Potential Effect of Glutamine in the Improvement of Intestinal Stem Cell Proliferation and the Alleviation of Burn-Induced Intestinal Injury via Activating YAP: A Preliminary Study
Abstract
:1. Introduction
2. Results
2.1. Gln Improves Crypt Survival and Mitigates Pathological Damage of Small Intestine after Burns in Mice
2.2. Gln Promotes Budding in Three-Dimensional (3D) Cultured Intestinal Organoids after Burns
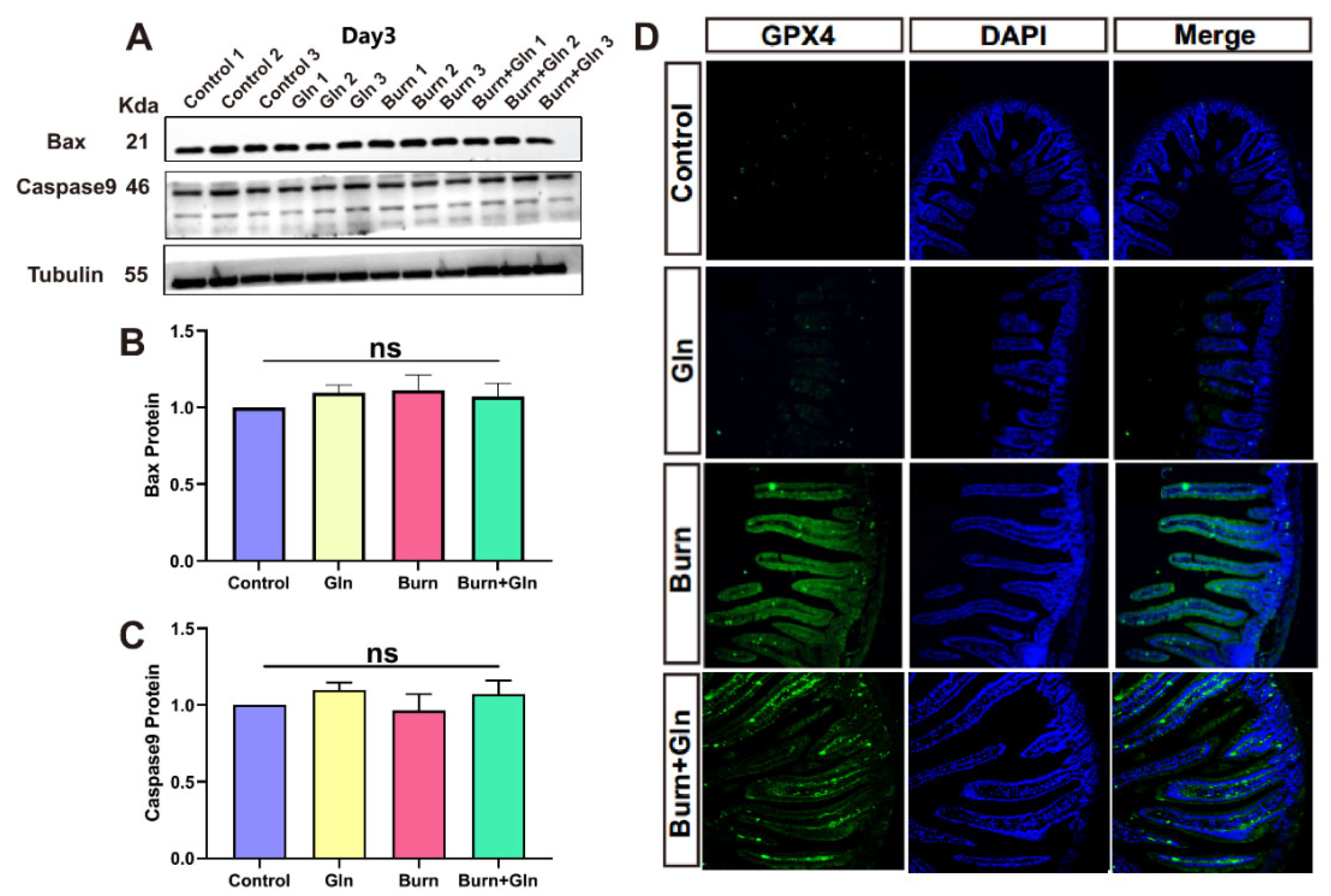
2.3. Effects of Gln on Endogenous Apoptosis and Ferroptosis of ISCs after Burns
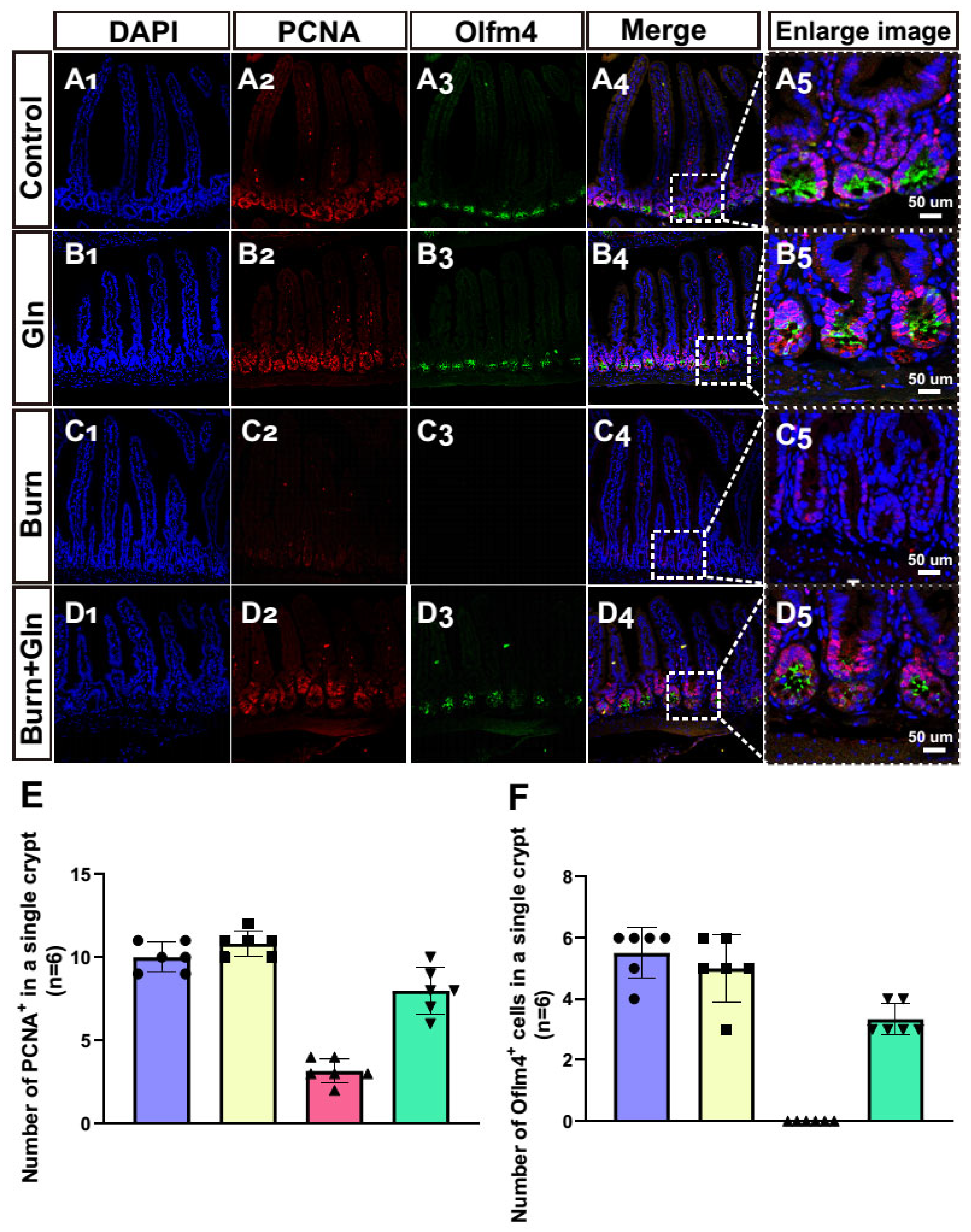
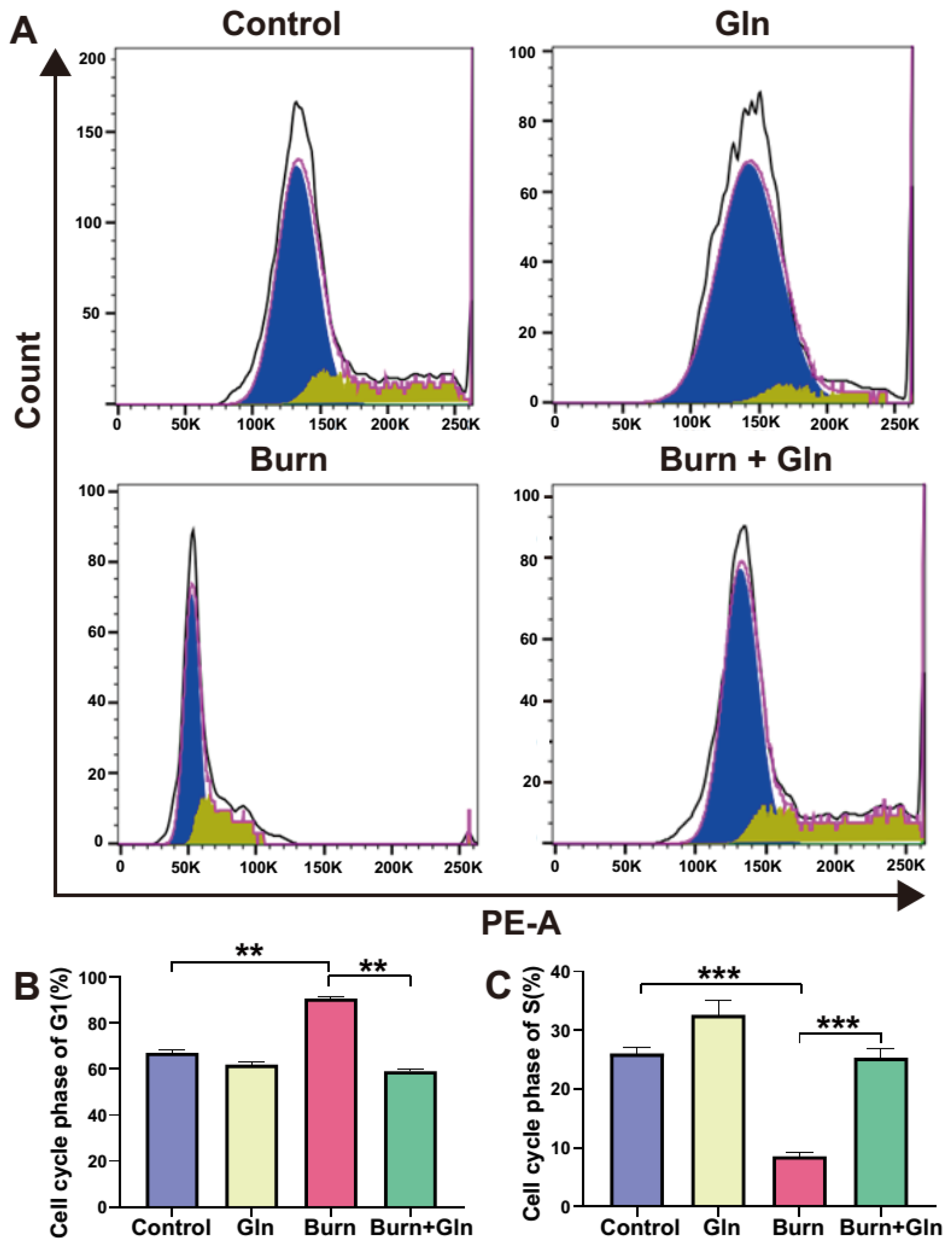
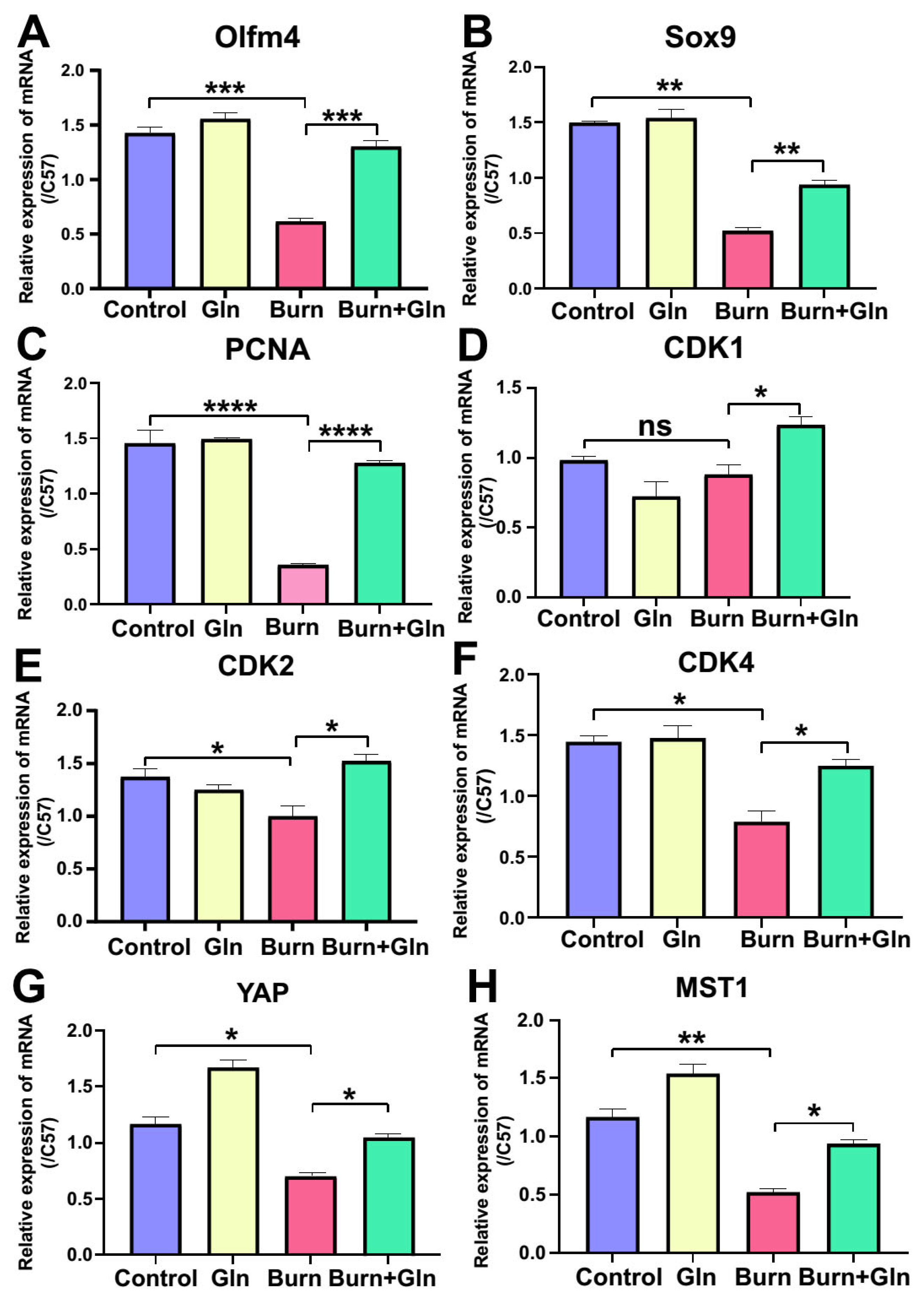
2.4. Gln Treatment Promotes Self-Renewal of ISCs by Speeding Up the Cell Cycle and Promoting Proliferation
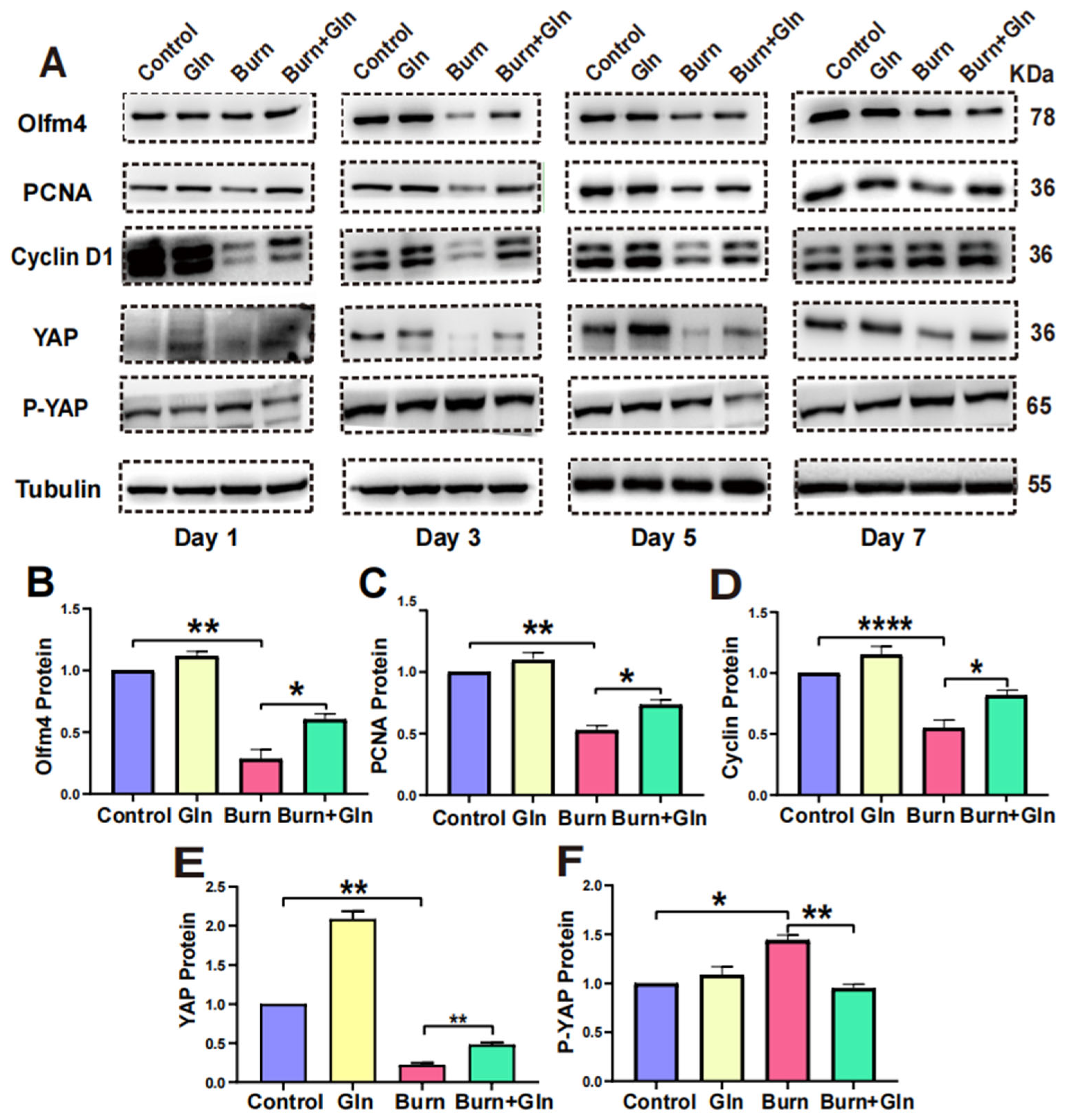
2.5. Gln Promotes Proliferation, Stemness and Modulates Cell Cycle Progression of ISCs via an Activation of YAP
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Experimental Animals
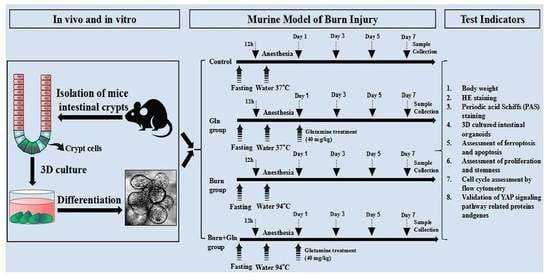
5.2. Murine Model of Burn Injury
5.3. H&E Staining and Determination of Villi Length and Crypt Depth
5.4. PAS Staining
5.5. Crypt Isolation and Culture
5.6. Protein Extraction and Western Blot Analysis
5.7. Immunofluorescence
5.8. Cell Cycle Detection
5.9. RNA Extraction and Quantitative Real-Time PCR Analysis
