Can Circadian Eating Pattern Adjustments Reduce Risk or Prevent Development of T2D?
Abstract
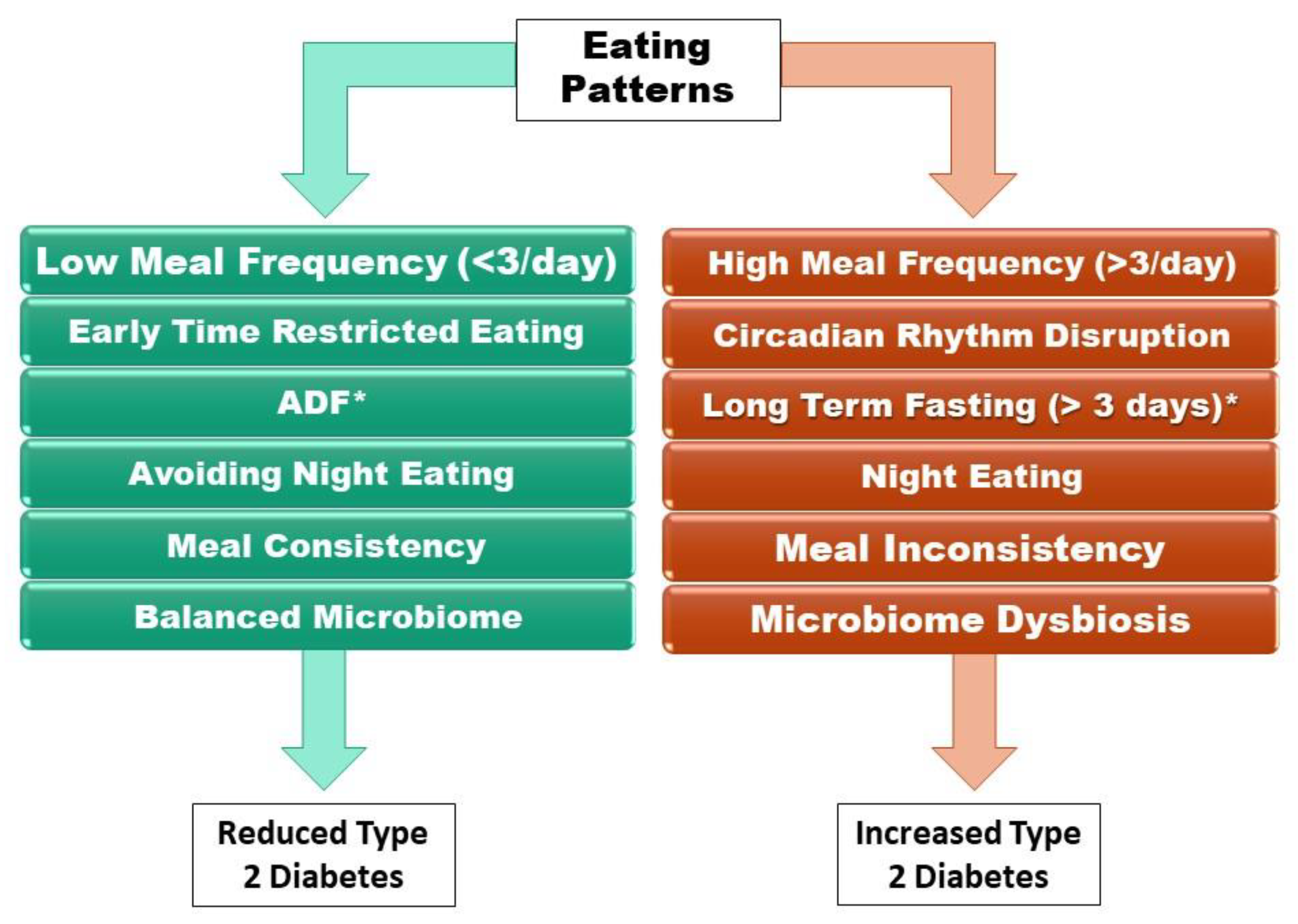
:1. Introduction
2. Modern Eating Patterns: The Cause of Type 2 Diabetes
2.1. History of Eating Patterns
2.2. High vs. Low Meal Frequency
3. The Circadian Clock, The Gut Microbiome, and Metabolic Hormones
3.1. The Gut Microbiome
3.2. Effects of Modern Eating Patterns on Hormone Secretion and Sensitivity
3.3. The Effects of Urbanization on Type 2 Diabetes
3.4. Reducing the Effects of Urbanization
4. Is Fasting a Treatment for Type 2 Diabetes?
4.1. Time-Restricted Eating
4.2. Alternate-Day Fasting
4.3. 5:2 Fasting
4.4. Long-Term Fasting
4.5. The Effects of Fasting on the Gut Microbiome
4.6. Fasting vs. Pharmacotherapy and Caloric Restriction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brunton, S. Pathophysiology of Type 2 Diabetes: The Evolution of Our Understanding. J. Fam. Pract. 2016, 65 (Suppl. S4). [Google Scholar] [PubMed]
- Gastaldelli, A. Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. S1), S60–S65. [Google Scholar] [CrossRef]
- Pratley, R.E.; Weyer, C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia 2001, 44, 929–945. [Google Scholar] [CrossRef] [Green Version]
- Sami, W.; Ansari, T.; Butt, N.S.; Ab Hamid, M.R. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. (Qassim) 2017, 11, 65–71. [Google Scholar] [PubMed]
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004, 88, 787–835, ix. [Google Scholar] [CrossRef] [PubMed]
- Cavaghan, M.K.; Ehrmann, D.A.; Polonsky, K.S. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J. Clin. Investig. 2000, 106, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; van der Schouw, Y.T.; Soedamah-Muthu, S.S.; Spijkerman, A.M.W.; Sluijs, I. Intake of dietary saturated fatty acids and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort: Associations by types, sources of fatty acids and substitution by macronutrients. Eur. J. Nutr. 2019, 58, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Hruby, A.; Manson, J.E.; Qi, L.; Malik, V.S.; Rimm, E.B.; Sun, Q.; Willett, W.C.; Hu, F.B. Determinants and Consequences of Obesity. Am. J. Public Health 2016, 106, 1656–1662. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, H.; Kimmerling, G.; Olefsky, J.M.; Reaven, G.M. Demonstration of insulin resistance in untreated adult onset diabetic subjects with fasting hyperglycemia. J. Clin. Investig. 1975, 55, 454–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A.; Ferrannini, E.; Simonson, D.C. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: Contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism 1989, 38, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Huecksteadt, T.P.; Matthaei, S.; Olefsky, J.M. Role of glucose transporters in the cellular insulin resistance of type II non-insulin-dependent diabetes mellitus. J. Clin. Investig. 1988, 81, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; Mintun, M.A.; Watkins, S.; Simoneau, J.A.; Jadali, F.; Fredrickson, A.; Beattie, J.; Theriault, R. The effect of non-insulin-dependent diabetes mellitus and obesity on glucose transport and phosphorylation in skeletal muscle. J. Clin. Investig. 1996, 97, 2705–2713. [Google Scholar] [CrossRef]
