMSE cohort. J. Allergy Clin. Immunol. 2014, 133, 580–582. [Google Scholar] [CrossRef]
- Wilson, J.M.; Workman, L.; Schuyler, A.J.; Rifas-Shiman, S.L.; McGowan, E.C.; Oken, E.; Gold, D.R.; Hamilton, R.G.; Platts-Mills, T.A.E. Allergen sensitization in a birth cohort at midchildhood: Focus on food component IgE and IgG(4) responses. J. Allergy Clin. Immunol. 2018, 141, 419–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakhlef, M.; Souiki, L. Contribution to the estimation of the prevalence of food allergy in schoolchildren in the city of Guelma (Algeria). Rev. Fr. D Allergol. 2021, 61, 573–578. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Pak, K.; Saito-Abe, M.; Yang, L.; Sato, M.; Irahara, M.; Mezawa, H.; Sasaki, H.; Nishizato, M.; Ishitsuka, K.; et al. Allergy and immunology in young children of Japan: The JECS cohort. World Allergy Organ. J. 2020, 13, 100479. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.Q.; Luo, J.Y.; Huang, H.M.; Zheng, P.Y.; Luo, W.T.; Wei, N.L.; Sun, B.Q. Food allergy and related risk factors in 2540 preschool children: An epidemiological survey in Guangdong Province, southern China. World J. Pediatr. WJP 2015, 11, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kårhus, L.L.; Skaaby, T.; Madsen, A.L.; Thuesen, B.H.; Schwarz, P.; Rumessen, J.J.; Linneberg, A. The association of celiac disease and allergic disease in a general adult population. United Eur. Gastroenterol. J. 2019, 7, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Palmu, S.; Heikkilä, P.; Uski, V.; Niitty, S.; Kurikka, S.; Korppi, M. National allergy programme had little impact on parent-reported food allergies in children aged 6–7 years. Acta. Paediatr. 2018, 107, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; He, M.; Shen, X.; Wu, X.; Yu, X.; He, F.; Li, M. Prevalence and influencing factors of food hypersensitivity in infants reported by parents in Chengdu City. Wei Sheng Yan Jiu = J. Hyg. Res. 2019, 48, 594–600. [Google Scholar]
- Zhang, Y.; Chen, Y.; Zhao, A.; Li, H.; Mu, Z.; Zhang, Y.; Wang, P. Prevalence of self-reported food allergy and food intolerance and their associated factors in 3–12 year-old children in 9 areas in China. Wei Sheng Yan Jiu = J. Hyg. Res. 2015, 44, 226–231. [Google Scholar]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008, 121, 1210–1218.e4. [Google Scholar] [CrossRef]
- Quirce, S.; Boyano-Martínez, T.; Díaz-Perales, A. Clinical presentation, allergens, and management of wheat allergy. Expert Rev. Clin. Immunol. 2016, 12, 563–572. [Google Scholar] [CrossRef] [Green Version]
- FAO/WHO. Risk Assessment of Food Allergens. Part 1—Review and Validation of Codex Alimentarius Priority Allergen List through Risk Assessment; Food & Agriculture Org.: Rome, Italy, 2022. [Google Scholar]
- Siripipattanamongkol, N.; Vichyanond, P.; Jirapongsananuruk, O.; Veskitkul, J.; Visitsunthorn, N.; Pacharn, P. Age of resolution from IgE-mediated wheat allergy. Asian Pac. J. Allergy Immunol. 2017, 35, 113–117. [Google Scholar]
- Christensen, M.J.; Eller, E.; Mortz, C.G.; Bindslev-Jensen, C. Patterns of suspected wheat-related allergy: A retrospective single-centre case note review in 156 patients. Clin. Transl. Allergy 2014, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.-S.; Rha, Y.-H.; Oh, I.-H.; Choi, Y.-S.; Choi, S.-H. Socioeconomic and sociodemographic factors related to allergic diseases in Korean adolescents based on the Seventh Korea Youth Risk Behavior Web-based Survey: A cross-sectional study. BMC Pediatr. 2016, 16, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Weger, W.W.; Sprikkelman, A.B.; Herpertz, C.E.M.; Meulen, G.N.; Vonk, J.M.; Kamps, A.W.A.; Koppelman, G.H. The dilemma of open or double-blind food challenges in diagnosing food allergy in children: Design of the ALDORADO trial. Pediatr. Allergy Immunol. 2022, 33, e13654. [Google Scholar] [CrossRef] [PubMed]









