The Role of Genetically Engineered Probiotics for Treatment of Inflammatory Bowel Disease: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Eligibility Criteria
- Population: rodents with colitis and patients with IBD;
- Intervention: supplementation with gm probiotics;
- Comparisons: placebo; wild-type probiotics, etc.;
- Outcomes: weight loss, colon length, disease activity, intestinal damage, anti- and pro-inflammatory cytokines, oxidative stress-related indicators, mucosal barrier function, etc.
- Study design: preclinical studies, randomized controlled trials, cohort studies, etc.
2.3. Exclusion Criteria
- Duplicated studies;
- In vitro studies or studies not related to our research topic;
- Papers published in a language other than English;
- Publication type: reviews, meta-analyses, and consensus papers
- Papers without the data we focused on or without full text.
2.4. Data Extraction and Risk of Bias
3. Results
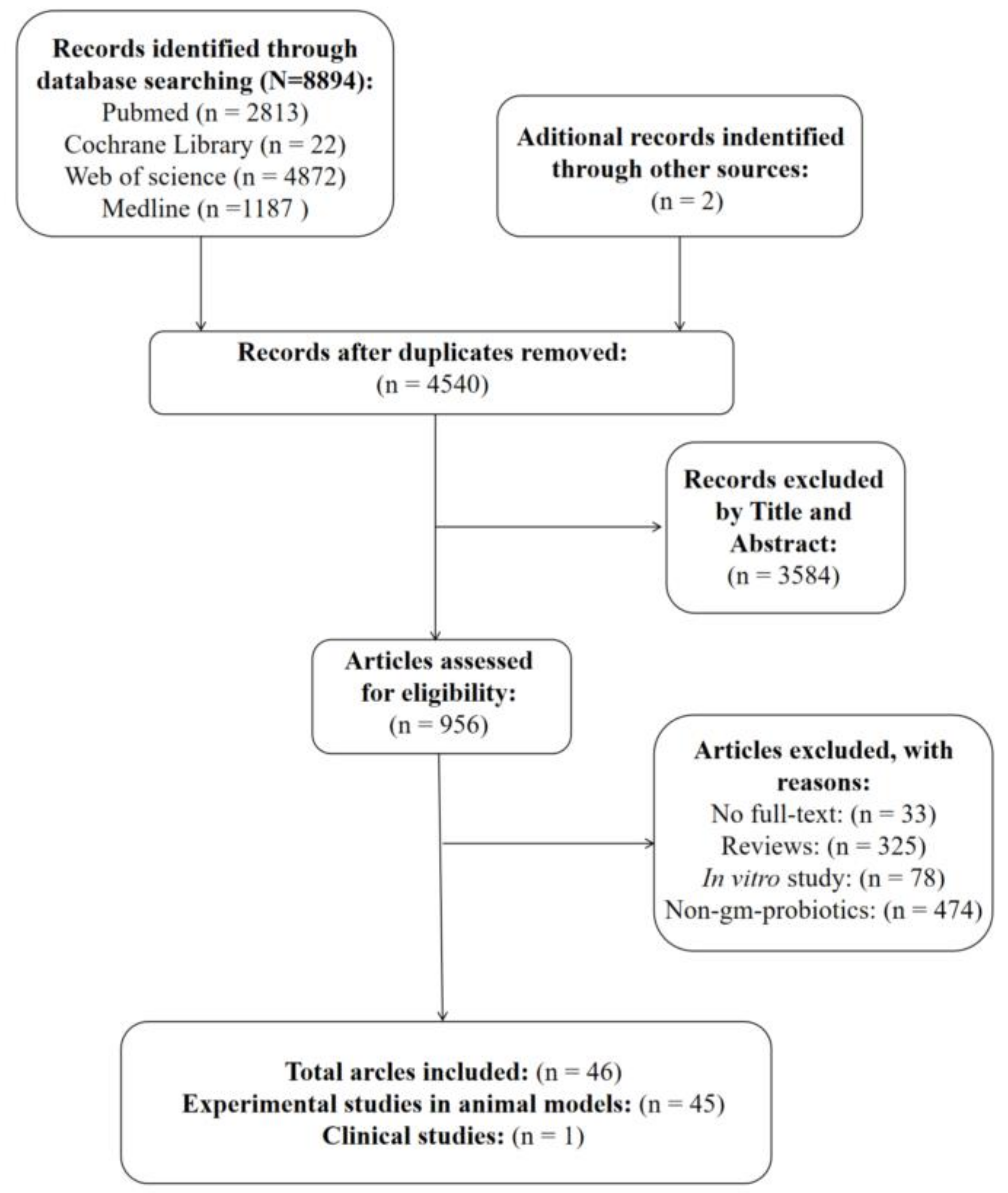
3.1. Study Selection
3.2. Qualitative Data
3.3. The Efficacy of Gm Probiotics Secreting Immunoregulatory Cytokines on Colitis Models and IBD Patients
3.3.1. IL-10
3.3.2. IL-27
3.3.3. IL-35
3.3.4. Growth Factors
3.3.5. Other Immunoregulatory Cytokines
3.3.6. Antibodies or Receptor Antagonist for Pro-Inflammatory Cytokines
3.3.7. Comparisons of Different Gm Probiotics
3.4. The Efficacy of Gm Probiotics Secreting Antioxidant Enzymes on Colitis Models
3.5. The Efficacy of Gm probiotics Secreting Antimicrobial Peptide on Colitis Models
3.6. The Efficacy of Gm Probiotics Promoting Production of Short-Chain Fatty Acids (SCFAs) or Related Organic Acids in GIT
3.7. The Efficacy of Gm Probiotics Secreting Alpha-Melanocyte-Stimulating Hormone (α-MSH) on Colitis Models
3.8. The Efficacy of Gm Probiotics Secreting Other Therapeutic Substances on Colitis Models
3.9. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit |
| DAI | Disease activity index |
| GIT | Gastrointestinal tract |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| DSS | Dextran sulphate sodium |
| TNBS | Trinitrobenzene sulfonic acid |
| DNBS | Dinitro-benzenesulfonic-acid |
| Gm probiotics | Genetically modified probiotics |
| IBD | Inflammatory bowel disease |
| MPO | Myeloperoxidase |
| SCFAs | Short-chain fatty acids |
References
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42, quiz e30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Zenlea, T.; Peppercorn, M.A. Immunosuppressive therapies for inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 3146–3152. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Barra, M.; Danino, T.; Garrido, D. Engineered Probiotics for Detection and Treatment of Inflammatory Intestinal Diseases. Front. Bioeng. Biotechnol. 2020, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in Cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef]
- Gazerani, P. Probiotics for Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4121. [Google Scholar] [CrossRef] [Green Version]
- Samer, A.; Toumi, R.; Soufli, I.; Touil-Boukoffa, C. Cell-free probiotic supernatant (CFS) treatment alleviates indomethacin-induced enterocolopathy in BALB/c mice by down-modulating inflammatory response and oxidative stress: Potential alternative targeted treatment. Inflammopharmacology 2022, 30, 1685–1703. [Google Scholar] [CrossRef]
- Wyszyńska, A.; Kobierecka, P.; Bardowski, J.; Jagusztyn-Krynicka, E.K. Lactic acid bacteria—20 years exploring their potential as live vectors for mucosal vaccination. Appl. Microbiol. Biotechnol. 2015, 99, 2967–2977. [Google Scholar] [CrossRef] [Green Version]
- Shigemori, S.; Shimosato, T. Applications of Genetically Modified Immunobiotics with High Immunoregulatory Capacity for Treatment of Inflammatory Bowel Diseases. Front. Immunol. 2017, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Steidler, L.; Hans, W.; Schotte, L.; Neirynck, S.; Obermeier, F.; Falk, W.; Fiers, W.; Remaut, E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000, 289, 1352–1355. [Google Scholar] [CrossRef] [Green Version]
