Korean Red Ginseng Potentially Improves Maintaining Antibodies after COVID-19 Vaccination: A 24-Week Longitudinal Study
Abstract
:1. Introduction
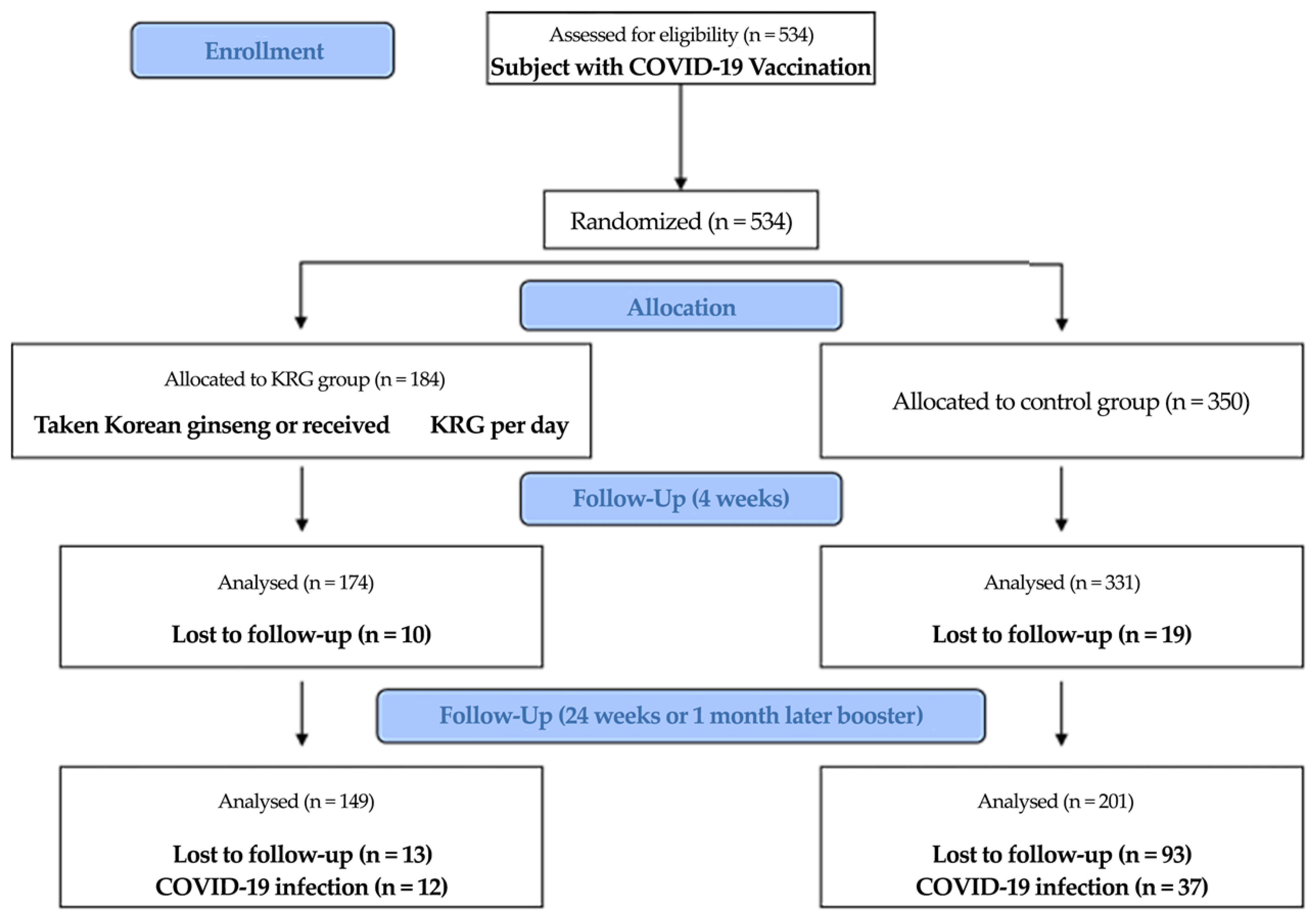
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Outcomes
2.3. Measurement of Anthropometric and Biochemical Parameters
2.4. Detection of Virus-Specific Antibodies
2.5. Statistical Analysis
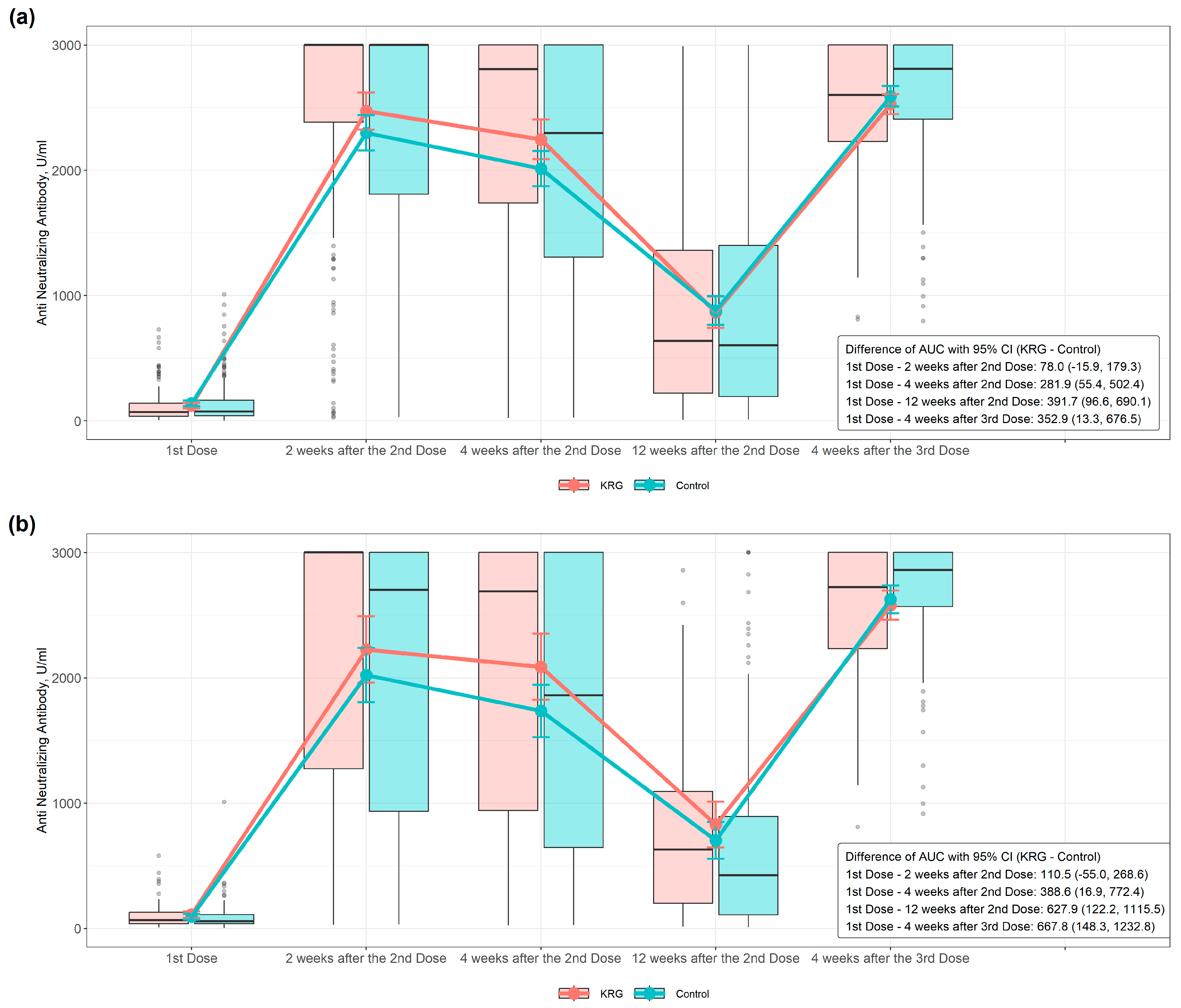
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, D.; Chang, X.; He, Y.; Tan, K.J.K. The determinants of COVID-19 morbidity and mortality across countries. Sci. Rep. 2022, 12, 5888. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-P.; Yang, M.; Lai, C.-L. COVID-19 vaccines: A review of the safety and efficacy of current clinical trials. Pharmaceuticals 2021, 4, 406. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and heterologous COVID-19 booster vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Zhang, D. Effectiveness of COVID-19 vaccine booster shot compared with non-booster: A meta-analysis. Vaccines 2022, 10, 1396. [Google Scholar] [CrossRef]
- Lassaunière, R.; Polacek, C.; Frische, A.; Boding, L.; Sækmose, S.G.; Rasmussen, M.; Fomsgaard, A. Neutralizing antibodies against the SARS-CoV-2 Omicron variant (BA.1) 1 to 18 weeks after the second and third doses of the BNT162b2 mRNA vaccine. JAMA Netw. Open 2022, 5, e2212073. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Alturaiki, W.; Mubarak, A.; Al Jurayyan, A.; Hemida, M.G. The pivotal roles of the host immune response in the fine-tuning the infection and the development of the vaccines for SARS-CoV-2. Hum. Vaccin. Immunother. 2021, 17, 3297–3309. [Google Scholar] [CrossRef]
