Effects of Immunonutrition on Cancer Patients Undergoing Surgery: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Oncologic Patient
3.2. Head and Neck Cancer Surgery
3.3. Hepatic Surgery
3.4. Bladder Surgery
3.5. Colorectal Surgery
3.6. Esophagogastric Surgery
3.7. Pancreatic Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Camblor-Álvarez, M.; Ocón-Bretón, M.J.; Luengo-Pérez, L.M.; Viruzuela, J.A.; Sendrós-Maroño, M.J.; Cervera-Peris, M.; Grande, E.; Álvarez-Hernández, J.; Jiménez-Fonseca, P. Nutritional support and parenteral nutrition in the oncological patient: An expert group consensus report. Nutr. Hosp. 2018, 35, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. Edinb. Scotl. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, R.A.; Wischmeyer, P.E. Clinical review: Optimizing enteral nutrition for critically ill patients—A simple data-driven formula. Crit. Care 2011, 15, 234. [Google Scholar] [CrossRef] [Green Version]
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G. A Randomized Trial of Glutamine and Antioxidants in Critically Ill Patients. N. Engl. J. Med. 2013, 368, 1489–1497. [Google Scholar] [CrossRef] [Green Version]
- Van Zanten, A.R.H.; Sztark, F.; Kaisers, U.X.; Zielmann, S.; Felbinger, T.W.; Sablotzki, A.R.; De Waele, J.J.; Timsit, J.-F.; Honing, M.L.H.; Keh, D.; et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: A randomized clinical trial. JAMA 2014, 312, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Mizock, B.A. Immunonutrition and critical illness: An update. Nutrition 2010, 26, 701–707. [Google Scholar] [CrossRef]
- Yu, J.; Liu, L.; Zhang, Y.; Wei, J.; Yang, F. Effects of omega-3 fatty acids on patients undergoing surgery for gastrointestinal malignancy: A systematic review and meta-analysis. BMC Cancer 2017, 17, 271. [Google Scholar] [CrossRef]
- Yu, K.; Zheng, X.; Wang, G.; Liu, M.; Li, Y.; Yu, P.; Yang, M.; Guo, N.; Ma, X.; Bu, Y.; et al. Immunonutrition vs Standard Nutrition for Cancer Patients: A Systematic Review and Meta-Analysis (Part 1). J. Parenter. Enteral Nutr. 2020, 44, 742–767. [Google Scholar] [CrossRef]
- Buzquurz, F.; Bojesen, R.D.; Grube, C.; Madsen, M.T.; Gögenur, I. Impact of oral preoperative and perioperative immunonutrition on postoperative infection and mortality in patients undergoing cancer surgery: Systematic review and meta-analysis with trial sequential analysis. BJS Open 2020, 4, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.D.P.; Howell, S.L.; Teixeira, F.J.; Pimentel, G.D. Dietary Amino Acids and Immunonutrition Supplementation in Cancer-Induced Skeletal Muscle Mass Depletion: A Mini-Review. Curr. Pharm. Des. 2020, 26, 970–978. [Google Scholar] [CrossRef]
- Howes, N.; Atkinson, C.; Thomas, S.; Lewis, S.J. Immunonutrition for patients undergoing surgery for head and neck cancer. Cochrane Database Syst. Rev. 2018, 8, CD010954. [Google Scholar] [CrossRef] [Green Version]
- Dechaphunkul, T.; Arundon, T.; Raungkhajon, P.; Jiratrachu, R.; Geater, S.L.; Dechaphunkul, A. Benefits of immunonutrition in patients with head and neck cancer receiving chemoradiation: A phase II randomized, double-blind study. Clin. Nutr. Edinb. Scotl. 2022, 41, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Luo, J.; Liu, Y.; Zhong, F.; Yang, X.; Gan, Y.; Su, S.; Li, B. Clinical Efficacy of Perioperative Immunonutrition Containing Omega-3-Fatty Acids in Patients Undergoing Hepatectomy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Nutr. Metab. 2020, 76, 375–386. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, B.; Jiao, A.; Li, F.; Wang, B.; Sun, N.; Zhang, J. The benefit of immunonutrition in patients undergoing hepatectomy: A systematic review and meta-analysis. Oncotarget 2017, 8, 86843–86852. [Google Scholar] [CrossRef] [Green Version]