- Polonsky, K.S.; Given, B.D.; Hirsch, L.; Shapiro, E.T.; Tillil, H.; Beebe, C.; Galloway, J.A.; Frank, B.H.; Karrison, T.; Van Cauter, E. Quantitative study of insulin secretion and clearance in normal and obese subjects. J. Clin. Investig. 1988, 81, 435–441. [Google Scholar] [CrossRef]
- Pick, A.; Clark, J.; Kubstrup, C.; Levisetti, M.; Pugh, W.; Bonner-Weir, S.; Polonsky, K.S. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 1998, 47, 358–364. [Google Scholar] [CrossRef]
- Cersosimo, E.; Gastaldelli, A.; Cervera, A.; Wajcberg, E.; Sriwijilkamol, A.; Fernandez, M.; Zuo, P.; Petz, R.; Triplitt, C.; Musi, N.; et al. Effect of exenatide on splanchnic and peripheral glucose metabolism in type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 2011, 96, 1763–1770. [Google Scholar] [CrossRef]
- Boden, G.; Cheung, P.; Stein, T.P.; Kresge, K.; Mozzoli, M. FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E12–E19. [Google Scholar] [CrossRef] [Green Version]
- Gastaldelli, A.; Miyazaki, Y.; Pettiti, M.; Matsuda, M.; Mahankali, S.; Santini, E.; DeFronzo, R.A.; Ferrannini, E. Metabolic effects of visceral fat accumulation in type 2 diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 5098–5103. [Google Scholar] [CrossRef] [Green Version]
- Pratipanawatr, W.; Pratipanawatr, T.; Cusi, K.; Berria, R.; Adams, J.M.; Jenkinson, C.P.; Maezono, K.; DeFronzo, R.A.; Mandarino, L.J. Skeletal muscle insulin resistance in normoglycemic subjects with a strong family history of type 2 diabetes is associated with decreased insulin-stimulated insulin receptor substrate-1 tyrosine phosphorylation. Diabetes 2001, 50, 2572–2578. [Google Scholar] [CrossRef] [Green Version]
- Bruce, D.G.; Chisholm, D.J.; Storlien, L.H.; Kraegen, E.W. Physiological importance of deficiency in early prandial insulin secretion in non-insulin-dependent diabetes. Diabetes 1988, 37, 736–744. [Google Scholar] [CrossRef]
- Eaton, S.B.; Konner, M. Paleolithic nutrition. A consideration of its nature and current implications. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [PubMed]
- Rubio-Ruiz, M.E.; Peredo-Escárcega, A.E.; Cano-Martínez, A.; Guarner-Lans, V. An Evolutionary Perspective of Nutrition and Inflammation as Mechanisms of Cardiovascular Disease. Int. J. Evol. Biol. 2015, 2015, 179791. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The Influence of Meal Frequency and Timing on Health in Humans: The Role of Fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.J.L.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar]
- Dashti, H.S.; Scheer, F.A.J.L.; Saxena, R.; Garaulet, M. Timing of Food Intake: Identifying Contributing Factors to Design Effective Interventions. Adv. Nutr. 2019, 10, 606–620. [Google Scholar]
- Lea, A.J.; Martins, D.; Kamau, J.; Gurven, M.; Ayroles, J.F. Urbanization and market integration have strong, nonlinear effects on cardiometabolic health in the Turkana. Sci. Adv. 2020, 6, eabb1430. [Google Scholar] [CrossRef]
- Manus, M.B. Evolutionary mismatch. Evol. Med. Public Health 2018, 2018, 190–191. [Google Scholar] [CrossRef]
- Kahleova, H.; Lloren, J.I.; Mashchak, A.; Hill, M.; Fraser, G.E. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J. Nutr. 2017, 147, 1722–1728. [Google Scholar]
- Mekary, R.A.; Giovannucci, E.; Willett, W.C.; Van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012, 95, 1182–1189. [Google Scholar]