| No | First Author, Published Year | Study Design | Study Period (Year, or Year and Month) | Sample Size | Country | Age of Population | Diagnostic Method * |
|---|---|---|---|---|---|---|---|
| 1 | S. Al-Hammadi., 2010 [17] | Cross-sectional study | December 2006 | 397 | The United Arab Emirates | Children | SRPD |
| 2 | Ali, F., 2017 [18] | Cross-sectional study | May 2015–January 2015 | 865 | Kuwait | Other | SR SRPD |
| 3 | Nora A. Althumiri., 2021 [19] | Cross-sectional study | January 2020 | 4709 | Saudi Arabia | Adults | SR |
| 4 | Basera, W., 2015 [20] | Cross-sectional study | February 2013–December 2014 | 512 | South Africa | Children | SPT |
| 5 | Beltrán-Cárdenas, C.E., 2021 [21] | Cross-sectional study | August 2019–September 2019 | 969 | Colombia | Children | SR |
| 6 | Botha, M., 2019 [22] | Cross-sectional study | February 2013–December 2016 | 1583 | South Africa | Children | SPT |
| 7 | da, S. Correia, J.A., 2009 [23] | Cross-sectional study | 2019–2020 | 412 | Brazil | Children | SR |
| 8 | Dean, T., 2007 [24] | Cohort study | September 2001–August 2002 | 807 | UK | Children | SPT |
| 9 | Dogruel, D., 2016 [25] | Cohort study | February 2010–February 2011 | 1377 | Turkey | Children | SPT sIgE |
| 10 | Gaspar-Marques, J., 2014 [26] | Cross-sectional study | NA | 1217 | Portugal | Children | SR |
| 11 | Goncalves, L.C.P., 2016 [27] | Cross-sectional study | March 2012–September 2013 | 9265 | Brazil | Other | SR SRPD |
| 12 | Grabenhenrich, L., 2020 [28] | Cohort study | 2013–2017 | 1341 1140 976 1570 1513 1556 1387 | Iceland UK Netherland Germany Poland Lithuania Spain | Children | SR |
| 13 | Grimshaw, K.E., 2016 [29] | Cohort study | 2006–2008 | 823 | UK | Children | FC |
| 14 | Gupta, Ruchi S., 2011 [30] | Cross-sectional study | June 2009–February 2010 | 38,480 | US | Children | SR |
| 15 | Gupta, Ruchi S., 2019 [31] | Cross-sectional study | October 2015–September 2016 | 40,443 | US | Adults | SR |
| 16 | Hoyos-Bachiloglu, R., 2014 [32] | Cross-sectional study | September 2011–December 2012 | 455 | Chile | Children | SR |
| 17 | Yan, H., 2010 [33] | Well health check | 2009 | 382 | China | Children | SPT |
| 18 | Irani, C., 2015 [34] | Cross-sectional study | July 2014 + 7 weeks | 506 | Lebanon | Other | SR |
| 19 | Kim, M., 2017 [35] | Cross-sectional study | September 2015 | 29,842 | Korea | Children | SR |
| 20 | Lao-araya, M., 2012 [36] | Cross-sectional study | 2010 | 446 | Thailand | Children | SR |
| 21 | Le, Thu T.K., 2019 [37] | Cross-sectional study | 2016 | 8620 | Vietnam | Children | SR SRPD |
| 22 | Jing, L., 2020 [38] | Cross-sectional study | September 2009–June 2016 | 6194 5139 12,997 5677 | China China Russia India | Children | SPT sIgE |
| 23 | Lyons, S.A., 2019 [39] | Cross-sectional study | 2005–2009 | 2229 935 2078 1497 | Sweden Spain Iceland Poland | Adults | sIgE |
| 24 | Mahesh, P.A., 2016 [40] | Cross-sectional study | 2005–2009 | 10,931 | India | Adults | sIgE |
| 25 | Matsyura, O., 2021 [41] | Cross-sectional study | 2016–2017 | 935 | Ukrine | Children | SR |
| 26 | McGowan, Emily C., 2013 [42] | Cross-sectional study | 2007–2010 | 2078 | US | Other | SR |
| 27 | Morillo-Argudo, D.A., 2020 [43] | Cross-sectional study | July 2013–July 2014 | 1497 | Ecuador | Children | SPT |
| 28 | Nachshon, L., 2019 [44] | Cross-sectional study | May-October 2016 | 12,592 | Israel | Children | SR |
| 29 | Okada, Y., 2017 [45] | Cross-sectional study | July 2014–February 2015 | 374 | Japan | Children | sIgE |
