Mid-Point of the Active Phase Is Better to Achieve the Natriuretic Effect of Acute Salt Load in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Diets
2.2. Metabolic Cage
2.3. Experimental Design
2.4. Urinary Measurements
2.5. Statistical Analysis
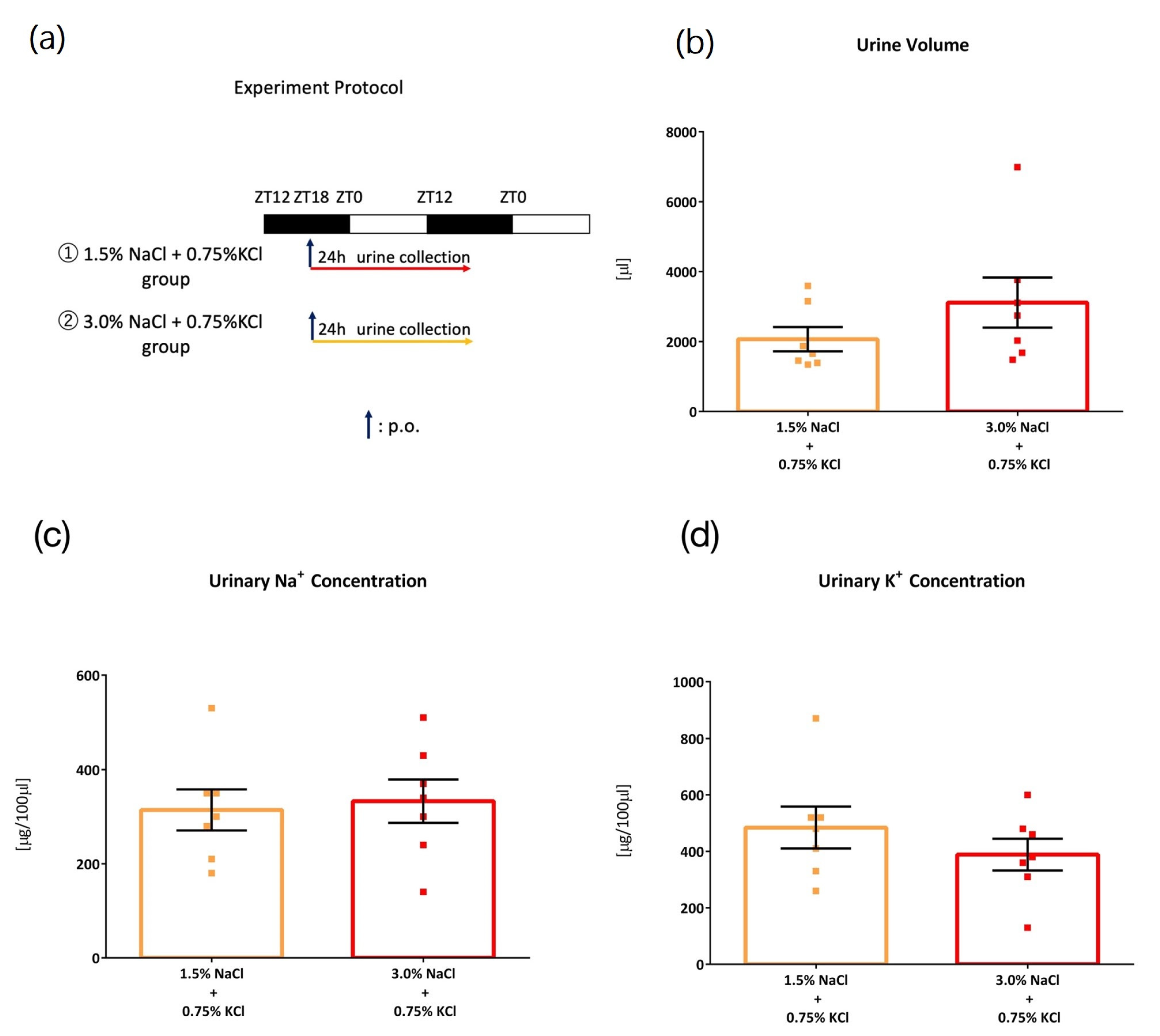
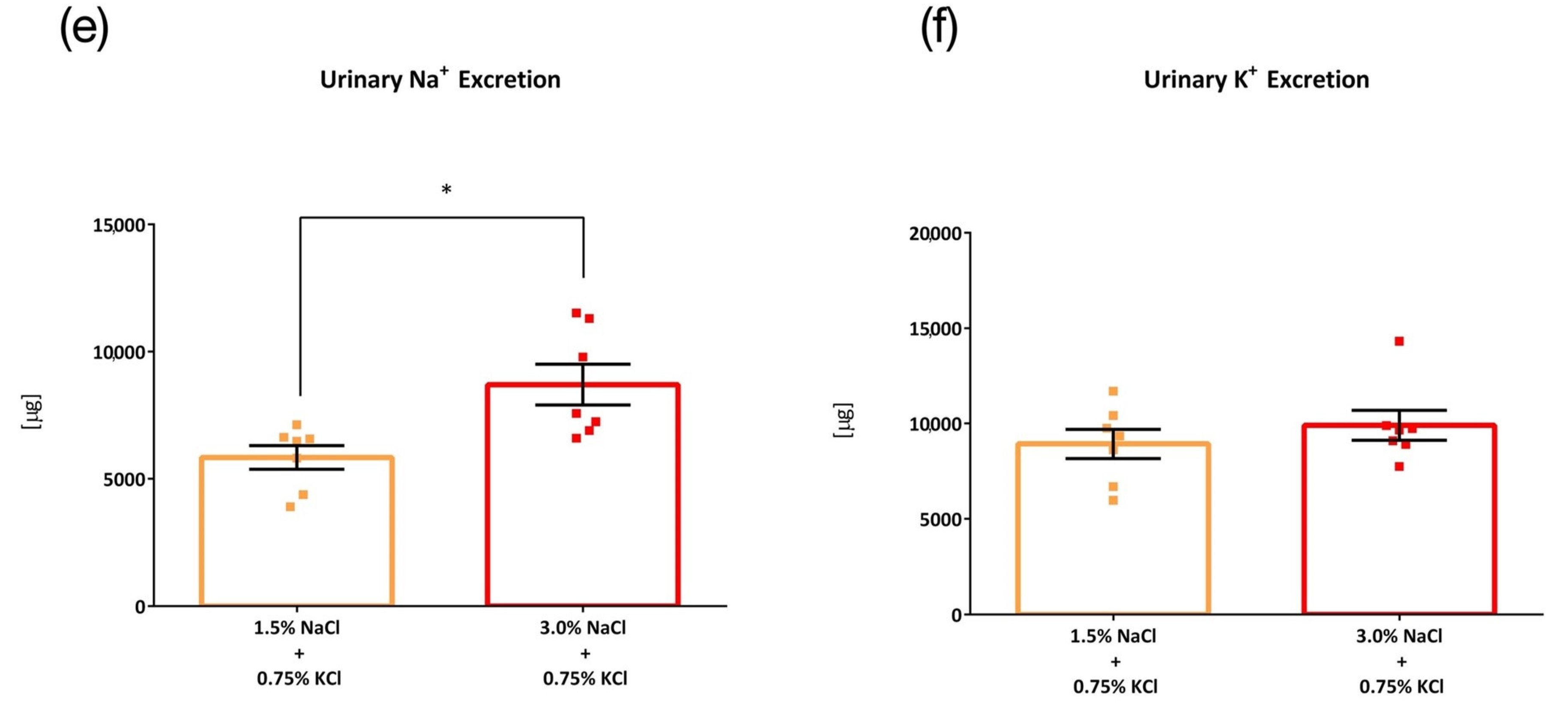
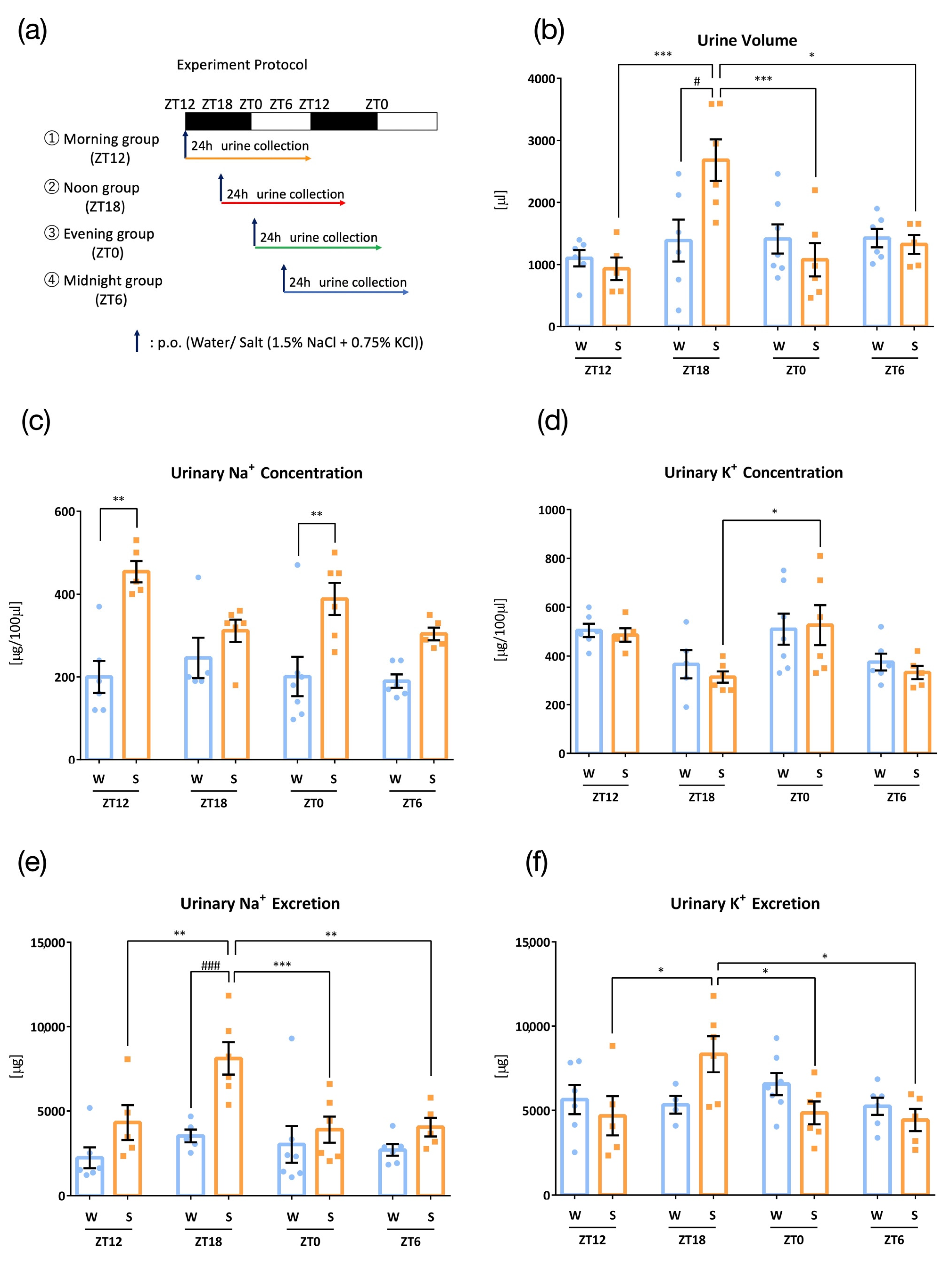
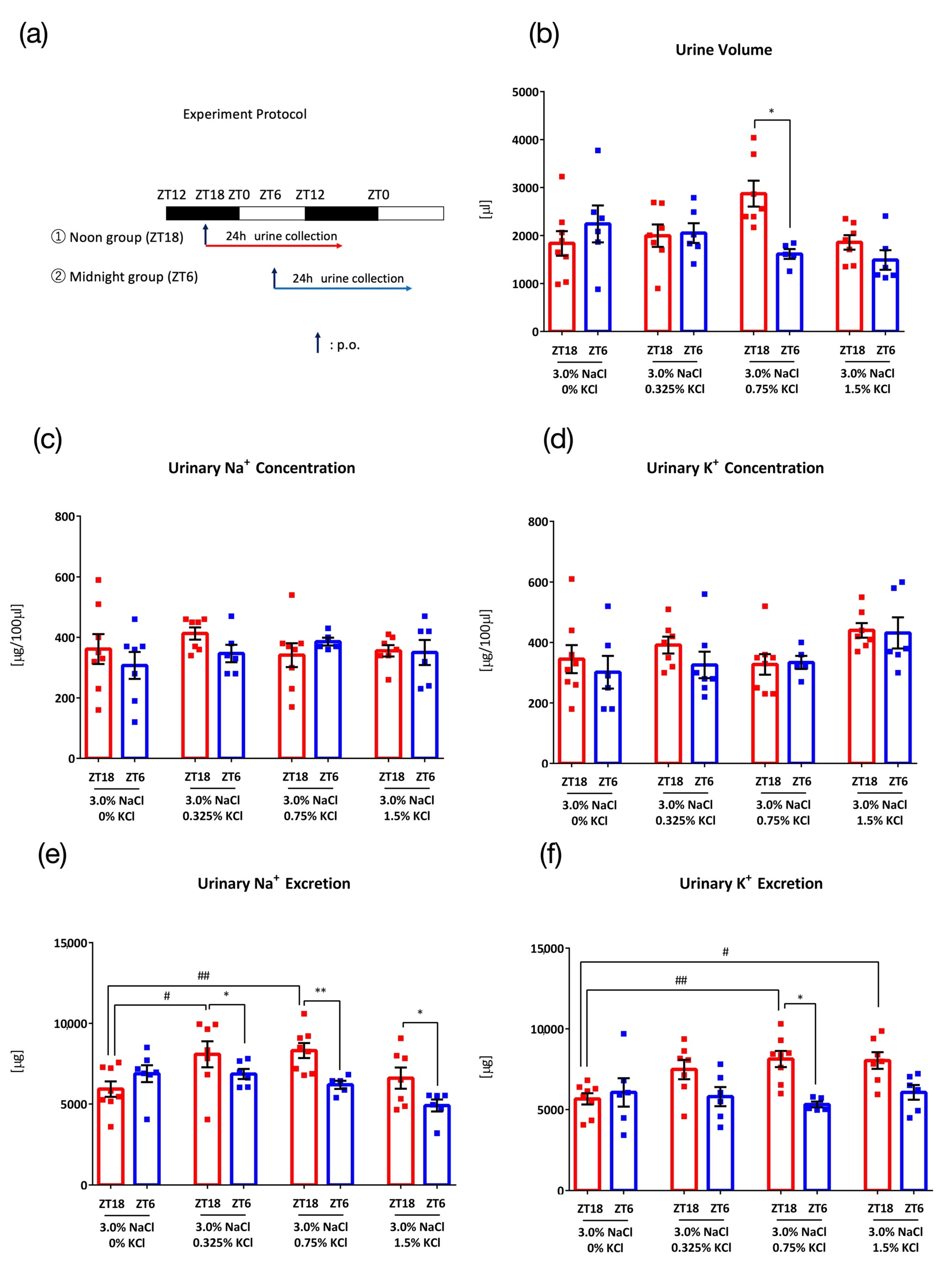
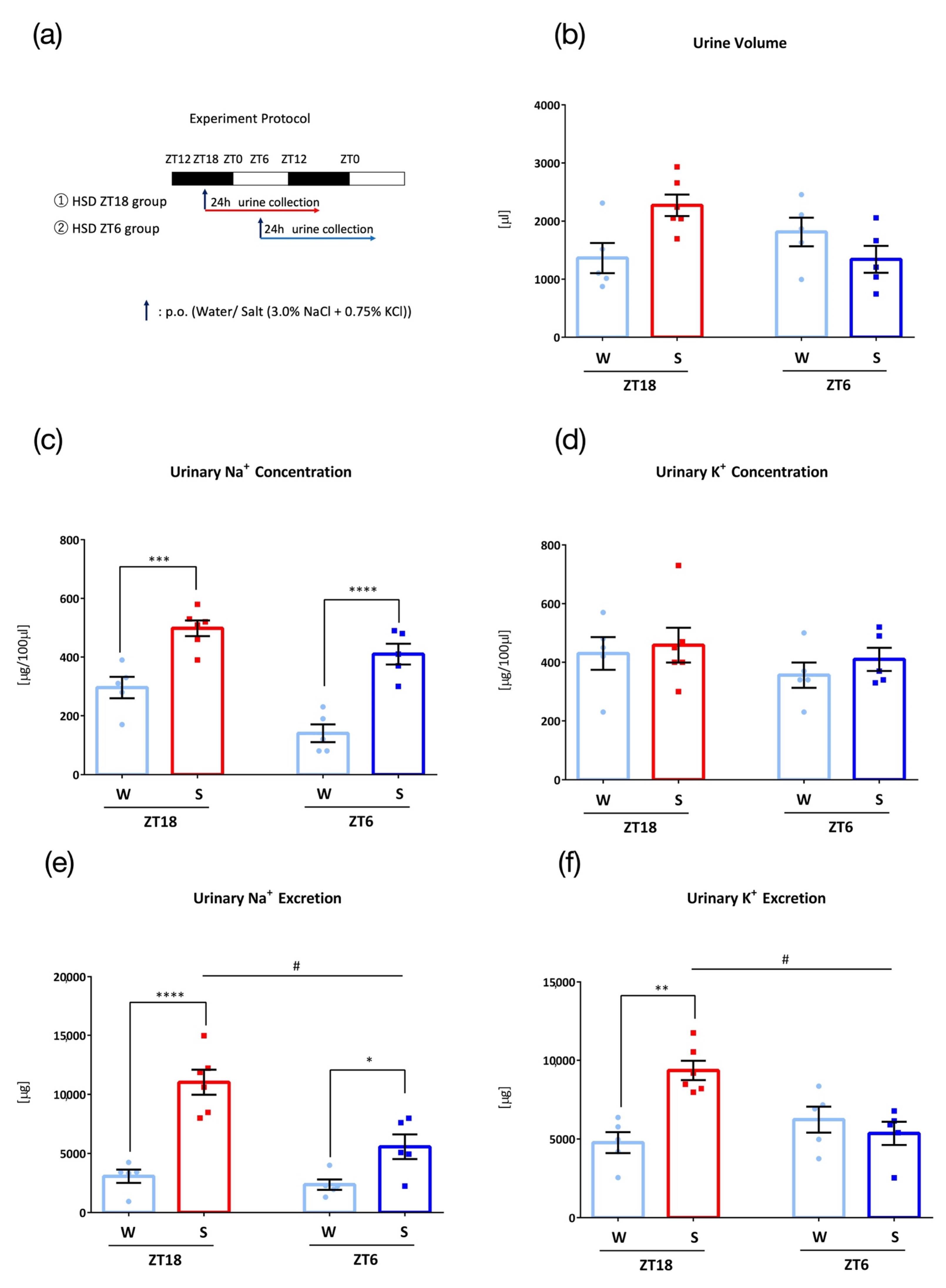
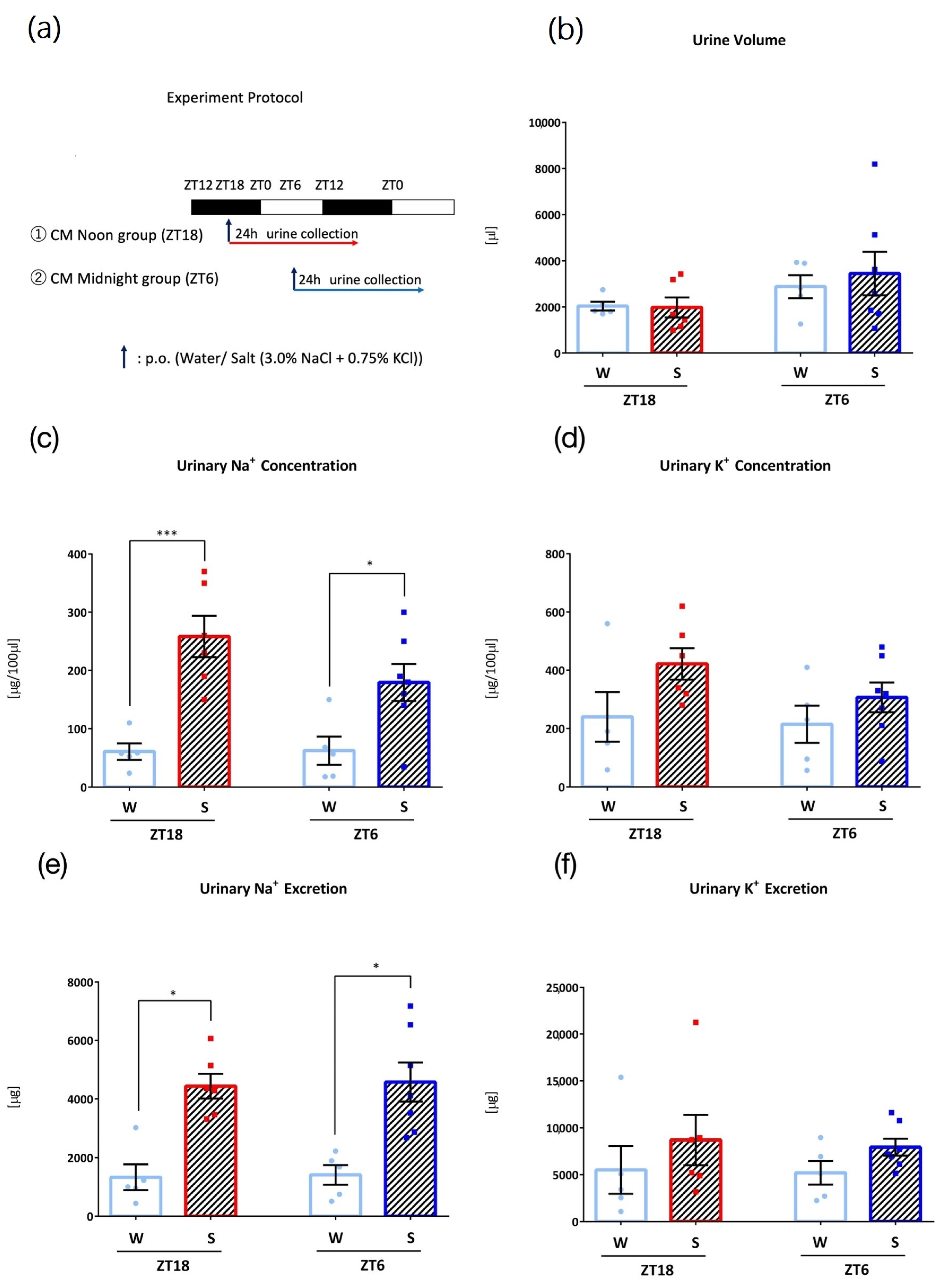