- Ciacio, O.; Voron, T.; Pittau, G.; Lewin, M.; Vibert, E.; Adam, R.; Cunha, A.S.; Cherqui, D.; Schielke, A.; Soubrane, O.; et al. Interest of preoperative immunonutrition in liver resection for cancer: Study protocol of the PROPILS trial, a multicenter randomized controlled phase IV trial. BMC Cancer 2014, 14, 980. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Immunonutrition suppresses acute inflammatory responses through modulation of resolvin E1 in patients undergoing major hepatobiliary resection. Surgery 2016, 160, 228–236. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.M.; Stanley, A.; Bechtel, M.D.; Yankee, T.M.; Chalise, P.; Hand, L.K.; Lee, E.K.; Smelser, W.; Mirza, M.; Wyre, H.; et al. Perioperative Immunonutrition Modulates Inflammatory Response after Radical Cystectomy: Results of a Pilot Randomized Controlled Clinical Trial. J. Urol. 2018, 200, 292–301. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.M.; Bechtel, M.D.; Hand, L.K.; Schleper, A.; Yankee, T.M.; Chalise, P.; Lee, E.K.; Mirza, M.; Wyre, H.; Griffin, J.; et al. Effects of Immunonutrition for Cystectomy on Immune Response and Infection Rates: A Pilot Randomized Controlled Clinical Trial. Eur. Urol. 2016, 69, 389–392. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.M.; Michel, C.; Robertson, H.; Camargo, J.T.; Linares, B.; Holzbeierlein, J.; Hamilton-Reeves, J.M. Optimizing Nutritional Status in Patients Undergoing Radical Cystectomy: A Systematic Scoping Review. Bladder Cancer 2021, 7, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Burden, S.; Billson, H.A.; Lal, S.; Owen, K.A.; Muneer, A. Perioperative nutrition for the treatment of bladder cancer by radical cystectomy. Cochrane Database Syst. Rev. 2019, 5, CD010127. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.; Park, H.-M.; Kim, C.H.; Kim, H.R. Impact of Preoperative Immunonutrition on the Outcomes of Colon Cancer Surgery: Results from a Randomized Controlled Trial. Ann. Surg. 2023, 277, 381–386. [Google Scholar] [CrossRef]
- Wierdak, M.; Surmiak, M.; Milian-Ciesielska, K.; Rubinkiewicz, M.; Rzepa, A.; Wysocki, M.; Major, P.; Kłęk, S.; Pędziwiatr, M. Immunonutrition Changes Inflammatory Response in Colorectal Cancer: Results from a Pilot Randomized Clinical Trial. Cancers 2021, 13, 1444. [Google Scholar] [CrossRef] [PubMed]
- Moya, P.; Soriano-Irigaray, L.; Ramirez, J.M.; Garcea, A.; Blasco, O.; Blanco, F.J.; Brugiotti, C.; Miranda, E.; Arroyo, A. Perioperative Standard Oral Nutrition Supplements Versus Immunonutrition in Patients Undergoing Colorectal Resection in an Enhanced Recovery (ERAS) Protocol: A Multicenter Randomized Clinical Trial (SONVI Study). Medicine 2016, 95, e3704. [Google Scholar] [CrossRef] [PubMed]
- Moya, P.; Miranda, E.; Soriano-Irigaray, L.; Arroyo, A.; Aguilar, M.-D.; Bellón, M.; Muñoz, J.-L.; Candela, F.; Calpena, R. Perioperative immunonutrition in normo-nourished patients undergoing laparoscopic colorectal resection. Surg. Endosc. 2016, 30, 4946–4953. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, X.; Xin, Q.; Cheng, Y.; Zhan, Z.; Zhang, J.; Wu, J. Effect of immunonutrition on colorectal cancer patients undergoing surgery: A meta-analysis. Int. J. Colorectal Dis. 2018, 33, 273–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Xiao, G.; Zhou, Y.; Ye, Y.; Wang, B. Impact of perioperative enteral immunonutrition in patients with gastrointestinal cancer undergoing elective surgery: A randomized controlled trial. Clin. Nutr. ESPEN 2021, 46, S776. [Google Scholar] [CrossRef]