- Vuksan, V.; Sievenpipper, J.; Jovanovski, E.; Jenkins, A.L. Current clinical evidence for Korean Red Ginseng in management of diabetes and dascular Disease: A Toronto’s Ginseng Clinical Testing Program. J. Ginseng Res. 2010, 34, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Park, B.-J.; Lee, Y.-J.; Lee, H.-R.; Jung, D.-H.; Na, H.-Y.; Kim, H.-B.; Shim, J.-Y. Effects of Korean Red Ginseng on cardiovascular risks in subjects with metabolic syndrome: A double-blind randomized controlled study. Korean J. Fam. Med. 2012, 33, 190–196. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Youn, S.H.; Kwak, Y.-S.; Han, C.-K.; Haidere, M.F.; Kim, J.K.; Min, H.; Jung, Y.-J.; Hosseinzadeh, H.; Hyun, S.H.; et al. Adaptogenic effects of Panax ginseng on modulation of immune functions. J. Ginseng Res. 2021, 45, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Ahn, H.-Y.; Kim, H.-J.; Kim, S.W.; So, S.-H.; In, G.; Park, C.-K.; Han, C.-K. Immuno-enhancement effects of Korean Red Ginseng in healthy adults: A randomized, double-blind, placebo-controlled trial. J. Ginseng Res. 2021, 45, 191–198. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.H.; Jo, S.; Cho, M.J.; Cho, Y.R.; Lee, Y.J.; Byun, S. Immunomodulatory functional foods and their molecular mechanisms. Exp. Mol. Med. 2022, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ju, Z.; Yang, Y.; Zhang, Y.; Yang, L.; Wang, Z. Phytochemical analysis of Panax species: A review. J. Ginseng Res. 2021, 45, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Kim, J.; Min, H. Ginseng, the natural effectual antiviral: Protective effects of Korean Red Ginseng against viral infection. J. Ginseng Res. 2016, 40, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.