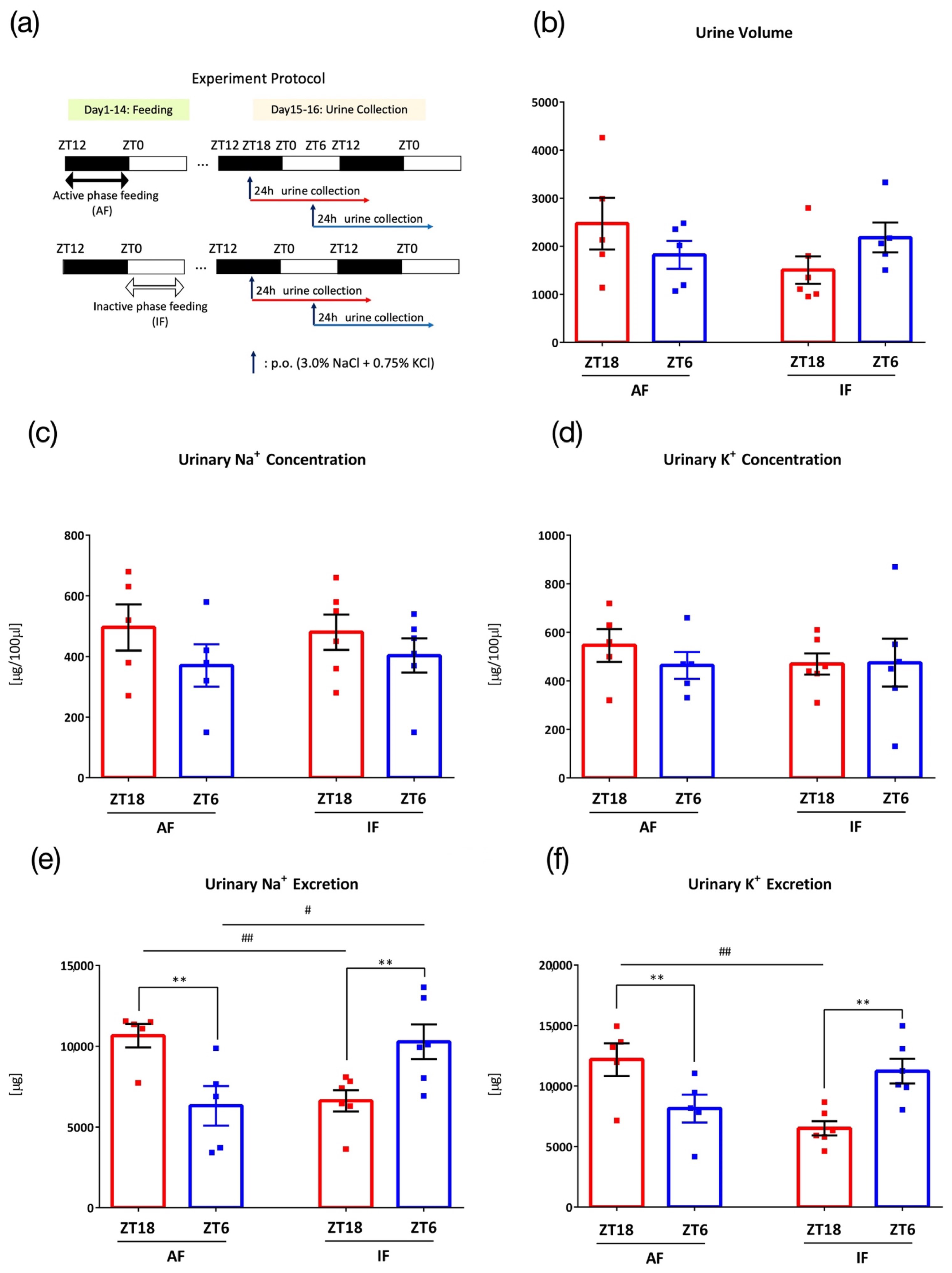
3. Results
3.1. Comparison of Four Intake Timings
3.2. Comparison of Salt Load with Different KCl Concentrations
3.3. Comparison of Salt Load with Different KCl Concentrations against Higher NaCl Concentration
3.4. Effects of High Salt Diet
3.5. Effects of Intake Timing on Clock Mutant Mice
3.6. Effects of Intake Timing on Mice under Time-Restricted Feeding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Aaron, K.J.; Sanders, P.W. Role of dietary salt and potassium intake in cardiovascular health and disease: A review of the evidence. Mayo Clin. Proc. 2013, 88, 987–995. [Google Scholar] [CrossRef]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef]
- WHO. Salt Reduction. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 10 October 2022).
- Ministry of Health, Labour and Welfare, Japan. The National Health and Nutrition Survey in Japan. 2019. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/r1-houkoku_00002.html (accessed on 25 October 2022).