- Cao, Y.; Han, D.; Zhou, X.; Han, Y.; Zhang, Y.; Li, H. Effects of preoperative nutrition on postoperative outcomes in esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2022, 35, doab028. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Zhang, L.; Wu, J.; Zhan, Z. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: A systematic review and meta-analysis. BMC Gastroenterol. 2018, 18, 11. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.-W.; Zhou, L.; Liu, Z.-Z.; Pei, D.-P.; Fan, W.-Q.; Ning, W. A Systematic Review and Meta-Analysis of the Effects of Perioperative Immunonutrition in Gastrointestinal Cancer Patients. Nutr. Cancer 2021, 73, 252–261. [Google Scholar] [CrossRef]
- Adiamah, A.; Rollins, K.E.; Kapeleris, A.; Welch, N.T.; Iftikhar, S.Y.; Allison, S.P.; Lobo, D.N. Postoperative arginine-enriched immune modulating nutrition: Long-term survival results from a randomised clinical trial in patients with oesophagogastric and pancreaticobiliary cancer. Clin. Nutr. Edinb. Scotl. 2021, 40, 5482–5485. [Google Scholar] [CrossRef]
- Li, X.-K.; Zhou, H.; Xu, Y.; Cong, Z.-Z.; Wu, W.-J.; Luo, J.; Jiang, Z.-S.; Shen, Y. Enteral immunonutrition versus enteral nutrition for patients undergoing oesophagectomy: A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.-G.; Luo, J.; Song, H.Y.D.T.N.; Alai, G.H.; Shen, X.; Lin, Y.D. Is immunonutrition superior to standard enteral nutrition in reducing postoperative complications in patients undergoing esophagectomy? A meta-analysis of randomized controlled trials. J. BUON Off. J. Balk. Union Oncol. 2021, 26, 204–210. [Google Scholar]
- Li, X.-K.; Cong, Z.-Z.; Wu, W.-J.; Xu, Y.; Zhou, H.; Wang, G.-M.; Qiang, Y.; Luo, L.-G.; Shen, Y. Enteral immunonutrition versus enteral nutrition for patients undergoing esophagectomy: A randomized controlled trial. Ann. Palliat. Med. 2021, 10, 1351–1361. [Google Scholar] [CrossRef]
- Kanekiyo, S.; Takeda, S.; Iida, M.; Nishiyama, M.; Kitahara, M.; Shindo, Y.; Tokumitsu, Y.; Tomochika, S.; Tsunedomi, R.; Suzuki, N.; et al. Efficacy of perioperative immunonutrition in esophageal cancer patients undergoing esophagectomy. Nutrition 2019, 59, 96–102. [Google Scholar] [CrossRef]
- Song, G.-M.; Liu, X.-L.; Bian, W.; Wu, J.; Deng, Y.-H.; Zhang, H.; Tian, X. Systematic review with network meta-analysis: Comparative efficacy of different enteral immunonutrition formulas in patients underwent gastrectomy. Oncotarget 2017, 8, 23376–23388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.S.; Aly, E.H. The effects of enteral immunonutrition in upper gastrointestinal surgery: A systematic review and meta-analysis. Int. J. Surg. 2016, 29, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Umeda, Y.; Yoshida, R.; Yagi, T.; Fujiwara, T. Systematic review on immunonutrition in partial pancreatoduodenectomy. Langenbecks Arch. Surg. 2020, 405, 585–593. [Google Scholar] [CrossRef]