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, W.; Wang, Y.; Wang, P.; Wang, F. Intestinal barrier dysfunction in severe burn injury. Burn. Trauma 2019, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hageman, J.H.; Heinz, M.C.; Kretzschmar, K.; van der Vaart, J.; Clevers, H.; Snippert, H.J.G. Intestinal Regeneration: Regulation by the Microenvironment. Dev. Cell 2020, 54, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Fujishima, K.; Kengaku, M. Modeling Intestinal Stem Cell Function with Organoids. Int. J. Mol. Sci. 2021, 22, 10912. [Google Scholar] [CrossRef] [PubMed]
- Jasper, H. Intestinal Stem Cell Aging: Origins and Interventions. Annu. Rev. Physiol. 2020, 82, 203–226. [Google Scholar] [CrossRef] [Green Version]
- Gehart, H.; Clevers, H. Tales from the crypt: New insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 19–34. [Google Scholar] [CrossRef]
- Sanberg, P.R.; Borlongan, C.V. The proliferation and differentiation of stem cell journals. Stem Cell Rev. Rep. 2010, 6, 497–499. [Google Scholar] [CrossRef]
- Masai, H.; Matsumoto, S.; You, Z.; Yoshizawa-Sugata, N.; Oda, M. Eukaryotic chromosome DNA replication: Where, when, and how? Annu. Rev. Biochem. 2010, 79, 89–130. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.H.; Tseng, B.J.; Tseng, S.H. Influence of Growth Hormone and Glutamine on Intestinal Stem Cells: A Narrative Review. Nutrients 2019, 11, 1941. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef] [Green Version]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [Green Version]
- Rogeri, P.S.; Gasparini, S.O.; Martins, G.L.; Costa, L.K.F.; Araujo, C.C.; Lugaresi, R.; Kopfler, M.; Lancha, A.H., Jr. Crosstalk Between Skeletal Muscle and Immune System: Which Roles Do IL-6 and Glutamine Play? Front. Physiol. 2020, 11, 582258. [Google Scholar] [CrossRef]