- Foligné, B.; Nutten, S.; Steidler, L.; Dennin, V.; Goudercourt, D.; Mercenier, A.; Pot, B. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: Technical and microbiological aspects. Dig. Dis. Sci. 2006, 51, 390–400. [Google Scholar] [CrossRef]
- Han, W.; Mercenier, A.; Ait-Belgnaoui, A.; Pavan, S.; Lamine, F.; van Swam, I.I.; Kleerebezem, M.; Salvador-Cartier, C.; Hisbergues, M.; Bueno, L.; et al. Improvement of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. Inflamm. Bowel Dis. 2006, 12, 1044–1052. [Google Scholar] [CrossRef]
- Yoon, S.W.; Lee, C.H.; Kim, J.Y.; Kim, J.Y.; Sung, M.H.; Poo, H. Lactobacillus casei secreting alpha-MSH induces the therapeutic effect on DSS-induced acute colitis in Balb/c Mice. J. Microbiol. Biotechnol. 2008, 18, 1975–1983. [Google Scholar]
- LeBlanc, J.G.; del Carmen, S.; Miyoshi, A.; Azevedo, V.; Sesma, F.; Langella, P.; Bermúdez-Humarán, L.G.; Watterlot, L.; Perdigon, G.; de Moreno de LeBlanc, A. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J. Biotechnol. 2011, 151, 287–293. [Google Scholar] [CrossRef]
- Gardlik, R.; Palffy, R.; Celec, P. Recombinant probiotic therapy in experimental colitis in mice. Folia Biol. 2012, 58, 238–245. [Google Scholar]
- Wong, C.C.; Zhang, L.; Li, Z.J.; Wu, W.K.; Ren, S.X.; Chen, Y.C.; Ng, T.B.; Cho, C.H. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J. Gastroenterol. Hepatol. 2012, 27, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Hamady, Z.Z. Novel xylan-controlled delivery of therapeutic proteins to inflamed colon by the human anaerobic commensal bacterium. Ann. R. Coll. Surg. Engl. 2013, 95, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, H.; Wang, Q.; Hou, C.; Thacker, P.; Qiao, S. Expression of catalase in Lactobacillus fermentum and evaluation of its anti-oxidative properties in a dextran sodium sulfate induced mouse colitis model. World J. Microbiol. Biotechnol. 2013, 29, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen, S.; de Moreno de LeBlanc, A.; Martin, R.; Chain, F.; Langella, P.; Bermúdez-Humarán, L.G.; LeBlanc, J.G. Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities. Appl. Environ. Microbiol. 2014, 80, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Carmen, S.; Martín Rosique, R.; Saraiva, T.; Zurita-Turk, M.; Miyoshi, A.; Azevedo, V.; de Moreno de LeBlanc, A.; Langella, P.; Bermúdez-Humarán, L.G.; LeBlanc, J.G. Protective effects of lactococci strains delivering either IL-10 protein or cDNA in a TNBS-induced chronic colitis model. J. Clin. Gastroenterol. 2014, 48 (Suppl. 1), S12–S17. [Google Scholar] [CrossRef]
- Martín, R.; Chain, F.; Miquel, S.; Natividad, J.M.; Sokol, H.; Verdu, E.F.; Langella, P.; Bermúdez-Humarán, L.G. Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. Hum. Vaccines Immunother. 2014, 10, 1611–1621. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-Humarán, L.G.; Motta, J.P.; Aubry, C.; Kharrat, P.; Rous-Martin, L.; Sallenave, J.M.; Deraison, C.; Vergnolle, N.; Langella, P. Serine protease inhibitors protect better than IL-10 and TGF-β anti-inflammatory cytokines against mouse colitis when delivered by recombinant lactococci. Microb. Cell Factories 2015, 14, 26. [Google Scholar] [CrossRef] [Green Version]
- Del Carmen, S.; Miyoshi, A.; Azevedo, V.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Evaluation of a Streptococcus thermophilus strain with innate anti-inflammatory properties as a vehicle for IL-10 cDNA delivery in an acute colitis model. Cytokine 2015, 73, 177–183. [Google Scholar] [CrossRef]
- Shigemori, S.; Watanabe, T.; Kudoh, K.; Ihara, M.; Nigar, S.; Yamamoto, Y.; Suda, Y.; Sato, T.; Kitazawa, H.; Shimosato, T. Oral delivery of Lactococcus lactis that secretes bioactive heme oxygenase-1 alleviates development of acute colitis in mice. Microb. Cell Factories 2015, 14, 189. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Guo, Q.; Li, S.; Liu, M.; Zhang, Q.; Xu, Z.; Sun, H. Anti-inflammatory properties of Bifidobacterium longum expressing human manganese superoxide dismutase using the TNBS-induced rats model of colitis. J. Microbiol. Biotechnol. 2017. ahead of print. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Lan, X.; Xu, X.; Zhang, X.; Li, X.; Zhao, Y.; Li, G.; Du, C.; Lu, S.; et al. Oral Escherichia coli expressing IL-35 meliorates experimental colitis in mice. J. Transl. Med. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Chiabai, M.J.; Almeida, J.F.; de Azevedo, M.G.D.; Fernandes, S.S.; Pereira, V.B.; de Castro, R.J.A.; Jerônimo, M.S.; Sousa, I.G.; de Souza Vianna, L.M.; Miyoshi, A.; et al. Mucosal delivery of Lactococcus lactis carrying an anti-TNF scFv expression vector ameliorates experimental colitis in mice. BMC Biotechnol. 2019, 19, 38. [Google Scholar] [CrossRef] [Green Version]