- Guan, H.; Chen, S.; Huang, Q. Effects of Enteral Immunonutrition in Patients Undergoing Pancreaticoduodenectomy: A Meta-Analysis of Randomized Controlled Trials. Ann. Nutr. Metab. 2019, 74, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-A.; Chen, Y.-C.; Tiong, C. Immunonutrition in Patients with Pancreatic Cancer Undergoing Surgical Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2798. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin. Nutr. Edinb. Scotl. 2020, 39, 2045–2054. [Google Scholar] [CrossRef]
- Kengsakul, M.; Nieuwenhuyzen-de Boer, G.M.; Udomkarnjananun, S.; Kerr, S.J.; Niehot, C.D.; van Beekhuizen, H.J. Factors predicting postoperative morbidity after cytoreductive surgery for ovarian cancer: A systematic review and meta-analysis. J. Gynecol. Oncol. 2022, 33, e53. [Google Scholar] [CrossRef]
- Schaefers, C.; Seidel, C.; Bokemeyer, F.; Bokemeyer, C. The prognostic impact of the smoking status of cancer patients receiving systemic treatment, radiation therapy, and surgery: A systematic review and meta-analysis. Eur. J. Cancer 2022, 172, 130–137. [Google Scholar] [CrossRef]
- Cereda, E.; Pisati, R.; Rondanelli, M.; Caccialanza, R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients 2022, 14, 1524. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Anand, L.; Vyas, A.K.; Premkumar, M.; Choudhury, A.K.; Trehanpati, N.; Benjamin, J.; Kumar, G.; Joshi, Y.K.; Sarin, S.K. Omega-3 fatty acid lipid emulsions are safe and effective in reducing endotoxemia and sepsis in acute-on-chronic liver failure: An open-label randomized controlled trial. J. Gastroenterol. Hepatol. 2021, 36, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Muñoz-Rodríguez, J.; Martos, R.; Parejo, V.; Prera, Á.; Tremps, C.; Bonfill, T.; del Pino, C.; Augé, A.; Prats, J. Progressive perioperative benefits of laparoscopy in combination with an ERAS (Enhanced Recovery After Surgery) protocol in radical cystectomy with ileal conduit. Actas Urol. Esp. 2021, 45, 289–299. [Google Scholar] [CrossRef]
- Wie, G.-A.; Cho, Y.-A.; Kim, S.-Y.; Kim, S.-M.; Bae, J.-M.; Joung, H. Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition 2010, 26, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Candela, C.G.; Milla, S.P.; Lozano, E.C.; Di Martino, M.; Alcolea, N.G.; Roldán, J.O.; de la Rica, A.S.; Lagunas, D.P. Immunonutrition in fast-track surgical patients—Evidence review and adapted algorithm. Nutr. Hosp. 2021, 38, 601–6021. [Google Scholar] [CrossRef]
- Bond-Smith, G.; Belgaumkar, A.P.; Davidson, B.R.; Gurusamy, K.S. Enhanced recovery protocols for major upper gastrointestinal, liver and pancreatic surgery. Cochrane Database Syst. Rev. 2016, 2, CD011382. [Google Scholar] [CrossRef] [Green Version]

| Set | Items | Terms |
|---|---|---|
| S1 | 294 | MJEMB(IMMUNONUTRITION) |
| S2 | 6759974 | EMB(SURGERY) OR EMB(PREOPERATIVE PERIOD) OR EMB.EXPLODE(SURGERY) OR EMB(POSTOPERATIVE CARE) OR EMB(ENHANCED RECOVERY AFTER SURGERY) OR (“FAST TRACK SURGERY”) OR EMB.EXPLODE(POSTOPERATIVE COMPLICATION) OR (“POSTOPERATIVE MORBIDITY”) OR EMB(SURGICAL MORTALITY) OR EMB(HOSPITAL READMISSION) OR EMB.EXPLODE(MALIGNANT NEOPLASM) OR EMB(CANCER SURGERY) |
| S3 | 707888 | EMB(TRAUMATIC BRAIN INJURY) OR EMB(SEPSIS) OR EMB(ACUTELY ILL PATIENT) OR EMB(BURN PATIENT) OR EMB(STEM CELL TRANSPLANTATION) OR EMB(BONE MARROW TRANSPLANTATION) OR EMB(INFLAMMATORY BOWEL DISEASE) OR EMB(CHRONIC INFLAMMATION) |
| S4 | 1557575 | EMB(SARCOPENIA) OR (“SKELETAL MUSCLE MASS”) OR EMB(FUNCTIONAL STATUS) OR EMB(CACHEXIA) OR EMB(MALNUTRITION) OR EMB(BODY COMPOSITION) OR (“BIOELECTRICAL IMPEDANCE”) OR (“PHASE ANGLE”) OR EMB(ECHOGRAPHY) OR EMB(COMPUTER ASSISTED TOMOGRAPHY) OR (“L3 MUSCLE”) OR EMB(DUAL-ENERGY X-RAY ABSORPTIOMETRY) |