- Neuhouser, M.L.; Wertheim, B.C.; Perrigue, M.M.; Hingle, M.; Tinker, L.F.; Shikany, J.M.; Johnson, K.C.; Waring, M.E.; Seguin-Fowler, R.A.; Vitolins, M.Z.; et al. Associations of Number of Daily Eating Occasions with Type 2 Diabetes Risk in the Women’s Health Initiative Dietary Modification Trial. Curr. Dev. Nutr. 2020, 4, nzaa126. [Google Scholar] [PubMed]
- Bertéus Forslund, H.; Torgerson, J.S.; Sjöström, L.; Lindroos, A.K. Snacking frequency in relation to energy intake and food choices in obese men and women compared to a reference population. Int. J. Obes. (Lond.) 2005, 29, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Błaszczyk-Bębenek, E.; Jagielski, P.; Bolesławska, I.; Jagielska, A.; Nitsch-Osuch, A.; Kawalec, P. Nutrition Behaviors in Polish Adults before and during COVID-19 Lockdown. Nutrients 2020, 12, 3084. [Google Scholar] [PubMed]
- Zeballos, E.; Todd, J.E. The effects of skipping a meal on daily energy intake and diet quality. Public Health Nutr. 2020, 23, 3346–3355. [Google Scholar] [CrossRef]
- Pot, G.K. Sleep and dietary habits in the urban environment: The role of chrono-nutrition. Proc. Nutr. Soc. 2018, 77, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Mekary, R.A.; Giovannucci, E.; Cahill, L.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in older women: Breakfast consumption and eating frequency. Am. J. Clin. Nutr. 2013, 98, 436–443. [Google Scholar]
- Froy, O. The relationship between nutrition and circadian rhythms in mammals. Front. Neuroendocrinol. 2007, 28, 61–71. [Google Scholar] [PubMed]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A.; et al. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 2019, 177, 896–909.e20. [Google Scholar]
- Díaz-Rizzolo, D.A.; Kostov, B.; López-Siles, M.; Serra, A.; Colungo, C.; González-De-Paz, L.; Martinez-Medina, M.; Sisó-Almirall, A.; Gomis, R. Healthy dietary pattern and their corresponding gut microbiota profile are linked to a lower risk of type 2 diabetes, independent of the presence of obesity. Clin. Nutr. 2020, 39, 524–532. [Google Scholar]
- Voigt, R.M.; Summa, K.C.; Forsyth, C.B.; Green, S.J.; Engen, P.; Naqib, A.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. The Circadian Clock Mutation Promotes Intestinal Dysbiosis. Alcohol Clin. Exp. Res. 2016, 40, 335–347. [Google Scholar]
- Alam, C.; Bittoun, E.; Bhagwat, D.; Valkonen, S.; Saari, A.; Jaakkola, U.; Eerola, E.; Huovinen, P.; Hänninen, A. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia 2011, 54, 1398–1406. [Google Scholar] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [PubMed] [Green Version]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, W.C.; Tsai, H.-J.; Chang, C.-C.; Chiu, Y.-W.; Hwang, S.-J.; Kuo, M.-C.; Chen, S.-C.; Dai, C.-Y.; Tsai, Y.-C. The Association of Targeted Gut Microbiota with Body Composition in Type 2 Diabetes Mellitus. Int. J. Med. Sci. 2021, 18, 511–519. [Google Scholar] [CrossRef]
- Bodosi, B.; Gardi, J.; Hajdu, I.; Szentirmai, E.; Obal, F.; Krueger, J.M. Rhythms of ghrelin, leptin, and sleep in rats: Effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1071–R1079. [Google Scholar] [CrossRef] [Green Version]