- Praveschotinunt, P.; Duraj-Thatte, A.M.; Gelfat, I.; Bahl, F.; Chou, D.B.; Joshi, N.S. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat. Commun. 2019, 10, 5580. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tian, M.; Li, W.; Hao, F. Preventative delivery of IL-35 by Lactococcus lactis ameliorates DSS-induced colitis in mice. Appl. Microbiol. Biotechnol. 2019, 103, 7931–7941. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, J.H.; Chang, Y.C.; Mou, K.Y. Treatment of murine colitis by Saccharomyces boulardii secreting atrial natriuretic peptide. J. Mol. Med. 2020, 98, 1675–1687. [Google Scholar] [CrossRef]
- Namai, F.; Shigemori, S.; Ogita, T.; Sato, T.; Shimosato, T. Microbial therapeutics for acute colitis based on genetically modified Lactococcus lactis hypersecreting IL-1Ra in mice. Exp. Mol. Med. 2020, 52, 1627–1636. [Google Scholar] [CrossRef]
- Zeng, L.; Tan, J.; Xue, M.; Liu, L.; Wang, M.; Liang, L.; Deng, J.; Chen, W.; Chen, Y. An engineering probiotic producing defensin-5 ameliorating dextran sodium sulfate-induced mice colitis via Inhibiting NF-kB pathway. J. Transl. Med. 2020, 18, 107. [Google Scholar] [CrossRef] [Green Version]
- Esposito, G.; Pesce, M.; Seguella, L.; Lu, J.; Corpetti, C.; Del Re, A.; De Palma, F.D.E.; Esposito, G.; Sanseverino, W.; Sarnelli, G. Engineered Lactobacillus paracasei Producing Palmitoylethanolamide (PEA) Prevents Colitis in Mice. Int. J. Mol. Sci. 2021, 22, 2945. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Pan, X.; Zhang, M.; Lv, Z.; Pan, L.L.; Sun, J. Recombinant CRAMP-producing Lactococcus lactis attenuates dextran sulfate sodium-induced colitis by colonic colonization and inhibiting p38/NF-κB signaling. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Park, Y.T.; Kim, T.; Ham, J.; Choi, J.; Lee, H.S.; Yeon, Y.J.; Choi, S.I.; Kim, N.; Kim, Y.R.; Seok, Y.J. Physiological activity of E. coli engineered to produce butyric acid. Microb. Biotechnol. 2022, 15, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.M.; Gutiérrez-Vázquez, C.; Sanmarco, L.M.; da Silva Pereira, J.A.; Li, Z.; Plasencia, A.; Hewson, P.; Cox, L.M.; O’Brien, M.; Chen, S.K.; et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Med. 2021, 27, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liao, Y.; Yang, R.; Zhu, Z.; Zhang, L.; Wu, Z.; Sun, X. An engineered probiotic secreting Sj16 ameliorates colitis via Ruminococcaceae/butyrate/retinoic acid axis. Bioeng. Transl. Med. 2021, 6, e10219. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, X.Y.; Zhang, D.; Zhang, Y.D.; Li, Z.H.; Liu, X.; Wu, F.; Chen, G.Q. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cell. Mol. Immunol. 2021, 18, 2344–2357. [Google Scholar] [CrossRef]
- Vandenbroucke, K.; de Haard, H.; Beirnaert, E.; Dreier, T.; Lauwereys, M.; Huyck, L.; Van Huysse, J.; Demetter, P.; Steidler, L.; Remaut, E.; et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010, 3, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Watterlot, L.; Rochat, T.; Sokol, H.; Cherbuy, C.; Bouloufa, I.; Lefèvre, F.; Gratadoux, J.J.; Honvo-Hueto, E.; Chilmonczyk, S.; Blugeon, S.; et al. Intragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in mice. Int. J. Food Microbiol. 2010, 144, 35–41. [Google Scholar] [CrossRef]
- Yao, J.; Wang, J.Y.; Lai, M.G.; Li, Y.X.; Zhu, H.M.; Shi, R.Y.; Mo, J.; Xun, A.Y.; Jia, C.H.; Feng, J.L.; et al. Treatment of mice with dextran sulfate sodium-induced colitis with human interleukin 10 secreted by transformed Bifidobacterium longum. Mol. Pharm. 2011, 8, 488–497. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Chen, J.; Chen, J.J.; Rong, L.; Ding, W.Q.; Yang, H.J.; Zhong, L. Effect of recombinant Lactobacillus casei expressing interleukin-10 in dextran sulfate sodium-induced colitis mice. J. Dig. Dis. 2013, 14, 76–83. [Google Scholar] [CrossRef]