- Chen, S.; Xia, Y.; Zhu, G.; Yan, J.; Tan, C.; Deng, B.; Deng, J.; Yin, Y.; Ren, W. Glutamine supplementation improves intestinal cell proliferation and stem cell differentiation in weanling mice. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Eyarefe, O.D.; Emikpe, B.O.; Akinloye, S.O.; Alonge, T.O.; Fayemi, O.E. Effects of honey, glutamine and their combination on canine small bowel epithelial cell proliferation following massive resection. Niger. J. Physiol. Sci. 2012, 27, 189–193. [Google Scholar]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Shin, J.E.; Park, H.W. The Role of Hippo Pathway in Cancer Stem Cell Biology. Mol. Cells 2018, 41, 83–92. [Google Scholar]
- Cheung, P.; Xiol, J.; Dill, M.T.; Yuan, W.C.; Panero, R.; Roper, J.; Osorio, F.G.; Maglic, D.; Li, Q.; Gurung, B.; et al. Regenerative Reprogramming of the Intestinal Stem Cell State via Hippo Signaling Suppresses Metastatic Colorectal Cancer. Cell Stem Cell 2020, 27, 590–604.e599. [Google Scholar] [CrossRef]
- LeBlanc, L.; Ramirez, N.; Kim, J. Context-dependent roles of YAP/TAZ in stem cell fates and cancer. Cell. Mol. Life Sci. 2021, 78, 4201–4219. [Google Scholar] [CrossRef]
- Enzo, E.; Santinon, G.; Pocaterra, A.; Aragona, M.; Bresolin, S.; Forcato, M.; Grifoni, D.; Pession, A.; Zanconato, F.; Guzzo, G.; et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015, 34, 1349–1370. [Google Scholar] [CrossRef]
