The Effects of Peanut Oligopeptides on Exercise-Induced Fatigue in Mice and Its Underlying Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animals and Experimental Design
2.3. Weight-Loaded Swimming Test
2.4. Blood Lactic Acid Determination
2.5. Determination of Liver and Muscle Glycogen Content
2.6. Biochemical Assay
2.7. Quantitative Real-Time PCR
2.8. Statistical Analysis
3. Results
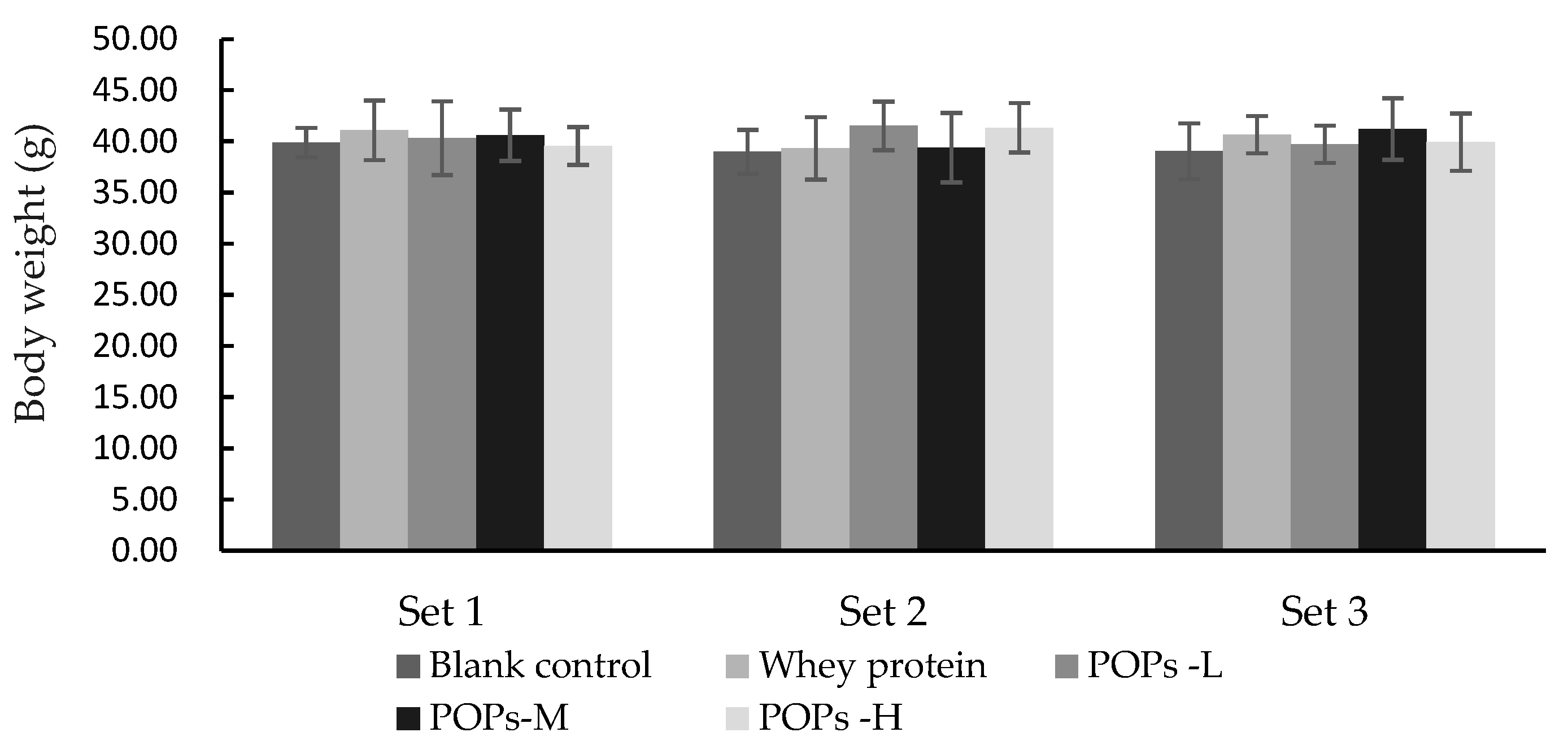
3.1. Effect of POPs on the Body Weight of the Mice
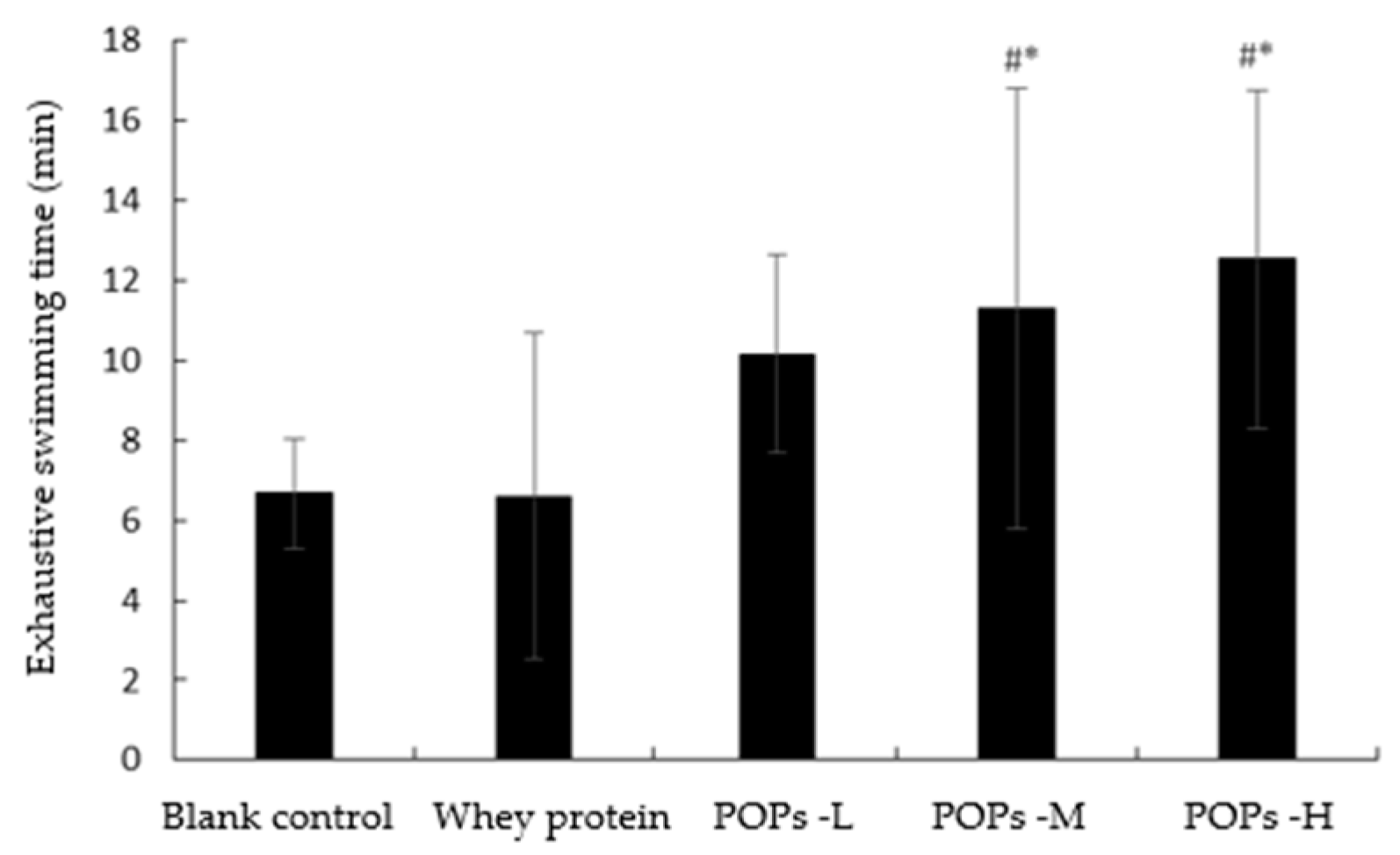
3.2. Effect of POPs on Exhaustive Swimming Time of Mice
3.3. Effect of POPs on Blood Lactate Concentration Levels in Mice
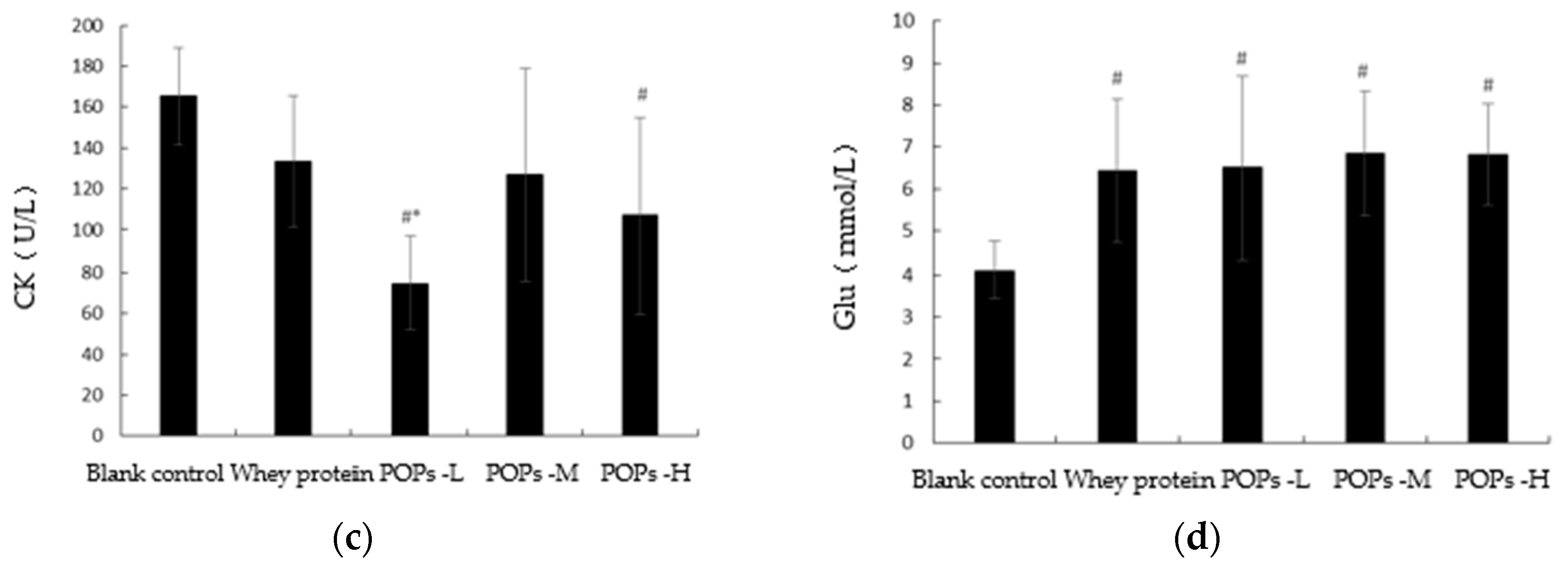
3.4. Effect of POPs on Serum Urea Nitrogen, Lactate Dehydrogenase, Creatine Kinase, and Blood Glucose Levels in Mice
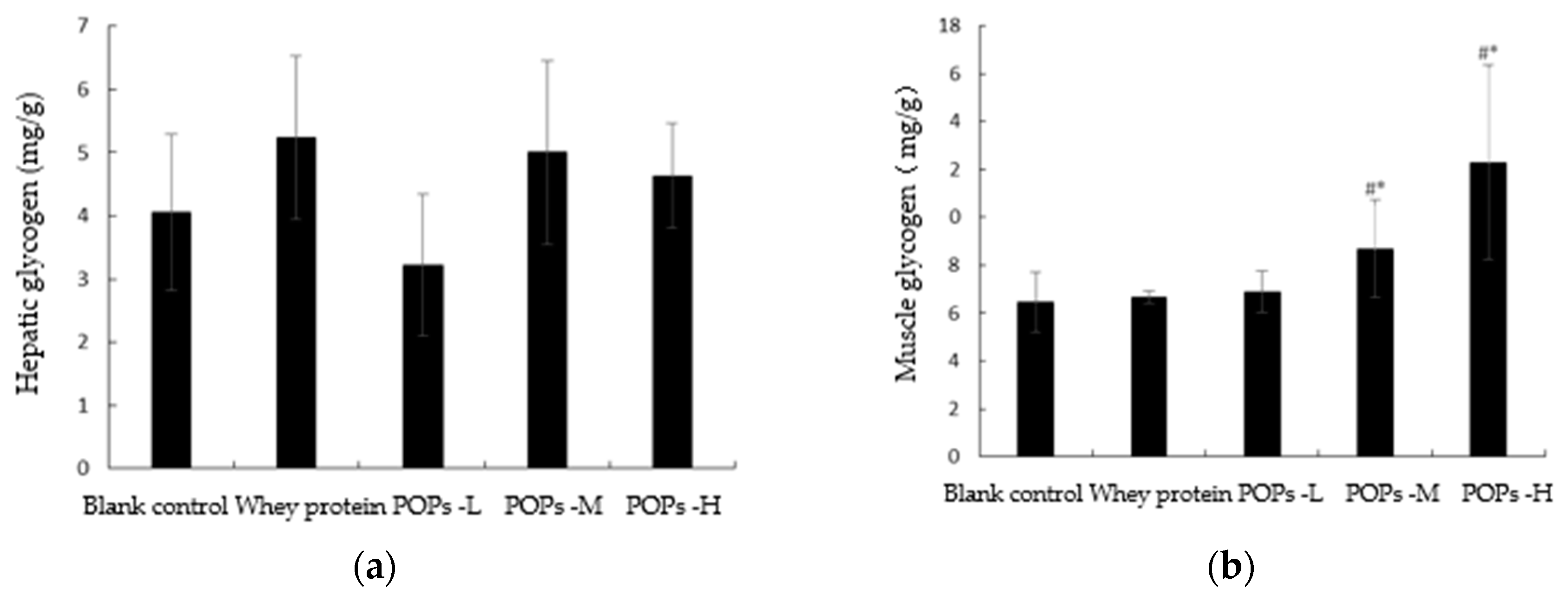
3.5. Effect of POPs on the Content of Hepatic and Muscle Glycogen in Mice
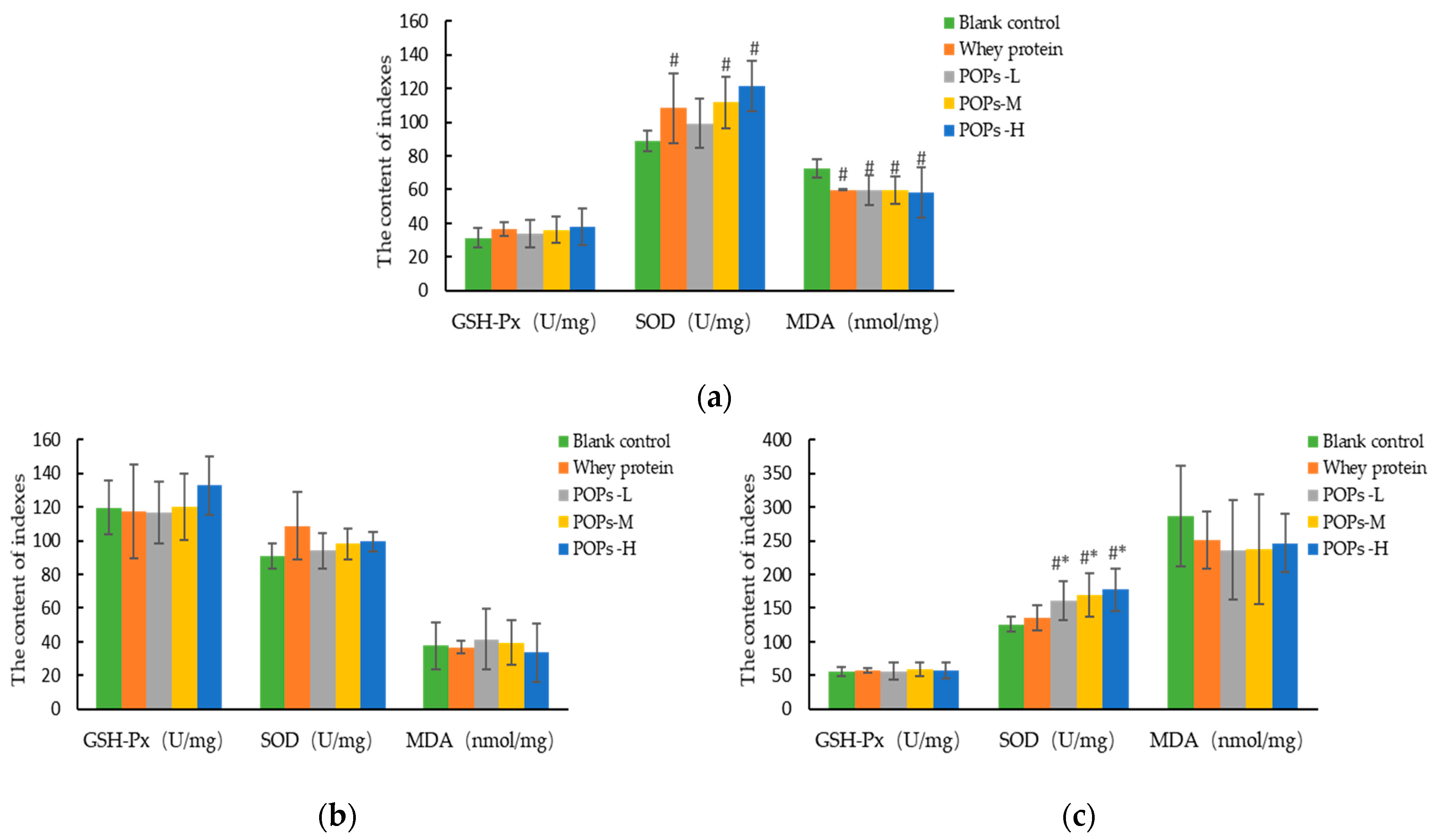
3.6. Effect of POPs on the Antioxidant Capacity of Mice
3.7. Effect of POPs on the Activities of Pyruvate Kinase, Malate Dehydrogenase, and Succinate Dehydrogenase in Mice
3.8. Effect of POPs on the Activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase in Mice
3.9. Effect of POPs on the Mitochondrial Function of Gastrocnemius Muscles in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, D.; Lian, J.; Wang, L.; Liu, X.; Wang, Y.; Zhao, X.; Zhang, X.; Hu, W. The anti-fatigue and anti-anoxia effects of Tremella extract. Saudi J. Biol. Sci. 2019, 26, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.A.; Thompson, L.A.; Lieberman, H.R. Fatigue and its management in the workplace. Neurosci. Biobehav. Rev. 2019, 96, 272–289. [Google Scholar] [CrossRef]