- Natalucci, G.; Riedl, S.; Gleiss, A.; Zidek, T.; Frisch, H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: Maintenance of a meal-related pattern. Eur. J. Endocrinol. 2005, 152, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Munsters, M.J.; Saris, W.H. Effects of meal frequency on metabolic profiles and substrate partitioning in lean healthy males. PLoS ONE 2012, 7, e38632. [Google Scholar] [CrossRef] [Green Version]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. (Lausanne) 2021, 12, 585887. [Google Scholar]
- Boden, G.; Chen, X.; Mozzoli, M.; Ryan, I. Effect of fasting on serum leptin in normal human subjects. J. Clin. Endocrinol. Metab. 1996, 81, 3419–3423. [Google Scholar]
- Kolaczynski, J.W.; Ohannesian, J.P.; Considine, R.V.; Marco, C.C.; Caro, J.F. Response of leptin to short-term and prolonged overfeeding in humans. J. Clin. Endocrinol. Metab. 1996, 81, 4162–4165. [Google Scholar] [PubMed] [Green Version]
- Pot, G.K.; Hardy, R.; Stephen, A.M. Irregular consumption of energy intake in meals is associated with a higher cardiometabolic risk in adults of a British birth cohort. Int. J. Obes. (Lond.) 2014, 38, 1518–1524. [Google Scholar]
- Adnan, D.; Trinh, J.; Bishehsari, F. Inconsistent eating time is associated with obesity: A prospective study. Excli. J. 2022, 21, 300–306. [Google Scholar]
- Bray, M.S.; Ratcliffe, W.F.; Grenett, M.H.; Brewer, R.A.; Gamble, K.; Young, M.E. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int. J. Obes. (Lond.) 2013, 37, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oike, H.; Oishi, K.; Kobori, M. Nutrients, Clock Genes, and Chrononutrition. Curr. Nutr. Rep. 2014, 3, 204–212. [Google Scholar] [PubMed] [Green Version]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar]
- Sonnier, T.; Rood, J.; Gimble, J.M.; Peterson, C.M. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J. Diabetes Complicat. 2014, 28, 836–843. [Google Scholar]
- Morgan, L.M.; Aspostolakou, F.; Wright, J.; Gama, R. Diurnal variations in peripheral insulin resistance and plasma non-esterified fatty acid concentrations: A possible link? Ann. Clin. Biochem. 1999, 36 Pt 4, 447–450. [Google Scholar]
- Pafili, Z.; Dimosthenopoulos, C. Novel trends and concepts in the nutritional management of glycemia in type 2 diabetes mellitus-beyond dietary patterns: A narrative review. Hormones (Athens) 2021, 20, 641–655. [Google Scholar]
- Wilhelmi de Toledo, F.; Grundler, F.; Sirtori, C.R.; Ruscica, M. Unravelling the health effects of fasting: A long road from obesity treatment to healthy life span increase and improved cognition. Ann. Med. 2020, 52, 147–161. [Google Scholar]
- Karatoprak, C.; Yolbas, S.; Cakirca, M.; Cinar, A.B.; Zorlu, M.; Kiskac, M.; Cikrikcioglu, M.A.; Erkoc, R.; Tasan, E. The effects of long term fasting in Ramadan on glucose regulation in type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2512–2516. [Google Scholar]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [PubMed] [Green Version]
- Cienfuegos, S.; McStay, M.; Gabel, K.; Varady, K.A. Time restricted eating for the prevention of type 2 diabetes. J. Physiol. 2022, 600, 1253–1264. [Google Scholar] [CrossRef]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 2007, 56, 1729–1734. [Google Scholar]
- Jamshed, H.; Beyl, R.A.; della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [PubMed] [Green Version]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [PubMed] [Green Version]