- Henderson, J.; Duffy, L.; Stratton, R.; Ford, D.; O’Reilly, S. Metabolic reprogramming of glycolysis and glutamine metabolism are key events in myofibroblast transition in systemic sclerosis pathogenesis. J. Cell. Mol. Med. 2020, 24, 14026–14038. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, M.M.; Zhang, Y.; Wang, Z.E.; Dan, W.; Fan, S.J.; Wei, Y.; Xia, L.; Peng, X. Effectiveness and mechanism study of glutamine on alleviating hypermetabolism in burned rats. Nutrition 2020, 79, 110934. [Google Scholar] [CrossRef]
- Wu, D.; Su, S.; Zha, X.; Wei, Y.; Yang, G.; Huang, Q.; Yang, Y.; Xia, L.; Fan, S.; Peng, X. Glutamine promotes O-GlcNAcylation of G6PD and inhibits AGR2 S-glutathionylation to maintain the intestinal mucus barrier in burned septic mice. Redox Biol. 2023, 59, 102581. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.K.; Strasser, A. The essentials of developmental apoptosis. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, P.M.; Oriá, R.B.; Maier, E.A.; Guedes, M.; de Azevedo, O.G.; Wu, D.; Willson, T.; Hogan, S.P.; Lima, A.A.; Guerrant, R.L.; et al. Alanyl-glutamine promotes intestinal epithelial cell homeostasis in vitro and in a murine model of weanling undernutrition. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 301, G612–G622. [Google Scholar] [CrossRef] [Green Version]
- Reicher, N.; Melkman-Zehavi, T.; Dayan, J.; Wong, E.A.; Uni, Z. Nutritional stimulation by in-ovo feeding modulates cellular proliferation and differentiation in the small intestinal epithelium of chicks. Anim. Nutr. 2022, 8, 91–101. [Google Scholar] [CrossRef]