| S5 | 1361498 | EMB(RANDOMIZED CONTROLLED TRIAL) OR EMB(SYSTEMATIC REVIEW) OR EMB(META ANALYSIS) |
| S6 | 7333881 | EMB(HUMAN) AND (LA(ENGLISH) OR LA(SPANISH)) AND PY (≥2016) |
| S7 | 65 | S1 AND (S2 OR S3 OR S4) AND S5 AND S6 |
| Article | Study Type | Number of Patients | Type of Patients | Intervention | Results | GRADE |
|---|---|---|---|---|---|---|
| ONCOLOGIC SURGERY | ||||||
| Yu K et al., 2020 [10] | Meta-analysis | 5983 (61 studies) | Oncologic surgery | IN (oral, enteral, parenteral, at least one of the following: arginine, glutamine, w3, nucleotides) vs. SN, conventional therapy, or fluids in the pre-, peri-, and PO period. | Reduced total infectious complications (RR 0.71 (0.64, 0.79)), wound infection (n = 4788, RR 0.72 (0.60, 0.87)), respiratory infections (n = 4919, RR 0.70 (0.59, 0.84)), UTI (n = 3686, RR 0.69 (0.51, 0.94)), anastomotic dehiscence (n = 3329, RR 0.70 (0.53, 0.91)), and hospital LOS (–2.12 (–2.72, −1.52) days). No differences in sepsis (n = 2322) or overall mortality. | Low (++oo) |
| Buzquurz F et al., 2020 [11] | Meta-analysis | 2159 (22 studies) | Oncologic surgery | IN from 30 days before surgery to at least 5 days before, which was allowed to continue after surgery, administered by oral or enteral route. The control group received standard care or placebo. | Reduced total infectious complications (n = 2068, RR 0.58 (0.48, 0.70)) and surgical site infection (n = 1958, RR 0.65 (0.50, 0.85)). No differences in mortality (n = 1641). | Moderate (+++o) |
| Article | Study Type | Number of Patients | Type of Patients | Intervention | Results | GRADE |
|---|---|---|---|---|---|---|
| HEAD AND NECK CANCER | ||||||
| Dechaphunkul T et al., 2022 [14] | RCT | 110 | Patients receiving chemoradiation | IN vs. isocaloric isonitrogenous formula 5 consecutive days before each chemotherapy session. | No difference in the proportion of patients with grade 3–4 oral mucositis between the two groups (62% vs. 67%, p = 0.690). | Low (++oo) |
| Howes N et al., 2018 [13] | Cochrane review | 1099 (19 RCT) | Surgery for head and neck cancer | Pre-, peri- or PO IN vs. SN or no ONS. | No difference in hospital LOS, wound infection, or overall mortality. Reduced fistula: RR 0.48 (95% CI 0.27 to 0.85; 10 studies, 747 participants; low-quality evidence). | Moderate (+++o) |
| Article | Study Type | Number of Patients | Type of Patients | Intervention | Results | GRADE |
|---|---|---|---|---|---|---|
| HEPATIC SURGERY | ||||||
| Gao B et al., 2020 [15] | Meta-analysis | 966 (9 studies) | Hepatectomy | Perioperative IN vs. SN, placebo or conventional diet (in 4 studies, ω-3 parenteral). | Reduced PO complications (RR 0.57 (0.34, 0.95)), total infections (RR 0.53 (0.37, 0.75)), wound infection (RR 0.50 (0.28, 0.89)), pneumonia (RR 0.60 (0.32, 1.12)), UTI (RR 1.30 (0.55, 3.08)), liver failure (RR 0.54 (0.23, 1.24)), mortality (RR 0.69 (0.26, 1.83)) and hospital LOS (–3.80 (–6.59, –1.02) days). | Low (++oo) |
| Zhang C et al., 2017 [16] | Meta-analysis | 805 (8 studies) | Hepatectomy | Perioperative IN (in 6 studies, ω-3 enteral or parenteral) vs. isocaloric isonitrogenous nutrition | Reduced PO complications (RR 0.59 (0.46, 0.75)), total infections (RR 0.46 (0.32, 0.68)). No differences in mortality. Hospital stay was shorter in the ω-3 group (–0.49 (–0.81, –0.16) days). | Low (++oo) |