- Baraniuk, J.N. Chronic fatigue syndrome prevalence is grossly overestimated using Oxford criteria compared to Centers for Disease Control (Fukuda) criteria in a U.S. population study. Fatigue Biomed. Health Behav. 2017, 5, 215–230. [Google Scholar] [CrossRef]
- Shi, J.; Shen, J.; Xie, J.; Zhi, J.; Xu, Y. Chronic fatigue syndrome in Chinese middle-school students. Medicine 2018, 97, e9716. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, L.; Du, Q.; Ren, J.-W.; Chen, Q.-H.; Li, D.; Mao, R.-X.; Liu, X.-R.; Li, Y. Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) and Their Anti-Fatigue Effects in Mice. Molecules 2018, 24, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Ren, J.-W.; Zhang, T.; Liu, R.; Wu, L.; Du, Q.; Li, Y. Anti-fatigue effects of small-molecule oligopeptides isolated from Panax quinquefolium L. in mice. Food Funct. 2018, 9, 4266–4273. [Google Scholar] [CrossRef]
- Bao, L.; Cai, X.; Wang, J.; Zhang, Y.; Sun, B.; Li, Y. Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice. Nutrients 2016, 8, 807. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M. Bioactive Peptides in Preventative Healthcare: An Overview of Bioactivities and Suggested Methods to Assess Potential Applications. Curr. Pharm. Des. 2021, 27, 1332–1341. [Google Scholar] [CrossRef]
- Chai, T.-T.; Ee, K.-Y.; Kumar, D.T.; Manan, F.A.; Wong, F.-C. Plant Bioactive Peptides: Current Status and Prospects Towards Use on Human Health. Protein Pept. Lett. 2021, 28, 623–642. [Google Scholar] [CrossRef]