-G.; Kim, M.-C.; Park, M.-K.; Song, J.-M.; Quan, F.-S.; Park, K.-M.; Cho, Y.-K.; Kang, S.-M. Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J. Med. Food 2012, 15, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Park, E.H.; Yum, J.; Ku, K.B.; Kim, H.M.; Kang, Y.M.; Kim, J.C.; Kim, J.A.; Kang, Y.K.; Seo, S.H. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J. Ginseng Res. 2014, 38, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-S.; Lee, J.-H.; Oh, M.; Choi, K.-M.; Jeong, M.R.; Park, J.-D.; Kwon, D.Y.; Ha, K.-C.; Park, E.-O.; Lee, N.; et al. Preventive effect of Korean Red Ginseng for acute respiratory illness: A randomized and double-blind clinical trial. J. Korean Med. Sci. 2012, 27, 1472–1478. [Google Scholar] [CrossRef]
- Lee, S.-O.; Lee, S.; Kim, S.-J.; Rhee, D.-K. Korean Red Ginseng enhances pneumococcal Δpep27 vaccine efficacy by inhibiting reactive oxygen species production. J. Ginseng Res. 2019, 43, 218–225. [Google Scholar] [CrossRef]
- Xu, M.L.; Kim, H.J.; Choi, Y.R.; Kim, H.-J. Intake of Korean Red Ginseng extract and saponin enhances the protection conferred by vaccination with inactivated Influenza A virus. J. Ginseng Res. 2012, 36, 396–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Althaus, T.; Tan, C.W.; Costantini, A.; Chia, W.N.; Chau, N.V.V.; Tan, L.V.; Mattiuzzo, G.; Rose, N.J.; Voiglio, E.; et al. WHO international standard for SARS-CoV-2 antibodies to determine markers of protection. Lancet Microbe 2022, 3, e81–e82. [Google Scholar] [CrossRef] [PubMed]
- Bailer, A.J. Testing for the equality of area under the curves when using destructive measurement techniques. J. Pharmacokinet. Biopharm. 1988, 16, 303–309. [Google Scholar] [CrossRef]