- Liu, L.; Michowski, W.; Kolodziejczyk, A.; Sicinski, P. The cell cycle in stem cell proliferation, pluripotency and differentiation. Nat. Cell Biol. 2019, 21, 1060–1067. [Google Scholar] [CrossRef]
- Biton, M.; Haber, A.L.; Rogel, N.; Burgin, G.; Beyaz, S.; Schnell, A.; Ashenberg, O.; Su, C.W.; Smillie, C.; Shekhar, K.; et al. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 2018, 175, 1307–1320.e1322. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.J.M.; Lo, Y.H.; Mah, A.T.; Kuo, C.J. The Intestinal Stem Cell Niche: Homeostasis and Adaptations. Trends Cell Biol. 2018, 28, 1062–1078. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lo, C.M.; Chen, L.; Ngan, E.S.; Xu, A.; Poon, R.Y. CDK1-PDK1-PI3K/Akt signaling pathway regulates embryonic and induced pluripotency. Cell Death Differ. 2017, 24, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Neganova, I.; Tilgner, K.; Buskin, A.; Paraskevopoulou, I.; Atkinson, S.P.; Peberdy, D.; Passos, J.F.; Lako, M. CDK1 plays an important role in the maintenance of pluripotency and genomic stability in human pluripotent stem cells. Cell Death Dis. 2014, 5, e1508. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zheng, Y.; Wang, Y.; Wang, J.; Sang, A.; Song, X.; Li, X. YAP1 alleviates sepsis-induced acute lung injury via inhibiting ferritinophagy-mediated ferroptosis. Front. Immunol. 2022, 13, 884362. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.L.; Brandquist, N.D.; Ouellette, C.Y.; Seshacharyulu, P.; Enke, C.A.; Ouellette, M.M.; Batra, S.K.; Yan, Y. PR55α regulatory subunit of PP2A inhibits the MOB1/LATS cascade and activates YAP in pancreatic cancer