- O’Connor, J.; Garcia-Vaquero, M.; Meaney, S.; Tiwari, B.K. Bioactive Peptides from Algae: Traditional and Novel Generation Strategies, Structure-Function Relationships, and Bioinformatics as Predictive Tools for Bioactivity. Mar. Drugs 2022, 20, 317. [Google Scholar] [CrossRef]
- Madhu, M.; Kumar, D.; Sirohi, R.; Tarafdar, A.; Dhewa, T.; Aluko, R.E.; Badgujar, P.C.; Awasthi, M.K. Bioactive peptides from meat: Current status on production, biological activity, safety, and regulatory framework. Chemosphere 2022, 307, 135650. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Niu, H.; Yang, T.; Lin, Q.; Luo, F.; Ma, M. Antioxidant and anti-fatigue activities of egg white peptides prepared by pepsin digestion. J. Sci. Food Agric. 2014, 94, 3195–3200. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, H.; Chi, Y.; Deng, R.; He, Q. Structural characterization, erythrocyte protection, and antifatigue effect of antioxidant collagen peptides from tilapia (Oreochromis nilotica L.) skin. Food Funct. 2020, 11, 10149–10160. [Google Scholar] [CrossRef]
- Toomer, O.T. Nutritional chemistry of the peanut (Arachis hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Bansode, R.R.; Randolph, P.D.; Plundrich, N.J.; Lila, M.A.; Williams, L.L. Peanut protein-polyphenol aggregate complexation suppresses allergic sensitization to peanut by reducing peanut-specific IgE in C3H/HeJ mice. Food Chem. 2019, 299, 125025. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Sun, C.; Zhao, Y.; Xiong, L.; Sun, Q. Purification and identification of antioxidant peptides from peanut protein isolate hydrolysates using UHR-Q-TOF mass spectrometer. Food Chem. 2014, 161, 148–154. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Lu, X.; Wei, S.; Sun, Q.; Jin, L.; Song, G.; You, J.; Li, F. Characterization of Peanut Protein Hydrolysate and Structural Identification of Umami-Enhancing Peptides. Molecules 2022, 27, 2853. [Google Scholar] [CrossRef]
- Zhang, J.; Sun-Waterhouse, D.; Feng, Y.; Su, G.; Zhao, M.; Lin, L. The umami intensity enhancement of peanut protein isolate hydrolysate and its derived factions and peptides by Maillard reaction and the analysis of peptide (EP) Maillard products. Food Res. Int. 2019, 120, 895–903. [Google Scholar] [CrossRef]
- Apostolovic, D.; Stanic-Vucinic, D.; de Jongh, H.H.J.; de Jong, G.A.H.; Mihailovic, J.; Radosavljevic, J.; Radibratovic, M.; Nordlee, J.A.; Baumert, J.L.; Milcic, M.; et al. Conformational stability of digestion-resistant peptides of peanut conglutins reveals the molecular basis of their allergenicity. Sci. Rep. 2016, 6, 29249. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, M.; Su, G.; Lin, L. Identification and taste characteristics of novel umami and umami-enhancing peptides separated from peanut protein isolate hydrolysate by consecutive chromatography and UPLC–ESI–QTOF–MS/MS. Food Chem. 2019, 278, 674–682. [Google Scholar] [CrossRef]
- Yu, G.; Klionsky, D.J. A “short-cut” response of autophagy to oxidative stress: Oxygen-dependent activity of a lysine demethylase guides the activity of ULK1 during hypoxia. Autophagy 2022, 18, 1749–1751. [Google Scholar] [CrossRef] [PubMed]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-mediated reinnervation of skeletal muscle in elderly people: An update. Eur. J. Transl. Myol. 2022, 32, 10416. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Q.; Meng, Q.; Wang, L.; Xiong, W.; Zhang, L. Anti-fatigue activity of polysaccharide fractions from Lepidium meyenii Walp. (maca). Int. J. Biol. Macromol. 2017, 95, 1305–1311. [Google Scholar] [CrossRef]
- Petersen, A.C.; Murphy, K.T.; Snow, R.J.; Leppik, J.A.; Aughey, R.J.; Garnham, A.P.; Cameron-Smith, D.; McKenna, M.J. Depressed Na+-K+-ATPase activity in skeletal muscle at fatigue is correlated with increased Na+-K+-ATPase mRNA expression following intense exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 289, R266–R274. [Google Scholar] [CrossRef]
- Kolling, J.; Scherer, E.B.S.; Siebert, C.; Hansen, F.; Torres, F.V.; Scaini, G.; Ferreira, G.; de Andrade, R.B.; Gonçalves, C.A.S.; Streck, E.L.; et al. Homocysteine induces energy imbalance in rat skeletal muscle: Is creatine a protector? Cell Biochem. Funct. 2013, 31, 575–584. [Google Scholar] [CrossRef]
- Zhao, X.-N.; Liang, J.-L.; Chen, H.-B.; Liang, Y.-E.; Guo, H.-Z.; Su, Z.-R.; Li, Y.-C.; Zeng, H.-F.; Zhang, X.-J. Anti-Fatigue and Antioxidant Activity of the Polysaccharides Isolated from Millettiae speciosae Champ. Leguminosae. Nutrients 2015, 7, 8657–8669. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Maes, M. Oxidative and Nitrosative Stress and Immune-inflammatory Pathways in Patients with Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Curr. Neuropharmacol. 2014, 12, 168–185. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, R.; Wei, C.; Xu, M.; Li, Y. Exogenous Nucleotides Improved the Oxidative Stress and Sirt-1 Protein Level of Brown Adipose Tissue on Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice. Nutrients 2022, 14, 2796. [Google Scholar] [CrossRef]
- Liu, R.; Hao, Y.-T.; Zhu, N.; Liu, X.-R.; Kang, J.-W.; Mao, R.-X.; Hou, C.; Li, Y. The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats. Nutrients 2020, 12, 1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Q.-H.; Ren, J.-W.; Sun, B.; Cai, X.-X.; Li, D.; Mao, R.-X.; Wu, X.; Li, Y. Ginseng (Panax ginseng Meyer) Oligopeptides Protect Against Binge Drinking-Induced Liver Damage through Inhibiting Oxidative Stress and Inflammation in Rats. Nutrients 2018, 10, 1665. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Liu, R.; Xu, M.-H.; Li, Y. Neuroprotective Actions of Different Exogenous Nucleotides in H2O2-Induced Cell Death in PC-12 Cells. Molecules 2023, 28, 1226. [Google Scholar] [CrossRef]
- González Fernández, Á.; de la Rubia Ortí, J.E.; Franco-Martinez, L.; Ceron, J.J.; Mariscal, G.; Barrios, C. Changes in Salivary Levels of Creatine Kinase, Lactate Dehydrogenase, and Aspartate Aminotransferase after Playing Rugby Sevens: The Influence of Gender. Int. J. Environ. Res. Public Health 2020, 17, 8165. [Google Scholar] [CrossRef] [PubMed]
- Barranco, T.; Tvarijonaviciute, A.; Tecles, F.; Carrillo, J.M.; Sánchez-Resalt, C.; Jimenez-Reyes, P.; Rubio, M.; García-Balletbó, M.; Cerón, J.J.; Cugat, R. Changes in creatine kinase, lactate dehydrogenase and aspartate aminotransferase in saliva samples after an intense exercise: A pilot