- Hanson, M.L.; Hixon, J.A.; Li, W.; Felber, B.K.; Anver, M.R.; Stewart, C.A.; Janelsins, B.M.; Datta, S.K.; Shen, W.; McLean, M.H.; et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology 2014, 146, 210–221.e213. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.L.; Zhang, J.; Liu, X.T.; Liu, H.; Zeng, X.F.; Qiao, S.Y. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-κB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J. Appl. Microbiol. 2014, 116, 1621–1631. [Google Scholar] [CrossRef]
- Whelan, R.A.; Rausch, S.; Ebner, F.; Günzel, D.; Richter, J.F.; Hering, N.A.; Schulzke, J.D.; Kühl, A.A.; Keles, A.; Janczyk, P.; et al. A transgenic probiotic secreting a parasite immunomodulator for site-directed treatment of gut inflammation. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 1730–1740. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, Y.; Deng, B.; Xu, Z. Recombinant Lactococcus lactis expressing porcine insulin-like growth factor I ameliorates DSS-induced colitis in mice. BMC Biotechnol. 2016, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Wei, P.; Yang, Y.; Ding, Q.; Li, X.; Sun, H.; Liu, Z.; Huang, J.; Gong, Y. Oral delivery of Bifidobacterium longum expressing α-melanocyte-stimulating hormone to combat ulcerative colitis. J. Med. Microbiol. 2016, 65, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Wei, P.; Yang, Y.; Liu, Z.; Huang, J.; Gong, Y.; Sun, H. Oral Bifidobacterium longum expressing alpha-melanocyte-stimulating hormone to fight experimental colitis. Drug Deliv. 2016, 23, 2058–2064. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Li, S.; Zhang, Q.; Xu, Z.; Wang, J.; Sun, H. Oral engineered Bifidobacterium longum expressing rhMnSOD to suppress experimental colitis. Int. Immunopharmacol. 2018, 57, 25–32. [Google Scholar] [CrossRef]
- Breyner, N.M.; Vilas Boas, P.B.; Fernandes, G.; de Carvalho, R.D.; Rochat, T.; Michel, M.L.; Chain, F.; Sokol, H.; de Azevedo, M.; Myioshi, A.; et al. Oral delivery of pancreatitis-associated protein by Lactococcus lactis displays protective effects in dinitro-benzenesulfonic-acid-induced colitis model and is able to modulate the composition of the microbiota. Environ. Microbiol. 2019, 21, 4020–4031. [Google Scholar] [CrossRef] [Green Version]
- Zurita-Turk, M.; Mendes Souza, B.; Prósperi de Castro, C.; Bastos Pereira, V.; Pecini da Cunha, V.; Melo Preisser, T.; Caetano de Faria, A.M.; Carmona Cara Machado, D.; Miyoshi, A. Attenuation of intestinal inflammation in IL-10 deficient mice by a plasmid carrying Lactococcus lactis strain. BMC Biotechnol. 2020, 20, 38. [Google Scholar] [CrossRef]
- Foligne, B.; Dessein, R.; Marceau, M.; Poiret, S.; Chamaillard, M.; Pot, B.; Simonet, M.; Daniel, C. Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology 2007, 133, 862–874. [Google Scholar] [CrossRef]
- Aubry, C.; Michon, C.; Chain, F.; Chvatchenko, Y.; Goffin, L.; Zimmerli, S.C.; Leguin, S.; Langella, P.; Bermudez-Humaran, L.; Chatel, J.M. Protective effect of TSLP delivered at the gut mucosa level by recombinant lactic acid bacteria in DSS-induced colitis mouse model. Microb. Cell Factories 2015, 14, 176. [Google Scholar] [CrossRef] [Green Version]
- Braat, H.; Rottiers, P.; Hommes, D.W.; Huyghebaert, N.; Remaut, E.; Remon, J.P.; van Deventer, S.J.; Neirynck, S.; Peppelenbosch, M.P.; Steidler, L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2006, 4, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Sasaoka, T.; Ito, M.; Yamashita, J.; Nakajima, K.; Tanaka, I.; Narita, M.; Hara, Y.; Hada, K.; Takahashi, M.; Ohno, Y.; et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am. J. Physiology. Gastrointest. Liver Physiol. 2011, 300, G568–G576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, S.S.; Thulesen, J.; Christensen, L.; Nexo, E.; Thim, L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. Gut 1999, 45, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J. Exp. Med. 2012, 209, 1595–1609. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Cheng, J.; Xi, S.; Qi, X.; Shen, S.; Ge, Y. Alginate/chitosan microcapsules for in-situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. e.V 2019, 137, 112–121. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Wang, G.; Ge, S.; Liu, H. Dendrobium Officinale Polysaccharides Protect against MNNG-Induced PLGC in Rats via Activating the NRF2 and Antioxidant Enzymes HO-1 and NQO-1. Oxidative Med. Cell. Longev. 2019, 2019, 9310245. [Google Scholar] [CrossRef] [Green Version]