cells. Oncogenesis 2019, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.; Park, S.Y.; Kim, H.S.; Nam, J.S. The Hippo-YAP Signaling as Guardian in the Pool of Intestinal Stem Cells. Biomedicines 2020, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, L.; Zhao, B. The regulation and function of YAP transcription co-activator. Acta Biochim. Biophys. Sin. 2015, 47, 16–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Wang, Y.; Yang, G.; Han, J.; Zhu, L.; Li, L.; Zhang, S. The role of the Hippo pathway in the pathogenesis of inflammatory bowel disease. Cell Death Dis. 2021, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Balasubramanian, I.; D’Agostino, L.; Bandyopadhyay, S.; Patel, R.; Avasthi, S.; Yu, S.; Goldenring, J.R.; Bonder, E.M.; Gao, N. RAB11A-mediated YAP localization to adherens and tight junctions is essential for colonic epithelial integrity. J. Biol. Chem. 2021, 297, 100848. [Google Scholar] [CrossRef]
- Lin, C.; Yao, E.; Chuang, P.T. A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth. Dev. Biol. 2015, 403, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Fu, W.; Liu, Y.; Wang, Q.; Wang, L.; Huang, Y. Agrin Promotes Limbal Stem Cell Proliferation and Corneal Wound Healing Through Hippo-Yap Signaling Pathway. Investig. Opthalmology Vis. Sci. 2020, 61, 7. [Google Scholar] [CrossRef]
- Mia, M.M.; Cibi, D.M.; Abdul Ghani, S.A.B.; Song, W.; Tee, N.; Ghosh, S.; Mao, J.; Olson, E.N.; Singh, M.K. YAP/TAZ deficiency reprograms macrophage phenotype and improves infarct healing and cardiac function after myocardial infarction. PLoS Biol. 2020, 18, e3000941. [Google Scholar] [CrossRef]