- Jaki, T.; Wolfsegger, M.J. A theoretical framework for estimation of AUCs in complete and incomplete sampling designs. Stat. Biopharm. Res. 2009, 1, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Jaki, T.; Wolfsegger, M.J. Estimation of pharmacokinetic parameters with the R package PK. Pharm. Stat. 2011, 10, 284–288. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [Green Version]
- Connors, J.; Bell, M.R.; Marcy, J.; Kutzler, M.; Haddad, E.K. The impact of immuno-aging on SARS-CoV-2 vaccine development. Geroscience 2021, 43, 31–51. [Google Scholar] [CrossRef]
- Agarwal, S.; Busse, P.J. Innate and adaptive immunosenescence. Ann. Allergy Asthma Immunol. 2010, 104, 183–190. [Google Scholar] [CrossRef]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; Simone, D.N.; Lippi, G. Total anti-SARS-CoV-2 antibodies measured 6 months after Pfizer-BioNTech COVID-19 vaccination in healthcare workers. J. Med. Biochem. 2022, 41, 199–203. [Google Scholar] [CrossRef] [PubMed]
- den Brok, M.H.; Büll, C.; Wassink, M.; de Graaf, A.M.; Wagenaars, J.A.; Minderman, M.; Thakur, M.; Amigorena, S.; Rijke, E.O.; Schrier, C.C.; et al. Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nat. Commun. 2016, 7, 13324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, M.; Gutama, K.P. Efficacy of the vaccines, their safety, and immune responses against SARS-CoV-2 infections. Am. J. Microbiol. Res. 2021, 9, 103–106. [Google Scholar] [CrossRef]
- Business Wire. Adjuvance Technologies Announces NIH Funding for COVID-19 Vaccine Research. Available online: https://businesswire.com/news/home/20200519005645/en/Adjuvance-Technologies-Announces-NIH-Funding-for-COVID-19-Vaccine-Research (accessed on 24 October 2022).