- Wehkamp, J.; Schmid, M.; Stange, E.F. Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2007, 23, 370–378. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Rajora, N.; Boccoli, G.; Catania, A.; Lipton, J.M. alpha-MSH modulates experimental inflammatory bowel disease. Peptides 1997, 18, 381–385. [Google Scholar] [CrossRef]
- Pesce, M.; D’Alessandro, A.; Borrelli, O.; Gigli, S.; Seguella, L.; Cuomo, R.; Esposito, G.; Sarnelli, G. Endocannabinoid-related compounds in gastrointestinal diseases. J. Cell. Mol. Med. 2018, 22, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Romano, B.; Petrosino, S.; Pagano, E.; Capasso, R.; Coppola, D.; Battista, G.; Orlando, P.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br. J. Pharmacol. 2015, 172, 142–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, B.P.; Quigley, E.M.M. Probiotics in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gao, M.; Cheng, X.; Kang, G.; Cao, X.; Huang, H. Engineered butyrate-producing bacteria prevents high fat diet-induced obesity in mice. Microb. Cell Factories 2020, 19, 94. [Google Scholar] [CrossRef]
- Song, A.A.; In, L.L.A.; Lim, S.H.E.; Rahim, R.A. A review on Lactococcus lactis: From food to factory. Microb. Cell Factories 2017, 16, 55. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, A. Animal models of inflammatory bowel disease. Prog. Mol. Biol. Transl. Sci. 2012, 105, 263–320. [Google Scholar] [CrossRef]

| Author | Year | Country | Lineage | Sex * | Age (Week) | Number of Groups | Number of Animals | Model | Acute/Chronic Course |
|---|---|---|---|---|---|---|---|---|---|
| Del Carmen et al. [29] | 2015 | Argentina | BALB/C mice | Female | 5 | 4 | 32 | TNBS-induced colitis | Acute |
| Gardlik et al. [21] | 2012 | Slovak Republic | C57BL/6 mice | Male | 10 | 6 | 60 | DSS-induced colitis | Acute |
| Foligné et al. [17] | 2006 | France | BALB/C mice | Female | 7–8 | 4 | 32–48 | TNBS-induced colitis | Acute |
| Del Carmen et al. [26] | 2014 | Argentina | BALB/C mice | Female | 5 | 5 | 90 | TNBS induced colitis | Chronic |
| Martín et al. [27] | 2014 | France | C57BL/6 mice | Male | 6–8 | 4 | 64 | DNBs induced colitis | Chronic |
| Steidler et al. [16] | 2000 | Belgium | BALB/C mice | Female | N.A. | 13 | 130 | DSS induced colitis | Chronic |
| 129 SvIEv IL-10−/− mice | Female | 3–7 | 3 | 15 | IL-10−/− mice | Chronic | |||
| Hamady et al. [23] | 2013 | Britain | C57BL/6 mice | Male | 8 | 7 | 56 | DSS induced colitis | Acute |
| Bermúdez-Humarán et al. [28] | 2015 | France | C57BL/6 mice | N.A. | 6–8 | 10 | 60–80 | DSS induced colitis | Acute |
| Liu et al. [36] | 2020 | Taiwan | C57BL/6JNarl mice | Male | 7–8 | 7 | 38 | DSS induced colitis | Acute |
| Chiabai et al. [33] | 2019 | Brazil | C57BL/6 mice | Female | 10 | 4 | 16–20 | DSS induced colitis | Acute |
| Namai et al. [37] | 2020 | Japan | C57BL/6 mice | Female | 7 | 2 | 36 | DSS induced colitis | Acute |
| Zhang et al. [32] | 2018 | China | BALB/C mice | Male | 6–8 | 4 | 40 | DSS induced colitis | Acute |
| Wang et al. [35] | 2019 | China | C57BL/6 mice | Female | 6–8 | 6 | 30 | DSS induced colitis | Acute |
| Zhang et al. [24] | 2013 | China | BALB/C mice | Female | 7 | 5 | 40 | DSS induced colitis | Acute |
| Xie et al. [31] | 2017 | China | Wistar rats | Male | 9–10 | 4 | 48 | TNBS induced colitis | Acute |
| LeBlanc et al. [20] | 2011 | Argentina | BALB/C mice | Female | 5 | 5 | 90 | TNBS induced colitis | Acute |
| Del Carmen et al. [25] | 2014 | Argentina | BALB/C mice | Female | 5 | 6 | 36 | TNBS induced colitis | Chronic |
| Han et al. [18] | 2006 | France | Wistar rats | Male | N.A. | 15 | 110 | TNBS induced colitis | Acute |