study. J. Sports Med. Phys. Fit. 2017, 58, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Oguma, Y.; Sato, Y.; Kobayashi, T.; Ito, E.; Tani, M.; Miyamoto, K.; Nishiwaki, Y.; Ishida, H.; Otani, T.; et al. Elevated Creatine Kinase and Lactic Acid Dehydrogenase and Decreased Osteocalcin and Uncarboxylated Osteocalcin are Associated with Bone Stress Injuries in Young Female Athletes. Sci. Rep. 2018, 8, 18019. [Google Scholar] [CrossRef] [Green Version]
- Ostojic, S.M. Exercise-induced mitochondrial dysfunction: A myth or reality? Clin. Sci. 2016, 130, 1407–1416. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in Myalgic Encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, D.; Sanislav, O.; Allan, C.; Smith, P.; Annesley, S.; Fisher, P. Dysregulated Provision of Oxidisable Substrates to the Mitochondria in ME/CFS Lymphoblasts. Int. J. Mol. Sci. 2021, 22, 2046. [Google Scholar] [CrossRef]
- Hsieh, P.-F.; Liu, S.-F.; Hung, T.-J.; Hung, C.-Y.; Liu, G.-Z.; Chuang, L.-Y.; Chen, M.-F.; Wang, J.-L.; Shi, M.-D.; Hsu, C.H.; et al. Elucidation of the therapeutic role of mitochondrial biogenesis transducers NRF-1 in the regulation of renal fibrosis. Exp. Cell Res. 2016, 349, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Serum albumin. Hepatology 1988, 8, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Crumpton, M.J.; Wilkinson, J.M. Amino acid compositions of human and rabbit γ-globulins and of the fragments produced by reduction. Biochem. J. 1963, 88, 228–234. [Google Scholar] [CrossRef] [Green Version]






| Reagent | Blank Control Tube (mL) | Standard Control Tube (mL) | Test Tube (mL) |