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Cronkite, D.A.; Strutt, T.M. The regulation of inflammation by innate and adaptive lymphocytes. J. Immunol. Res. 2018, 2018, 1467538. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021, 36, 1095041. [Google Scholar] [CrossRef]
- So, S.-H.; Lee, J.W.; Kim, Y.-S.; Hyun, S.H.; Han, C.-K. Red ginseng monograph. J. Ginseng Res. 2018, 42, 549–561. [Google Scholar] [CrossRef]
- Shanshan, Y.; Zhou, X.; Li, F.; Xu, C.; Zheng, F.; Li, J.; Zhao, H.; Dai, Y.; Liu, S.; Feng, Y. Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti-inflammatory activity of ginseng. Sci. Rep. 2017, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Song, S.-W.; Kim, H.-N.; Shim, J.-Y.; Yoo, B.-Y.; Kim, D.-H.; Lee, S.-H.; Park, J.-S.; Kim, M.-J.; Yoo, J.-H.; Cho, B.; et al. Safety and tolerability of Korean Red Ginseng in healthy adults: A multicenter, double-blind, randomized, placebo-controlled trial. J. Ginseng Res. 2018, 42, 571–576. [Google Scholar] [CrossRef]
- Ng, O.-W.; Chia, A.; Tan, A.T.; Jadi, R.S.; Leong, H.N.; Bertoletti, A.; Tan, Y.-J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 2016, 34, 2008–2014. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.H.; Lee, S.M.; Han, C.-K.; In, G.; Park, C.-K.; Hyun, S.H. Immune activity of polysaccharide fractions isolated from Korean Red Ginseng. Molecules 2020, 25, 3569. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lee, H. Stimulatory effect of Korean Red-Ginseng extract on the proliferation and cellular activity of lymphocytes. J. Ginseng Res. 1998, 22, 60–65. [Google Scholar]
- Suh, S.O.; Jeung, C.H.; Cho, M.Y.; Soon, G.S. The effect of red ginseng for postoperative immune response in gastrointestinal carcinoma. J. Ginseng Res. 1998, 22, 32–42. [Google Scholar]
- Saba, E.; Lee, Y.Y.; Kim, M.; Kim, S.-H.; Hong, S.-B.; Rhee, M.H. A comparative study on immune-stimulatory and antioxidant activities of various types of ginseng extracts in murine and rodent models. J. Ginseng Res. 2018, 42, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Shin, B.-K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Jong, S.K.; Lee, Y.Y.; In, G.; Park, C.-K.; Han, C.-K. Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. J. Ginseng Res. 2020, 44, 527–537. [Google Scholar] [CrossRef]
- Velikova, T.; Fabbri, A.; Infante, M. The role of vitamin D as a potential adjuvant for COVID-19 vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-D.; Lin, C.-H.; Lei, W.-T.; Chang, H.-Y.; Lee, H.-C.; Yeung, C.-Y.; Chiu, N.-C.; Chi, H.; Liu, J.-M.; Hsu, R.-J.; et al. Does vitamin D deficiency affect the immunogenic responses to influenza vaccination? A systematic review and meta-analysis. Nutrients 2018, 10, 409. [Google Scholar] [CrossRef] [Green Version]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Domínguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef]
- Chillon, T.S.; Demircan, K.; Heller, R.A.; Hirschbil-Bremer, I.M.; Diegmann, J.; Bachmann, M.; Moghaddam, M.; Schomburg, L. Relationship between vitamin D status and antibody response to COVID-19 mRNA vaccination in healthy adults. Biomedicines 2021, 9, 1714. [Google Scholar] [CrossRef]