- Filippini, T.; Violi, F.; D’Amico, R.; Vinceti, M. The effect of potassium supplementation on blood pressure in hypertensive subjects: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 230, 127–135. [Google Scholar] [CrossRef]
- Wei, K.Y.; Gritter, M.; Vogt, L.; de Borst, M.H.; Rotmans, J.I.; Hoorn, E.J. Dietary potassium and the kidney: Lifesaving physiology. Clin. Kidney J. 2020, 13, 952–968. [Google Scholar] [CrossRef]
- Penton, D.; Czogalla, J.; Loffing, J. Dietary potassium and the renal control of salt balance and blood pressure. Pflug. Arch. Eur. J. Physiol. 2015, 467, 513–530. [Google Scholar] [CrossRef]
- Nomura, N.; Shoda, W.; Uchida, S. Clinical importance of potassium intake and molecular mechanism of potassium regulation. Clin. Exp. Nephrol. 2019, 23, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Costello, H.M.; Johnston, J.G.; Juffre, A.; Crislip, G.R.; Gumz, M.L. Circadian clocks of the kidney: Function, mechanism, and regulation. Physiol. Rev. 2022, 102, 1669–1701. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006, 15, R271–R277. [Google Scholar] [CrossRef]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef]
- Shea, S.A.; Hilton, M.F.; Hu, K.; Scheer, F.A. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ. Res. 2011, 108, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, S.; Pradervand, S.; Centeno, G.; Zavadova, V.; Tokonami, N.; Maillard, M.; Bonny, O.; Firsov, D. The circadian clock modulates renal sodium handling. J. Am. Soc. Nephrol. JASN 2012, 23, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Zuber, A.M.; Centeno, G.; Pradervand, S.; Nikolaeva, S.; Maquelin, L.; Cardinaux, L.; Bonny, O.; Firsov, D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc. Natl. Acad. Sci. USA 2009, 106, 16523–16528. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Tahara, Y.; Whittaker, D.S.; Wang, H.B.; Yamaji, T.; Wakui, H.; Haraguchi, A.; Yamazaki, M.; Miyakawa, H.; Hama, K.; et al. The circadian clock is disrupted in mice with adenine-induced tubulointerstitial nephropathy. Kidney Int. 2020, 97, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.; Weder, A.B. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension 2006, 48, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tahara, Y.; Hirao, A. The adjustment and manipulation of biological rhythms by light, nutrition, and abused drugs. Adv. Drug Deliv. Rev. 2010, 62, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Hara, R.; Wan, K.; Wakamatsu, H.; Aida, R.; Moriya, T.; Akiyama, M.; Shibata, S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells Devoted Mol. Cell. Mech. 2001, 6, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Kuroda, H.; Saito, K.; Nakajima, Y.; Kubo, Y.; Ohnishi, N.; Seo, Y.; Otsuka, M.; Fuse, Y.; Ohura, Y.; et al. In vivo monitoring of peripheral circadian clocks in the mouse. Curr. Biol. 2012, 22, 1029–1034. [Google Scholar] [CrossRef]
- Ikeda, Y.; Sasaki, H.; Ohtsu, T.; Shiraishi, T.; Tahara, Y.; Shibata, S. Feeding and adrenal entrainment stimuli are both necessary for normal circadian oscillation of peripheral clocks in mice housed under different photoperiods. Chronobiol. Int. 2015, 32, 195–210. [Google Scholar] [CrossRef]
- Imamura, M.; Sasaki, H.; Shinto, T.; Tahara, Y.; Makino, S.; Kuwahara, M.; Tada, A.; Abe, N.; Michie, M.; Shibata, S. Association Between Na, K, and Lipid Intake in Each Meal and Blood Pressure. Front. Nutr. 2022, 9, 853118. [Google Scholar] [CrossRef]
- Johnston, J.G.; Speed, J.S.; Jin, C.; Pollock, D.M. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am. J. Physiol. Ren. Physiol. 2016, 311, F991–F998. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Shibata, S. Time-of-Day-Dependent Physiological Responses to Meal and Exercise. Front. Nutr. 2020, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Tamagawa, T.; Kawashima, M.; Mito, N.; Shibata, S. Attenuating effect of clock mutation on triglyceride contents in the ICR mouse liver under a high-fat diet. J. Biol. Rhythm. 2007, 22, 312–323. [Google Scholar] [CrossRef]
- Palmer, B.F. Regulation of Potassium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1050–1060. [Google Scholar] [CrossRef]
- Susa, K.; Sohara, E.; Isobe, K.; Chiga, M.; Rai, T.; Sasaki, S.; Uchida, S. WNK-OSR1/SPAK-NCC signal cascade has circadian rhythm dependent on aldosterone. Biochem. Biophys. Res. Commun. 2012, 427, 743–747. [Google Scholar] [CrossRef]
- Layton, A.T.; Gumz, M.L. Sex differences in circadian regulation of kidney function of the mouse. Am. J. Physiol. Ren. Physiol. 2022, 323, F675–F685. [Google Scholar] [CrossRef]