| Wong et al. [22] | 2012 | China | BALB/C mice | Male | 6–8 | 10 | 94 | DSS induced colitis | Acute |
| Li et al. [40] | 2021 | China | C57BL/6 mice | Male | 7–8 | 6 | 30 | DSS induced colitis | Acute |
| Zeng et al. [38] | 2020 | China | C57BL/6 mice | Male | 6–8 | 4 | 28 | DSS induced colitis | Acute |
| Esposito et al. [39] | 2021 | Italy | C57BL/6J mice | Male | 6 | 7 | 70 | DSS induced colitis | Acute |
| Park et al. [41] | 2021 | Korea | C57BL/6J mice | Male | 8 | 6 | 60 | DSS induced colitis | Acute |
| Yan et al. [45] | 2021 | China | C57BL/6J mice | Male | 7 | 5 | 25 | DSS induced colitis | Acute |
| Yoon et al. [19] | 2008 | Korea | BALB/C mice | Female | 6 | 4 | 20 | DSS induced colitis | Acute |
| Shigemori et al. [30] | 2015 | Japan | C57BL/6 mice | Female | 7 | 4 | 39 | DSS induced colitis | Acute |
| Praveschotinunt et al. [34] | 2019 | USA | C57BL/6NCrl mice | Female | 8–9 | 8 | 38–49 | DSS induced colitis | Acute |
| Sun et al. [43] | 2021 | China | C57BL/6 mice | Male | 6–8 | 4 | 26 | DSS induced colitis | Acute |
| Scott at al [42] | 2021 | Canada | C57BL/6J mice | Male | 8–10 | 4 | 36 | TNBS induced colitis | Acute |
| Female | 8–10 | 4 | 41 | DSS induced colitis | Chronic | ||||
| Female | 8–10 | 5 | 29 | Anti-CD3 antibody-induced enteritis | Acute | ||||
| Wang et al. [44] | 2021 | China | BALB/C mice | Male | 6 | 9 | 37–53 | DSS-induced colitis | Acute |
| Wei et al. [54] | 2016 | China | SD rats | Male and Female | N.A. | 4 | 48 | DSS-induced colitis | Acute |
| Wei et al. [55] | 2016 | China | BALB/c mice | Male | 6–12 | 4 | 40 | DSS-induced colitis | Acute |
| Vandenbroucke et al. [46] | 2010 | Belgium | BALB/c mice | Female | 11 | 5 | 50 | DSS-induced colitis | Chronic |
| IL10 knockout mice | N.A. | 20 | 10 | 87 | IL10 knockout mice | Chronic | |||
| Liu et al. [53] | 2016 | China | BALB/c mice | Female | 8 | 5 | 40 | DSS-induced colitis | Acute |
| Zurita-Turk et al. [58] | 2020 | Brazil | IL-10−/− mice and wild-type mice | N.A. | 2 | 4 | About 36 | IL-10−/− | Chronic |
| Qiu et al. [49] | 2013 | China | BALB/c mice | Female | 4–6 | 8 | 64 | DSS-induced colitis | Acute |
| Yao et al. [48] | 2011 | China | BALB/c mice | Male | 6 | 5 | 50 | DSS-induced colitis | Acute |
| Hanson et al. [50] | 2014 | USA | C57BL/6 and Rag1−/− | Male | 7.5 | N.A. | N.A. | Transfer of CD4 + CD45RBhi T cells-induced colitis | Chronic |
| Whelan et al. [52] | 2014 | Germany | C57BL/6 mice | Male | 9–11 | 4 | 45 | DSS induced colitis | Acute |
| Breyner et al. [57] | 2019 | France | C57BL/6 mice | N.A. | 6–8 | 4 | N.A. | DNBS-induced colitis | Acute |
| 4 | N.A. | DSS-induced colitis | Acute | ||||||
| Liu et al. [56] | 2018 | China | SD rats | Male | N.A. | 4 | 48 | DSS-induced colitis | Acute |
| Hou et al. [51] | 2014 | China | BALB/c mice | Female | 6 | 4 | 60 | TNBS-induced colitis | Acute |
| Watterlot et al. [47] | 2010 | France | BALB/c mice | Male | 7 | 5 | 50 | DSS-induced colitis | Acute |
| Aubry et al. [60] | 2015 | France | C57BL/6 mice | N.A. | 6 | 6 | DSS-induced colitis | Acute | |
| Foligne et al. [59] | 2007 | France | BALB/c and C57BL/6 | Female | 7–9 | 4 | 40 | TNBS-induced colitis | Acute |
| 4 | 40 | IL-10−/− and TNBS-induced colitis | Acute | ||||||
| 8 | 80 | DSS-induced colitis | Acute |
| Author | Year | Wild-Type Probiotic Strain | Constructed Plasmid with Function | Recombinant Probiotic Name | Secretions | Administration | Dose/Day | Length |
|---|---|---|---|---|---|---|---|---|
| del Carmen et al. [29] | 2015 | S. thermophilus CRL807 | pValac::il-10 | S. thermophilus CRL 807pValac::il-10 | IL-10 | Gastric gavage | 108 CFU | 12 days |
| Gardlik et al. [21] | 2012 | E. coli Nissle 1917 | pMEC-IL10 | Nissle 1917/pMEC-IL10 | IL-10 | Gastric gavage | 109 bacteria | 7 days |
| L. lactis | pMEC-IL10 | Lactococcus lactis/pMEC-IL10 | IL-10 | Gastric gavage | 109 bacteria | 7 days | ||
| Foligné et al. [17] | 2006 | L. lactis MG1363 | N.A. | LL-mIL-10 | mIL-10 | Gastric gavage | 105 to 109 CFU | 14 days |
| del Carmen et al. [26] | 2014(a) | L. lactis MG1363 | pValac:il-10 | LL-pValac:IL-10 | mIL-10 | Gastric gavage | 109 CFU | 14 days |