- Cannon, A.R.; Akhtar, S.; Hammer, A.M.; Morris, N.L.; Javorski, M.J.; Li, X.; Kennedy, R.H.; Gamelli, R.L.; Choudhry, M.A. Effects of Mesalamine Treatment on Gut Barrier Integrity after Burn Injury. J. Burn. Care Res. 2016, 37, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Barrett, L.W.; Fear, V.S.; Foley, B.; Audsley, K.; Barnes, S.; Newnes, H.; McDonnell, A.; Wood, F.M.; Fear, M.W.; Waithman, J. Non-severe burn injury increases cancer incidence in mice and has long-term impacts on the activation and function of T cells. Burn. Trauma 2022, 10, tkac016. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Furukawa, K.; Bailey, C.A.; Wu, G. Oxidation of amino acids, glucose, and fatty acids as metabolic fuels in enterocytes of post-hatching developing chickens. J. Anim. Sci. 2022, 100, skac053. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.E.; Hattori, K.; Levy, L.; Imada, S.; Goto, N.; Vukovic, M.; Sze, D.; Kummerlowe, C.; Matute, J.D.; Duan, J.; et al. Screening for modulators of the cellular composition of gut epithelia via organoid models of intestinal stem cell differentiation. Nat. Biomed. Eng. 2022, 6, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Bai, Y.; Chen, Y.; Wang, K. Knockout of the Transducin-Like Enhancer of Split 6 Gene Affects the Proliferation and Cell Cycle Process of Mouse Spermatogonia. Int. J. Mol. Sci. 2020, 21, 5827. [Google Scholar] [CrossRef]