| Ciacio O et al., 2021 [17] | RCT | 399 | Liver resection for cancer (not cirrhosis) | Oral IN 7 days before vs. isocaloric isonitrogenous formula | No differences in 30-day morbidity rate (Clavien–Dindo >2), infectious and non-infectious complications, LOS, or duration of antibiotic treatment. | High (++++) |
| Uno H et al., 2016 [18] | RCT | 40 | Major hepatobiliary resection in cancer | Oral IN 5 days before (1000 kcal/day of IEN formula) vs. isocaloric diet | Reduced infections at 30 days (wound infection, abscesses, pneumonia, sepsis, 40 vs. 75% (p < 0.05)), shorter LOS (36.9 vs. 53.9 days, p < 0.01), lessened severity of complications (p < 0.05) (Clavien–Dindo scale), increased EPA/AA and resolvin E1, and decreased IL6 (same PCR results in both groups). | Moderate (+++o) |
| Article | Study Type | Number of Patients | Type of Patients | Intervention | Results | GRADE |
|---|---|---|---|---|---|---|
| BLADDER SURGERY | ||||||
| Hamilton-Reeves JM et al., 2018 [19] | RCT | 29 | Radical cystectomy (bladder cancer, not metastatic, not severe malnutrition) | Pre-IN (5 days) and post- IN (5 days) or HC HP ONS | Increased the T-helper lymphocytes (Th1–Th2) balance, decreased interleukin 6 (IL6), plasma arginine was maintained. No difference in appendicular muscle loss. | Low (++oo) |
| Hamilton-Reeves JM et al., 2016 [20] | RCT | 29 | Radical cystectomy (bladder cancer, not metastatic, no severe malnutrition) | Pre-IN (5 days) and post-IN (5 days) or HC HP ONS | 33% reduction in PO complications at 90 days (RR 0.31, 95% CI 0.08 to 1.23, p:0.060), without differences in hospital LOS. | Low (++oo) |
| Alam SM et al., 2021 [21] | Systematic review | 17 studies (6 with IN) | Radical cystectomy | 2 studies compared IN with standard care and without ONS, 3 compared it with ONS, and in 1 the information was not available. | 2 studies showed a reduction in PO complications, 1 study found immunological changes and a reduction in some inflammatory mediators, and 1 study found no differences in infectious complications. The other 2 studies are pending results. | Very Low (+ooo) |
| Burden S et al., 2019 [22] | Cochrane review | 500 (8 RCT) IN 1 study with 29 patients | Radical cystectomy for bladder cancer | Perioperative nutrition | Limited evidence for a perioperative nutrition benefit from the interventions. IN reduced 90-day PO complications (RR 0.31, 95% CI 0.08 to 1.23; low-quality evidence). Similar hospital LOS. | Very low (+ooo) |
| Article | Study Type | Number of Patients | Type of Patients | Intervention | Results | GRADE |
|---|---|---|---|---|---|---|
| COLORECTAL SURGERY | ||||||
| Lee SY et al., 2021 [23] | RCT | 176 | Colon cancer | Preoperatory IN (400 mL/day) for 7 days vs. standard diet. | No differences in PO infectious and noninfectious complications, overall complications, 30-day readmission, hospital LOS, or weight. | Moderate (+++o) |
| Wierdak M et al., 2021 [24] | RCT | 26 | Colorectal cancer | 14 days before surgery IN (400 mL/day) vs. ONS. | Changed inflammatory response ((tumoral necrosis factor-α) TNF-α, interleukin 8 (IL-8), C-X-C Motif Chemokine Ligand 1 (CXCL1)), superficial neutrophil infiltration. No changes in morbidity, LOS, or readmissions. | Moderate (+++o) |
| Moya P et al., 2016 [25] | RCT | 122 | Laparoscopic colorectal resection (not malnourish) | IN (400 mL/day) for 7 days before and 5 days after surgery vs. dietetic recommendations. | Reduced cases of wound infection (11.50 vs. 0.00%, p = 0.006). No differences in hospital LOS. | Moderate (+++o) |