- Liu, J.; Yi, P.; Liu, F. The Effect of Early Time-Restricted Eating vs. Later Time-Restricted Eating on Weight Loss and Metabolic Health: A Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2023, 1–11. [Google Scholar] [CrossRef]
- Peeke, P.M.; Greenway, F.L.; Billes, S.K.; Zhang, D.; Fujioka, K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: Results of a randomized, controlled, virtual clinical trial. Nutr. Diabetes 2021, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity (Silver Spring) 2020, 28, 860–869. [Google Scholar]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhang, W.; Yun, D.; Li, L.; Zhao, W.; Li, Y.; Liu, X.; Liu, Z. Alternate-day fasting alleviates diabetes-induced glycolipid metabolism disorders: Roles of FGF21 and bile acids. J. Nutr. Biochem. 2020, 83, 108403. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Donahoo, W.T. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring) 2016, 24, 1874–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stice, E.; Davis, K.; Miller, N.P.; Marti, C.N. Fasting increases risk for onset of binge eating and bulimic pathology: A 5-year prospective study. J. Abnorm. Psychol. 2008, 117, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Tsintzas, K.; Macdonald, I.A.; Cordon, S.M.; Taylor, M.A. Effects of intermittent (5:2) or continuous energy restriction on basal and postprandial metabolism: A randomised study in normal-weight, young participants. Eur. J. Clin. Nutr. 2022, 76, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Witjaksono, F.; Prafiantini, E.; Rahmawati, A. Effect of intermittent fasting 5:2 on body composition and nutritional intake among employees with obesity in Jakarta: A randomized clinical trial. BMC Res. Notes 2022, 15, 323. [Google Scholar] [CrossRef]
- Wilhelmi de Toledo, F.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, e0209353. [Google Scholar] [CrossRef] [Green Version]
- Michalsen, A.; Kuhlmann, M.K.; Lüdtke, R.; Bäcker, M.; Langhorst, J.; Dobos, G.J. Prolonged fasting in patients with chronic pain syndromes leads to late mood-enhancement not related to weight loss and fasting-induced leptin depletion. Nutr. Neurosci. 2006, 9, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.C.; Khatri, A.; Kifayat, A.; Cho, R.; Aronow, W.S. Starvation Ketoacidosis due to the Ketogenic Diet and Prolonged Fasting—A Possibly Dangerous Diet Trend. Am. J. Case Rep. 2019, 20, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.B.; Wasinski, F.; Donato, J., Jr. Prolonged fasting induces long-lasting metabolic consequences in mice. J. Nutr. Biochem. 2020, 84, 108457. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Zhang, Y.; O’Keefe, J.; Pesaresi, T.; Lun, M.; Lawney, B.; Steinhauser, M.L. Prolonged fasting drives a program of metabolic inflammation in human adipose tissue. Mol. Metab. 2020, 42, 101082. [Google Scholar] [CrossRef]
- Qatanani, M.; Tan, Y.; Dobrin, R.; Greenawalt, D.M.; Hu, G.; Zhao, W.; Olefsky, J.M.; Sears, D.D.; Kaplan, L.M.; Kemp, D.M. Inverse regulation of inflammation and mitochondrial function in adipose tissue defines extreme insulin sensitivity in morbidly obese patients. Diabetes 2013, 62, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Bolondi, L.; Gaiani, S.; Testa, S.; Labo, G. Gall bladder sludge formation during prolonged fasting after gastrointestinal tract surgery. Gut 1985, 26, 734–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.; Cooper, C.; Sayer, A.A. Nutrition and sarcopenia: A review of the evidence and implications for preventive strategies. J. Aging Res. 2012, 2012, 510801. [Google Scholar] [CrossRef]