- Genoud, V.; Stortz, M.; Waisman, A.; Berardino, B.G.; Verneri, P.; Dansey, V.; Salvatori, M.; Remes Lenicov, F.; Levi, V. Extraction-free protocol combining proteinase K and heat inactivation for detection of SARS-CoV-2 by RT-qPCR. PLoS ONE 2021, 16, e0247792. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, P.; Zhang, Y.; Fan, S.; Wei, Y.; Yang, Z.; Wang, F.; Peng, X. Potential Effect of Glutamine in the Improvement of Intestinal Stem Cell Proliferation and the Alleviation of Burn-Induced Intestinal Injury via Activating YAP: A Preliminary Study. Nutrients 2023, 15, 1766. https://doi.org/10.3390/nu15071766
Chen X, Zhang P, Zhang Y, Fan S, Wei Y, Yang Z, Wang F, Peng X. Potential Effect of Glutamine in the Improvement of Intestinal Stem Cell Proliferation and the Alleviation of Burn-Induced Intestinal Injury via Activating YAP: A Preliminary Study. Nutrients. 2023; 15(7):1766. https://doi.org/10.3390/nu15071766
Chicago/Turabian StyleChen, Xia, Panyang Zhang, Yajuan Zhang, Shijun Fan, Yan Wei, Zhifan Yang, Fengchao Wang, and Xi Peng. 2023. "Potential Effect of Glutamine in the Improvement of Intestinal Stem Cell Proliferation and the Alleviation of Burn-Induced Intestinal Injury via Activating YAP: A Preliminary Study" Nutrients 15, no. 7: 1766. https://doi.org/10.3390/nu15071766
APA StyleChen, X., Zhang, P., Zhang, Y., Fan, S., Wei, Y., Yang, Z., Wang, F., & Peng, X. (2023). Potential Effect of Glutamine in the Improvement of Intestinal Stem Cell Proliferation and the Alleviation of Burn-Induced Intestinal Injury via Activating YAP: A Preliminary Study. Nutrients, 15(7), 1766. https://doi.org/10.3390/nu15071766