| L. lactis MG1363 | pGroeESL:il-10 | LL-pGroESL:IL-10 | mIL-10 | Gastric gavage | 109 CFU | 14 days | ||
| Martín et al. [27] | 2014 | L. lactis MG1363 | pLB350 | LL-IL10 | mIL-10 | Gastric gavage | 109 CFU | 10 days |
| Steidle et al. [16] | 2000 | L. lactis | N.A. | LL-mIL-10 | mIL-10 | Gastric gavage | 2 × 107 CFU or 2 × 109 CFU | 2 weeks or 4 weeks |
| Hamady et al. [23] | 2013 | B. ovatus | N.A. | BO-KGF | KGF-2 | Gastric gavage | 2 × 108 CFU | 5 days |
| B. ovatus | N.A. | BO-TGF | TGF-β1 | Gastric gavage | 2 × 108 CFU | 5 days | ||
| Bermúdez-Humarán et al. [28] | 2015 | L. lactis MG1363 | pSEC:mIL-10 | LL-IL-10 | mIL-10 | Gastric gavage | 5 × 109 CFU | 7 days |
| L. lactis MG1363 | pSEC:mTGF-β | LL-TGF-β | TGF-β | Gastric gavage | 5 × 109 CFU | 7 days | ||
| L. lactis MG1363 | pSEC:elafin | L. lactis Elafin | Elafin | Gastric gavage | 5 × 109 CFU | 7 days | ||
| L. lactis MG1363 | pSEC: mSLPI | L. lactis SLPI | SLPI | Gastric gavage | 5 × 109 CFU | 7 days | ||
| Liu et al. [36] | 2020 | S. boulardii | N.A. | N.A. | IL-10 | Gastric gavage | 109 CFU | 5 days |
| S. boulardii | N.A. | N.A. | TNFR1-ECD | Gastric gavage | 109 CFU | 5 days | ||
| S. boulardii | N.A. | N.A. | AP | Gastric gavage | 109 CFU | 5 days | ||
| S. boulardii | N.A. | N.A. | ANP | Gastric gavage | 109 CFU | 5 days | ||
| S. boulardii | N.A. | N.A. | ANPm | Gastric gavage | 109 CFU | 5 days | ||
| Chiabai et al. [33] | 2019 | L. lactis MG1363 FnBPA + (LL-F) | pValac::anti-TNFα | LL-FT | scFv of anti-TNFα antibody | Gastric gavage | 2.0–2.5 × 109 CFU | 4 days |
| Namai et al. [37] | 2020 | L. lactis NZ9000 | pNZ8148#2:SEC-IL1Ra | NZ-IL1Ra | mIL-1Ra | Gastric gavage | 1010 CFU | 12 days |
| Zhang et al. [32] | 2018 | E. coli BL21(DE3) | pET-28a(+)-IL35 | E. coli/IL-35 | IL-35 | Gastric gavage | 1010 CFU | 5 days |
| Wang et al. [35] | 2019 | L. lactis NZ9000 | pNZ8148+IL-35 | NZ9000/IL-35 | mIL-35 | Gastric gavage | 109 CFU | 14 days (3 times weekly) |
| Zhang et al. [24] | 2013 | L. fermentum I5007 | pLK126 | L. fermentum P126 | CAT | Gastric gavage | 109 CFU | 7 days |
| Xie et al. [31] | 2017 | B. longum HB15 | pBsSOD | B. longum-rhMnSOD | MnSOD | Gastric gavage | 2 × 109 CFU | 7 days |
| LeBlanc et al. [20] | 2011 | L. casei BL23 | pLEM415-mnkat | Lb. casei BL23 pLEM415-mnkat | CAT | Gastric gavage | 109 CFU | 24 days |
| L. casei BL23 | pLEM415-sodA | Lb. casei BL23 pLEM415-sodA | SOD | Gastric gavage | 109 CFU | 24 days | ||
| del Carmen et al. [25] | 2014(b) | S. thermophilus CRL807 | pIL253-sodA | S. thermophilus CRL 807:SOD | SOD | Gastric gavage | 109 CFU or 3 × 1010 CFU | 14 days |
| S. thermophilus CRL807 | pIL253-mnkat | S. thermophilus CRL 807:CAT | CAT | Gastric gavage | 109 CFU or 3 × 1010 CFU | 14 days | ||
| Han et al. [18] | 2006 | L. lactis NZ9800 | pNZ8048sodA | L. lactis SOD+ | SOD | Gastric gavage | 109 CFU | 8 days |
| L. plantarum NCIMB8826 | pNZ8048sodA | L. plantarum SOD+ | SOD | Gastric gavage | 109 CFU | 8 days | ||
| Wong et al. [22] | 2012 | L. lactis NZ3900 (N0) | N.A. | N4 | mCRAMP | Gastric gavage | 108 or 1010 CFU | 7 days |
| Li et al. [40] | 2021 | L. lactis NZ9000 | pMG36e-Usp45-CRAMP | L.L-pMU45CR | CRAMP | Gastric gavage | 1010 CFU | 4 days |
| L. lactis NZ9000 | pNZ8148-Usp45-CRAMP | L.L- pNU45CR | CRAMP | Gastric gavage | 1010 CFU | 4 days | ||
| Zeng et al. [38] | 2020 | L. lactis NZ9000 | pN8148-SHD-5 | NZ9000SHD-5 | HD-5 | Gastric gavage | N.A. | 7 days |
| Esposito et al. [39] | 2021(a) | L. paracasei F19 | pTRKH3-slp-NAPE-PLD | pNAPE-LP | NAPE-PLD | Gastric gavage | 0.8–1.2 × 108 CFU | 5 days |
| Park et al. [41] | 2021 | E. coli MG1655 | pACYC184-BCD-BUT | MG1655-BCD-BUT | BCD and BUT | Gastric gavage | 0.2 × 109 CFU | 9 days |
| E. coli Nissle 1917 | pACYC184-BCD-BUT | EcN-BCD-BUT | BCD and BUT | Gastric gavage | 0.2 × 109 CFU | 9 days | ||
| Yan et al. [45] | 2021 | E. coli Nissle 1917 | pYX50 | EcNL4 (EcNΔldhA) | 3HB | Gastric gavage | 5 × 1010 cells | 7 days |
| Yoon et al. [19] | 2008 | L. casei BLS | pLUAT-ssMSH | L. casei-alpha-MSH | alpha-MSH | Gastric gavage | 1010 CFU | 7 days |
| Shigemori et al. [30] | 2015 | L. lactis NZ9000 | pNZ8148#2:SEC-mHO-1 | NZ-HO | rmHO-1 | Gastric gavage | 5 × 109 CFU | 7 days |