| 30 | Ontiveros, N., 2021 [46] | Cross-sectional study | October 2020 | 1058 | Paraguayan | Adults | SR |
| 31 | Ontiveros, N., 2016 [47] | Cross-sectional study | September 2014–August 2015 | 1049 | Mexico | Children | SR |
| 32 | Ostblom, E., 2008 [48] | Cohort study | 1994–1996 | 3742 | Sweden | Children | sIgE |
| 33 | Ostblom, E., 2008 [49] | Cohort study | 1994–1996 | 3742 | Sweden | Children | SR |
| 34 | Osterballe, M., 2009 [50] | Cross-sectional study | NA | 1094 | Denmark | Adults | SR |
| 35 | Pyrhonen, K., 2009 [51] | Cross-sectional study | April 2001–March 2005 | 3308 | Finland | Children | SR SRPD |
| 36 | Sasaki, M., 2018 [52] | Cross-sectional study | 2011–2014 | 9663 | Australia | Children | FC |
| 37 | Schmitz, R., 2013 [53] | Cross-sectional study | May 2003–May 2006 | 12,998 | Germany | Children | sIgE |
| 38 | Sha, L., 2019 [54] | Cross-sectional study | 2010 | 13,073 | China | Children | SR |
| 39 | Soller, L., 2012 [55] | Cross-sectional study | May 2008–March 2009 | 9667 | Canada | Other | SR |
| 40 | Soller, L., 2015 [56] | Cross-sectional study | 2010–2011 | 15,022 | Canada | Other | SR SRPD |
| 41 | Strinnholm, A., 2014 [57] | Cohort study | 2006–2010 | 2585 | Sweden | Children | SR |
| 42 | Tsai, H.J., 2009 [58] | Cross-sectional study | August 2005–May 2008 | 2004 | US | Other | sIgE |
| 43 | Venkataraman, D., 2018 [59] | Cohort study | 2007 | 1290 | UK | Adults | SR |
| 44 | Venter, C., 2016 [60] | Cohort study | 2011–2012 | 827 | UK | Children | FC |
| 45 | Venter, C., 2018 [61] | Cohort study | 2011–2012 | 827 | UK | Children | SPT |
| 46 | Verrill, L., 2015 [62] | Cross-sectional study | 2007–2010 | 44,778 | US | Adults | SR SRPD |
| 47 | Vierk, K.A., 2007 [63] | Cross-sectional study | April 2001–August 2011 | 4568 | US | Adults | SR SRPD |
| 48 | Wickman, M., 2014 [64] | Cohort study | 2010–2012 | 1699 | Sweden | Children | sIgE |
| 49 | Wilson, J.M., 2018 [65] | Cohort study | 1999–2002 | 1279 | US | Children | sIgE |
| 50 | Yakhlef, M., 2021 [66] | Cross-sectional study | 15 April 2018–29 April 2018 | 528 | Algeria | Children | SR |
| 51 | Yamamoto-Hanada, K., 2020 [67] | Cross-sectional study | January 2011–March 2014 | 92,945 | Japan | Children | SR |
| 52 | Zeng, G.Q., 2015 [68] | Cross-sectional study | January 2013–December 2013 | 2540 | China | Children | SR |
| 53 | Karhus, L, L., 2019 [69] | Cross-sectional study | June 2006–June 2008 | 3405 | Denmark | Adults | sIgE |
| 54 | Palmu.S., 2018 [70] | Cross-sectional study | May 2016–September 2016 | 1937 | Finland | Children | SR |
| 55 | Maolin, W., 2019 [71] | Cross-sectional study | March 2014–March 2015 | 923 | China | Children | SR |
| 56 | Yarong, Z., 2015 [72] | Cross-sectional study | November 2011–April 2012 | 1792 | China | Children | SR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wu, Y.; Wang, J.; Wang, Z.; Gao, J.; Yuan, J.; Chen, H. A Meta-Analysis of the Prevalence of Wheat Allergy Worldwide. Nutrients 2023, 15, 1564. https://doi.org/10.3390/nu15071564
Liu W, Wu Y, Wang J, Wang Z, Gao J, Yuan J, Chen H. A Meta-Analysis of the Prevalence of Wheat Allergy Worldwide. Nutrients. 2023; 15(7):1564. https://doi.org/10.3390/nu15071564
Chicago/Turabian StyleLiu, Wenfeng, Yong Wu, Jian Wang, Zhongliang Wang, Jinyan Gao, Juanli Yuan, and Hongbing Chen. 2023. "A Meta-Analysis of the Prevalence of Wheat Allergy Worldwide" Nutrients 15, no. 7: 1564. https://doi.org/10.3390/nu15071564
APA StyleLiu, W., Wu, Y., Wang, J., Wang, Z., Gao, J., Yuan, J., & Chen, H. (2023). A Meta-Analysis of the Prevalence of Wheat Allergy Worldwide. Nutrients, 15(7), 1564. https://doi.org/10.3390/nu15071564