| Moya P et al., 2016 [26] | RCT | 244 | Colorectal resection (not malnourished) | IN (400 mL/day) for 7 days before and 5 days after surgery vs. isocaloric isonitrogenous formula. | Reduced overall complications, mainly due to a decrease in the number of infectious complications (23.8% vs. 0.7%, p = 0.0007). Surgical site infection number was lower (16.4% vs. 5.7%, p = 0.0008). No differences in hospital LOS. | Moderate (+++o) |
| Xu J et al., 2018 [27] | Systematic Review | 6 Studies with enteral immunonutrition | Colorectal surgery | RCT Enteral Immunonutrition vs Standard formula | Immunonutrition can reduce the length of hospital stay (OR 2.53 (1.29–2.41)) and number of infectious complications (OR 0.33; (0.21–0.53)) | Low (++oo) |
| Article | Study Type | Number of Patients | Type of Patients | Intervention | Results | GRADE |
|---|---|---|---|---|---|---|
| ESOPHAGOGASTRIC SURGERY | ||||||
| Cao Y et al., 2022 [29] | Meta-analysis | 1864 (15 studies, RCT and observational) | Esophageal cancer | Preoperatory IN vs. isocaloric isonitrogenous formula | Reduced infectious complications (OR = 0.51, 95% CI (0.26, 0.98) and length of hospital stay (MD = −2.10 days), 95% CI (−3.72, −0.47)). No differences in overall complications, in-hospital mortality, or anastomotic leaks. | Very Low (+ooo) |
| Cheng Y et al., 2018 [30] | Meta-analysis | 583 (7 studies) | Gastric cancer | PO IN vs. EN | Increased levels of CD4+, CD4+/ CD8+, IgM, IgG, lymphocytes, and prealbumin (when administered for >7 days), reduced systemic inflammatory response syndrome (MD, –0.89 days; 95% CI, –1.40 to–0.39; p = 0.005) and postoperative complications (RR, 0.29; 95% CI, 0.14–0.60; p = 0.001). Pulmonary infection, length of hospitalization, CD8+, and other serum proteins were not improved. | Low (++oo) |
| Niu JW et al., 2021 [31] | Meta-analysis | 16 studies | Gastrointestinal cancer | Perioperative IN vs. SN | Decreased the risk of surgical site infection, hospital stay (in addition to early enteral nutrition after surgical resection of gastric cancer), WBC, and CRP. No changes in CD4+ or inflammatory cytokines. | Low (++oo) |
| Adiamah et al., 2021 [32] | RCT | 108 | Esophagogastric or pancreaticobiliary cancer (not metastatic nor chemotherapy) | PO (10–15 days), jejunostomy, IN vs. isocaloric isonitrogenous formula | No differences in long-term survival | High (++++) |
| Li XK et al., 2020 [33] | Meta-analysis | 320 (6 studies) | Esophageal cancer | Perioperative IN vs. SN | No improved clinical outcomes or immune indices. | High (++++) |
| Zhuo ZG et al., 2021 [34] | Meta-analysis | 638 (6 studies) | Esophageal cancer | Perioperative IN vs. SN. | No significant differences in PO complications. | High (++++) |
| Li XK et al., 2021 [35] | RCT | 112 | Esophageal cancer | Preoperatory (7 days) IN vs. oral nutrition and PO jejunostomy EIN vs. EN. | Reduced the rate of CD8/CD3 at POD 3, increased the rate of CD4/CD8 at POD 3, IgM at POD 3 and 7, and the rates of NK and IgA at PDD 30. No significant differences in 2-year progression-free survival or overall survival. | Moderate (+++o) |
| Kanekiyo S et al., 2019 [36] | RCT | 40 | Esophageal cancer | Preoperatory (7 days) IN vs. oral nutrition and PO (7 days) jejunostomy EIN vs. EN. | Increased RBP, decreased infectious complications and changes to therapeutic antibiotics. No differences in ICU or hospital LOS, 5-year progression-free survival, or overall survival. | High (++++) |