- Laurens, C.; Grundler, F.; Damiot, A.; Chery, I.; Le Maho, A.L.; Zahariev, A.; Le Maho, Y.; Bergouignan, A.; Gauquelin-Koch, G.; Simon, C.; et al. Is muscle and protein loss relevant in long-term fasting in healthy men? A prospective trial on physiological adaptations. J. Cachexia Sarcopenia Muscle 2021, 12, 1690–1703. [Google Scholar] [CrossRef]
- Carter, S.; Clifton, P.M.; Keogh, J.B. Effect of Intermittent Compared With Continuous Energy Restricted Diet on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Noninferiority Trial. JAMA Netw Open 2018, 1, e180756. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Zhang, X.; Ma, M.; Xie, Z.; Pan, Q.; Ma, Z.; Peppelenbosch, M.P. Remodeling of the gut microbiome during Ramadan-associated intermittent fasting. Am. J. Clin. Nutr. 2021, 113, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Mesnage, R.; Grundler, F.; Schwiertz, A.; Le Maho, Y.; de Toledo, F.W. Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting. J. Nutr. Sci. 2019, 8, e36. [Google Scholar] [CrossRef] [Green Version]
- Dantas Machado, A.C.; Brown, S.D.; Lingaraju, A.; Sivaganesh, V.; Martino, C.; Chaix, A.; Zhao, P.; Pinto, A.F.; Chang, M.W.; Richter, R.A.; et al. Diet and feeding pattern modulate diurnal dynamics of the ileal microbiome and transcriptome. Cell Rep. 2022, 40, 111008. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Ko, G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014, 80, 5935–5943. [Google Scholar] [CrossRef] [Green Version]
- Morales-Suarez-Varela, M.; Sánchez, E.C.; Peraita-Costa, I.; Llopis-Morales, A.; Soriano, J.M. Intermittent Fasting and the Possible Benefits in Obesity, Diabetes, and Multiple Sclerosis: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 3179. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef]
- Liu, B.; Hutchison, A.T.; Thompson, C.H.; Lange, K.; Heilbronn, L.K. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes. Res. Clin. Pract. 2019, 13, 408–415. [Google Scholar] [CrossRef]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl. Res. 2014, 164, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Devlin, B.L.; Hawley, J.A. Perspective: Time-Restricted Eating-Integrating the What with the When. Adv. Nutr. 2022, 13, 699–711. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, C.; Czaja, K. Can Circadian Eating Pattern Adjustments Reduce Risk or Prevent Development of T2D? Nutrients 2023, 15, 1762. https://doi.org/10.3390/nu15071762
Harris C, Czaja K. Can Circadian Eating Pattern Adjustments Reduce Risk or Prevent Development of T2D? Nutrients. 2023; 15(7):1762. https://doi.org/10.3390/nu15071762
Chicago/Turabian StyleHarris, Carlee, and Krzysztof Czaja. 2023. "Can Circadian Eating Pattern Adjustments Reduce Risk or Prevent Development of T2D?" Nutrients 15, no. 7: 1762. https://doi.org/10.3390/nu15071762
APA StyleHarris, C., & Czaja, K. (2023). Can Circadian Eating Pattern Adjustments Reduce Risk or Prevent Development of T2D? Nutrients, 15(7), 1762. https://doi.org/10.3390/nu15071762