| Praveschotinunt et al. [34] | 2019 | E. coli Nissle 1917 | pBbB8k-CsgA-TFF3 | PBP8 CsgA-TFF3 | Trefoil factors 3 | Rectal administration | 108 CFU | 14 days |
| Sun et al. [43] | 2021 | S. cerevisiae BY4741 | N.A. | S. cerevisiae 39# | Lactic acid | Gastric gavage | 2 × 108 CFU | 7 days |
| Scott at al [42] | 2021 | S. cerevisiae (CB008) | TM-3 Strain mfa2::HIS3-pFUS1 RROP1 | APTM-3 | Human P2Y2 purinergic receptor | Gastric gavage | 2 × 108 CFU | 11 days |
| S. cerevisiae (CB008) | TM-3 Strain mfa2::HIS3-pFUS1 RROP1 | APTM-3 | Human P2Y2 purinergic receptor | Gastric gavage | 2 × 108 CFU | 21 days | ||
| S. cerevisiae (CB008) | TM-3 Strain mfa2::HIS3-pFUS1 RROP1 | APTM-3 | Human P2Y2 purinergic receptor | Gastric gavage | 2 × 108 CFU | N.A. | ||
| Wang et al. [44] | 2021 | E. coli Nissle 1917 | pGEX-4T-1-Sj16-AsBD and pGEX-4T-1-Sj16-GFP-AsBD | EcN-Sj16 | Sj16 | Gastric gavage | 1 × 109 CFU | 3 days (Days 0, 4, and 8) |
| Wei et al. [54] | 2016 | B. longum HB15 | pDGMSH | B. longum-a-MSH | alpha-MSH | Gastric gavage | 2 × 1010 CFU | 7 days |
| Wei et al. [55] | 2016 | B. longum HB15 | pBDMSH | B. longum-a-MSH | alpha-MSH | Gastric gavage | 1 × 1010 CFU | 9 days |
| Vandenbroucke et al. [46] | 2010 | L. lactis MG1363 | N.A. | LL–MT1–MT1 | MT1–MT1 Nanobody | Gastric gavage | 2 × 109 CFU | 14 or 21 days |
| L. lactis MG1363 | N.A. | LL–MT1 | MT1 Nanobody | Gastric gavage | 3 × 109 CFU | 21 days | ||
| L. lactis MG1363 | pT1mIL10 | LL–Mil10 | IL-10 | Gastric gavage | 4 × 109 CFU | 14 or 21 days | ||
| Liu et al. [53] | 2016 | L. lactis NZ9000 | pNZ8148-pIGF-I3 | L. lactis NZ9000 (pNZ8148-pIGF-I3) | IGF-I | Gastric gavage | 4 × 1012 CFU | 10 days |
| Zurita-Turk et al. [58] | 2020 | L. lactis MG1363 | pValac:il-10 | L. lactis MG1363 FnBPA+ (pValac:il-10) | IL-10 | Gastric gavage | 2 × 109 CFU | 6 weeks |
| Qiu et al. [49] | 2013 | L. casei CECT 5276 | pIlac-sp-IL10 | N.A. | IL-10 | Gastric gavage | 0.6 × 107 or 0.6 × 108 or 0.6 × 109 CFU | 10 days |
| Yao et al. [48] | 2011 | B. longum NCC 2705 | pBBADs-hIL-10 | BL-hIL-10 | IL-10 | Gastric gavage | 1.2 × 108 CFU | 7 days |
| Hanson et al. [50] | 2014 | L. lactis | N.A. | LL-IL-27 | IL-27 | Gastric gavage | 2 × 108 CFU | 14 days |
| Whelan et al. [52] | 2014 | E. coli Nissle 1917 | pMU13 -AvCys | EcN-AvCys | Nematode cystatin | Gastric gavage | 2 × 109 CFU | 4 days |
| Breyner et al. [57] | 2019 | L. lactis NZ9000 | pSEC:PAP | LL-PAP | PAP | Gastric gavage | 5 × 109 CFU | 9 or 17 days |
| Liu et al. [56] | 2018 | B. longum HB25 | pBDMnSOD | B. longum-PEP-1-rhMn-SOD | rhMn-SOD | Gastric gavage | 2 × 109 CFU | 7 days |
| Hou et al. [51] | 2014 | L. fermentum I5007 | pMF009 | L. fermentum (pMF009) | SOD | Gastric gavage | 5 × 109 CFU | 6 days |
| Watterlot et al. [47] | 2010 | L. casei | pILKSsodA | Lb. casei pILKSsodA | SOD | Gastric gavage | 5 × 109 CFU | 9 days |
| L. casei | pVE3874 | Lb. casei BL23 pVE3874 | CAT | Gastric gavage | 5 × 109 CFU | 9 days | ||
| Aubry et al. [60] | 2015 | L. lactis MG1363 | pGroESL-TSLP | LL-TSLP | TSLP | Gastric gavage | 1–5 × 109 CFU | 4 or 12 or 17 days |
| Foligne et al. [59] | 2007 | L. lactis MG1363 | pMEC237 | LL-LcrV | Immunomodulatory Yersinia LcrV Protein | Gastric gavage | 2 × 108 CFU | 5 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zhang, J.; Duan, L. The Role of Genetically Engineered Probiotics for Treatment of Inflammatory Bowel Disease: A Systematic Review. Nutrients 2023, 15, 1566. https://doi.org/10.3390/nu15071566
Zhang T, Zhang J, Duan L. The Role of Genetically Engineered Probiotics for Treatment of Inflammatory Bowel Disease: A Systematic Review. Nutrients. 2023; 15(7):1566. https://doi.org/10.3390/nu15071566
Chicago/Turabian StyleZhang, Tao, Jindong Zhang, and Liping Duan. 2023. "The Role of Genetically Engineered Probiotics for Treatment of Inflammatory Bowel Disease: A Systematic Review" Nutrients 15, no. 7: 1566. https://doi.org/10.3390/nu15071566
APA StyleZhang, T., Zhang, J., & Duan, L. (2023). The Role of Genetically Engineered Probiotics for Treatment of Inflammatory Bowel Disease: A Systematic Review. Nutrients, 15(7), 1566. https://doi.org/10.3390/nu15071566