- Bankir, L.; Perucca, J.; Norsk, P.; Bouby, N.; Damgaard, M. Relationship between Sodium Intake and Water Intake: The False and the True. Ann. Nutr. Metab. 2017, 70 (Suppl. S1), 51–61. [Google Scholar] [CrossRef]
- Murai, I.; Sugimoto, M.; Ikeda, S.; Kume, S. Effects of high potassium chloride supplementation on water intake, urine volume and nitrogen balance in mice. Anim. Sci. J. Nihon Chikusan Gakkaiho 2010, 81, 80–84. [Google Scholar] [CrossRef]
- Jensen, I.S.; Larsen, C.K.; Leipziger, J.; Sørensen, M.V. Na(+) dependence of K(+) -induced natriuresis, kaliuresis and Na(+)/Cl(−) cotransporter dephosphorylation. Acta Physiol. 2016, 218, 49–61. [Google Scholar] [CrossRef]
- Speed, J.S.; Hyndman, K.A.; Roth, K.; Heimlich, J.B.; Kasztan, M.; Fox, B.M.; Johnston, J.G.; Becker, B.K.; Jin, C.; Gamble, K.L.; et al. High dietary sodium causes dyssynchrony of the renal molecular clock in rats. Am. J. Physiol. Ren. Physiol. 2018, 314, F89–F98. [Google Scholar] [CrossRef]
- Oike, H.; Nagai, K.; Fukushima, T.; Ishida, N.; Kobori, M. High-salt diet advances molecular circadian rhythms in mouse peripheral tissues. Biochem. Biophys. Res. Commun. 2010, 402, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gizowski, C.; Bourque, C.W. Sodium regulates clock time and output via an excitatory GABAergic pathway. Nature 2020, 583, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, M.K.; Speed, J.S.; Roth, K.J.; Zhang, D.; Jin, C.; Gamble, K.L.; Pollock, D.M. Short-term daytime restricted feeding in rats with high salt impairs diurnal variation of Na(+) excretion. Am. J. Physiol. Ren. Physiol. 2022, 322, F335–F343. [Google Scholar] [CrossRef]
- Iwamoto, T.; Torimoto, K.; Gotoh, D.; Onishi, S.; Hori, S.; Morizawa, Y.; Nakai, Y.; Miyake, M.; Fujimoto, K. Reduced salt intake partially restores the circadian rhythm of bladder clock genes in Dahl salt-sensitive rats. Life Sci. 2022, 306, 120842. [Google Scholar] [CrossRef]
- Alli, A.; Yu, L.; Holzworth, M.; Richards, J.; Cheng, K.Y.; Lynch, I.J.; Wingo, C.S.; Gumz, M.L. Direct and indirect inhibition of the circadian clock protein Per1: Effects on ENaC and blood pressure. Am. J. Physiol. Ren. Physiol. 2019, 316, F807–F813. [Google Scholar] [CrossRef]
- Ihara, T.; Mitsui, T.; Nakamura, Y.; Kira, S.; Miyamoto, T.; Nakagomi, H.; Sawada, N.; Hirayama, Y.; Shibata, K.; Shigetomi, E.; et al. The Clock mutant mouse is a novel experimental model for nocturia and nocturnal polyuria. Neurourol. Urodyn. 2017, 36, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Negoro, H.; Kanematsu, A.; Doi, M.; Suadicani, S.O.; Matsuo, M.; Imamura, M.; Okinami, T.; Nishikawa, N.; Oura, T.; Matsui, S.; et al. Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat. Commun. 2012, 3, 809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Colson, J.C.; Jin, C.; Becker, B.K.; Rhoads, M.K.; Pati, P.; Neder, T.H.; King, M.A.; Valcin, J.A.; Tao, B.; et al. Timing of Food Intake Drives the Circadian Rhythm of Blood Pressure. Function 2021, 2, zqaa034. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Joo, Y.; Kim, M.S.; Choe, H.K.; Tong, Q.; Kwon, O. Effects of Intermittent Fasting on the Circulating Levels and Circadian Rhythms of Hormones. Endocrinol. Metab. 2021, 36, 745–756. [Google Scholar] [CrossRef]
- Ivy, J.R.; Oosthuyzen, W.; Peltz, T.S.; Howarth, A.R.; Hunter, R.W.; Dhaun, N.; Al-Dujaili, E.A.; Webb, D.J.; Dear, J.W.; Flatman, P.W.; et al. Glucocorticoids Induce Nondipping Blood Pressure by Activating the Thiazide-Sensitive Cotransporter. Hypertension 2016, 67, 1029–1037. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, D.; Aoki, N.; Tanaka, M.; Aoyama, S.; Shibata, S. The circadian clock controls fluctuations of colonic cell proliferation during the light/dark cycle via feeding behavior in mice. Chronobiol. Int. 2015, 32, 1145–1155. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, M.; Sasaki, H.; Hayashi, K.; Shibata, S. Mid-Point of the Active Phase Is Better to Achieve the Natriuretic Effect of Acute Salt Load in Mice. Nutrients 2023, 15, 1679. https://doi.org/10.3390/nu15071679
Imamura M, Sasaki H, Hayashi K, Shibata S. Mid-Point of the Active Phase Is Better to Achieve the Natriuretic Effect of Acute Salt Load in Mice. Nutrients. 2023; 15(7):1679. https://doi.org/10.3390/nu15071679
Chicago/Turabian StyleImamura, Momoko, Hiroyuki Sasaki, Katsuki Hayashi, and Shigenobu Shibata. 2023. "Mid-Point of the Active Phase Is Better to Achieve the Natriuretic Effect of Acute Salt Load in Mice" Nutrients 15, no. 7: 1679. https://doi.org/10.3390/nu15071679
APA StyleImamura, M., Sasaki, H., Hayashi, K., & Shibata, S. (2023). Mid-Point of the Active Phase Is Better to Achieve the Natriuretic Effect of Acute Salt Load in Mice. Nutrients, 15(7), 1679. https://doi.org/10.3390/nu15071679