| Song GM et al., 2017 [37] | Meta-analysis | 840 (11 RCT) | Gastric cancer | Perioperative IN vs. SN. Comparison of different IN formulas. | Decreased IC and LOS. Arg+RNA+ω-3-FAs was superior to Arg+RNA and Arg+Gln for IC. | Moderate (+++o) |
| Wong CS et al., 2016 [38] | Meta-analysis | 2016 (19 RCT) | Upper gastrointestinal surgery | Perioperative IN vs. SN. | Reduced wound infections (RR 0.59, 95% CI 0.40–0.88; p = 0.009) and hospital LOS (MD −2.92 days, 95% CI −3.89 to −1.95; p < 0.00001). | Moderate (+++o) |
| Article | Study Type | Number of Patients | Type of Patients | Intervention | Results | GRADE |
|---|---|---|---|---|---|---|
| PANCREATIC SURGERY | ||||||
| Takagi K et al., 2020 [39] | Meta-analysis | 349 (5 studies) | Partial pancreatoduodenectomy | IN vs. SN, PN, or no intervention | Reduced overall PO complications and infections. No effects on major complications, mortality, fistula, or delayed gastric emptying. | Moderate (+++o) |
| Guan H et al., 2019 [40] | Meta-analysis | 299 (4 RCT) | Pancreatoduodenectomy | Perioperative IN vs. SN. | Reduced PO infectious complications (RR 0.58, 95% CI 0.37–0.92; p = 0.02) and hospital LOS (MD −1.79, 95% CI −3.40 to 0.18; p = 0.03). No differences in overall PO complications, non-infectious complications, or PO mortality. | Moderate (+++o) |
| Yang F et al., 2020 [41] | Meta-analysis | 368 (6 RCT) | Pancreatic cancer | Perioperative IN vs. SN. | Decreased the rate of infectious complications (RR = 0.47, 95% CI (0.23, 0.94), p = 0.03) and the hospital LOS (MD = −1.90, 95% CI (−3.78, −0.02), p = 0.05), especially in the preoperative group. | High (++++) |
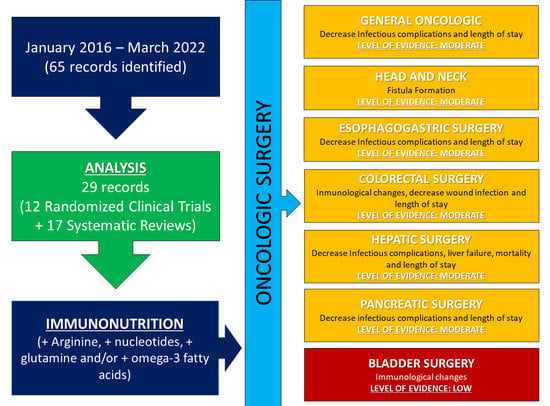
| Localization of Surgery | Effect of Immunonutrition | Level of Evidence |
|---|---|---|
| General oncologic | Decreased infectious complications and length of stay | MODERATE |
| Head and neck | Fistula formation | MODERATE |
| Esophagogastric surgery | Decreased infectious complications and length of stay | MODERATE |
| Colorectal surgery | Immunological changes, decreased wound infections and length of stay | MODERATE |
| Hepatic surgery | Decreased infectious complications, liver failure, mortality, and length of stay | MODERATE |
| Pancreatic surgery | Decreased infectious complications and length of stay | MODERATE |
| Bladder surgery | Immunological changes | LOW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Malpartida, K.; Aragón-Valera, C.; Botella-Romero, F.; Ocón-Bretón, M.J.; López-Gómez, J.J. Effects of Immunonutrition on Cancer Patients Undergoing Surgery: A Scoping Review. Nutrients 2023, 15, 1776. https://doi.org/10.3390/nu15071776
García-Malpartida K, Aragón-Valera C, Botella-Romero F, Ocón-Bretón MJ, López-Gómez JJ. Effects of Immunonutrition on Cancer Patients Undergoing Surgery: A Scoping Review. Nutrients. 2023; 15(7):1776. https://doi.org/10.3390/nu15071776
Chicago/Turabian StyleGarcía-Malpartida, Katherine, Carmen Aragón-Valera, Francisco Botella-Romero, María Julia Ocón-Bretón, and Juan J. López-Gómez. 2023. "Effects of Immunonutrition on Cancer Patients Undergoing Surgery: A Scoping Review" Nutrients 15, no. 7: 1776. https://doi.org/10.3390/nu15071776
APA StyleGarcía-Malpartida, K., Aragón-Valera, C., Botella-Romero, F., Ocón-Bretón, M. J., & López-Gómez, J. J. (2023). Effects of Immunonutrition on Cancer Patients Undergoing Surgery: A Scoping Review. Nutrients, 15(7), 1776. https://doi.org/10.3390/nu15071776