- Kim, D.H.; Xu, Y.H.; Kim, Y.C.; Bang, K.H.; Kim, J.U.; Cha, S.W.; He, Z.M.; Yang, H.; Jang, I.B.; Zhang, L.X. Clinical study on food safety evaluation of Panax ginseng. Korean J. Med. Crop Sci. 2015, 23, 185–189. [Google Scholar] [CrossRef]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential antibody response to mRNA COVID-19 vaccines in healthy subjects. Microbiol. Spectr. 2021, 9, e0034121. [Google Scholar] [CrossRef]
| Variables | KRG 1 (N = 149) | Control (N = 201) | p-Value | |
|---|---|---|---|---|
| Age, years | 47.2 ± 9.0 | 50.3 ± 9.8 | 0.002 * | |
| Sex (male, %) | 104 (69.8) | 80 (39.4) | 0.001 | |
| Body mass index | 24.5 ± 3.2 | 24.6 ± 4.0 | 0.814 * | |
| Glucose, mg/dL | 99.8 ± 19.1 | 102.2 ± 22.0 | 0.285 * | |
| Total Cholesterol, mg/dL | 191.6 ± 37.8 | 177.8 ± 37.8 | 0.986 * | |
| Triglyceride, mg/dL | 116.0 (74.0–173.0) | 109.0 (72.0–165.0) | 0.193 | |
| HDL 2 cholesterol, mg/dL | 55.2 ± 16.3 | 54.1 ± 14.5 | 0.539 * | |
| LDL 3 cholesterol, mg/dL | 123.9 ± 35.6 | 111.1 ± 35.4 | 0.001 * | |
| Ig E | 65.7 (21.1–165.0) | 46.0 (18.5–102.0) | 0.152 | |
| Vitamin D | 22.5 ± 9.14 | 24.1 ± 11.0 | 0.017 * | |
| White blood cells (×103 L) | 5.830 ± 1.38 | 6.081 ± 1.541 | 0.153 * | |
| AST 4 (IU/L) | 24.2 ± 20.1 | 23.5 ± 14.2 | 0.001 * | |
| ALT 5 (IU/L) | 24.8 ± 15.4 | 23.7 ± 16.6 | 0.343 * | |
| Hypertension, (%) | 25 (16.1) | 46 (22.9) | 0.152 | |
| Diabetes, (%) | 6 (4.0) | 31 (15.4) | 0.001 | |
| First dose | Second dose | 0.027 | ||
| Az 6 | Az 6 | 17 (11.4) | 36 (17.9) | - |
| Az 6 | Pfizer | 22 (14.8) | 15 (7.5) | - |
| Moderna | Moderna | 40 (26.8) | 41 (20.4) | - |
| Pfizer | Pfizer | 70 (47.0) | 109 (54.2) | - |
| Variables | Subjects | |
|---|---|---|
| r 1 | p-Value * | |
| Age, years | −0.279 | 0.001 |
| Body mass index | −0.016 | 0.715 |
| Glucose, mg/dL | 0.022 | 0.617 |
| Total cholesterol, mg/dL | 0.038 | 0.388 |
| Triglyceride, mg/dL | −0.021 | 0.635 |
| HDL 2 cholesterol, mg/dL | 0.017 | 0.689 |
| LDL 3 cholesterol, mg/dL | 0.029 | 0.505 |
| Ig E | 0.010 | 0.822 |
| Vitamin D | −0.147 | 0.001 |
| White Blood Cells (×103 L) | 0.097 | 0.028 |
| Anti-S-Ab 4 | 0.736 | 0.001 |
| AST 5 (IU/L) | 0.078 | 0.078 |
| ALT 6 (IU/L) | 0.066 | 0.133 |
| COVID-19 Antibody (Anti-N-Ab 2) | KRG 1 (N = 149) | Control (N = 201) | p-Value * |
|---|---|---|---|
| 2 weeks after the second dose—first dose (baseline) | 2350.74 ± 898.48 | 2160.30 ± 995.34 | 0.042 |
| 4 weeks after the second dose—first dose (baseline) | 2125.56 ± 949.85 | 1874.02 ± 989.24 | 0.014 |
| 12 weeks after the second dose—first dose (baseline) | 745.39 ± 709.05 | 742.81 ± 770.13 | 0.739 |
| 4 weeks after the third dose— first dose (baseline) | 2405.77 ± 512.71 | 2451.61 ± 611.84 | 0.796 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.; Park, B.; Kim, H.; Choi, S.; Jung, D. Korean Red Ginseng Potentially Improves Maintaining Antibodies after COVID-19 Vaccination: A 24-Week Longitudinal Study. Nutrients 2023, 15, 1584. https://doi.org/10.3390/nu15071584
Yoon J, Park B, Kim H, Choi S, Jung D. Korean Red Ginseng Potentially Improves Maintaining Antibodies after COVID-19 Vaccination: A 24-Week Longitudinal Study. Nutrients. 2023; 15(7):1584. https://doi.org/10.3390/nu15071584
Chicago/Turabian StyleYoon, Jihyun, Byoungjin Park, Heejung Kim, Seungjun Choi, and Donghyuk Jung. 2023. "Korean Red Ginseng Potentially Improves Maintaining Antibodies after COVID-19 Vaccination: A 24-Week Longitudinal Study" Nutrients 15, no. 7: 1584. https://doi.org/10.3390/nu15071584