|---|---|---|---|
| Protein precipitant–NaF mixture | 0.5 | ||
| Lactic acid standard-application solution | 0.5 | ||
| Supernatant solution | 0.5 | ||
| 4%CuSO4 | 0.1 | 0.1 | 0.1 |
| Concentrated sulfuric acid | 3 | 3 | 3 |
| Placed in boiling water for 5 min after being mixed; then placed in cold water for 10 min | |||
| 1.5% p-hydroxybiphenyl solution | 0.1 | 0.1 | 0.1 |
| Objective Gene | Primer | Sequences | Length | Temperature (°C) |
|---|---|---|---|---|
| β-actin | Forward | GATTACTGCTCTGGCTCCTAG | 147 bp | 62 |
| Reverse | GACTCATCGTACTCCTGCTTGC | |||
| NRF-1 | Forward | TATGGCGGAAGTAATGAAAGACG | 101 bp | 60 |
| Reverse | CAACGTAAGCTCTGCCTTGTT | |||
| mtTFA | Forward | AGGTCCAGCTCACTAACTGC | 217 bp | 62 |
| Reverse | TGTATGCTGTGGTTTCCCAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Li, Z.; Yu, X.-C.; Hu, J.-N.; Zhu, N.; Liu, X.-R.; Hao, Y.-T.; Kang, J.-W.; Li, Y. The Effects of Peanut Oligopeptides on Exercise-Induced Fatigue in Mice and Its Underlying Mechanism. Nutrients 2023, 15, 1743. https://doi.org/10.3390/nu15071743
Liu R, Li Z, Yu X-C, Hu J-N, Zhu N, Liu X-R, Hao Y-T, Kang J-W, Li Y. The Effects of Peanut Oligopeptides on Exercise-Induced Fatigue in Mice and Its Underlying Mechanism. Nutrients. 2023; 15(7):1743. https://doi.org/10.3390/nu15071743
Chicago/Turabian StyleLiu, Rui, Zhen Li, Xiao-Chen Yu, Jia-Ni Hu, Na Zhu, Xin-Ran Liu, Yun-Tao Hao, Jia-Wei Kang, and Yong Li. 2023. "The Effects of Peanut Oligopeptides on Exercise-Induced Fatigue in Mice and Its Underlying Mechanism" Nutrients 15, no. 7: 1743. https://doi.org/10.3390/nu15071743






